Pele ramseyi, Ng, Peter K. L., 2011
|
publication ID |
https://doi.org/10.5281/zenodo.202261 |
|
DOI |
https://doi.org/10.5281/zenodo.6183146 |
|
persistent identifier |
https://treatment.plazi.org/id/901E8781-FFEB-311C-FF70-FE55FB09F8CC |
|
treatment provided by |
Plazi |
|
scientific name |
Pele ramseyi |
| status |
gen. nov. |
Pele ramseyi View in CoL , new genus, new species
( Figs. 2–9 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 )
Material. Holotype: male (9.32 × 4.99 mm) ( BPBM S15980 View Materials ), anchialine lava caves of Ahihi-Kina‘u Natural Area Reserve, Maui, Hawaii, tide 0.4 m, coll. M. Ramsey, 31 July 2010, 1800 hours. Paratype: 1 female (11.53 × 6.14 mm) ( ZRC 2010.0322), same data as holotype.
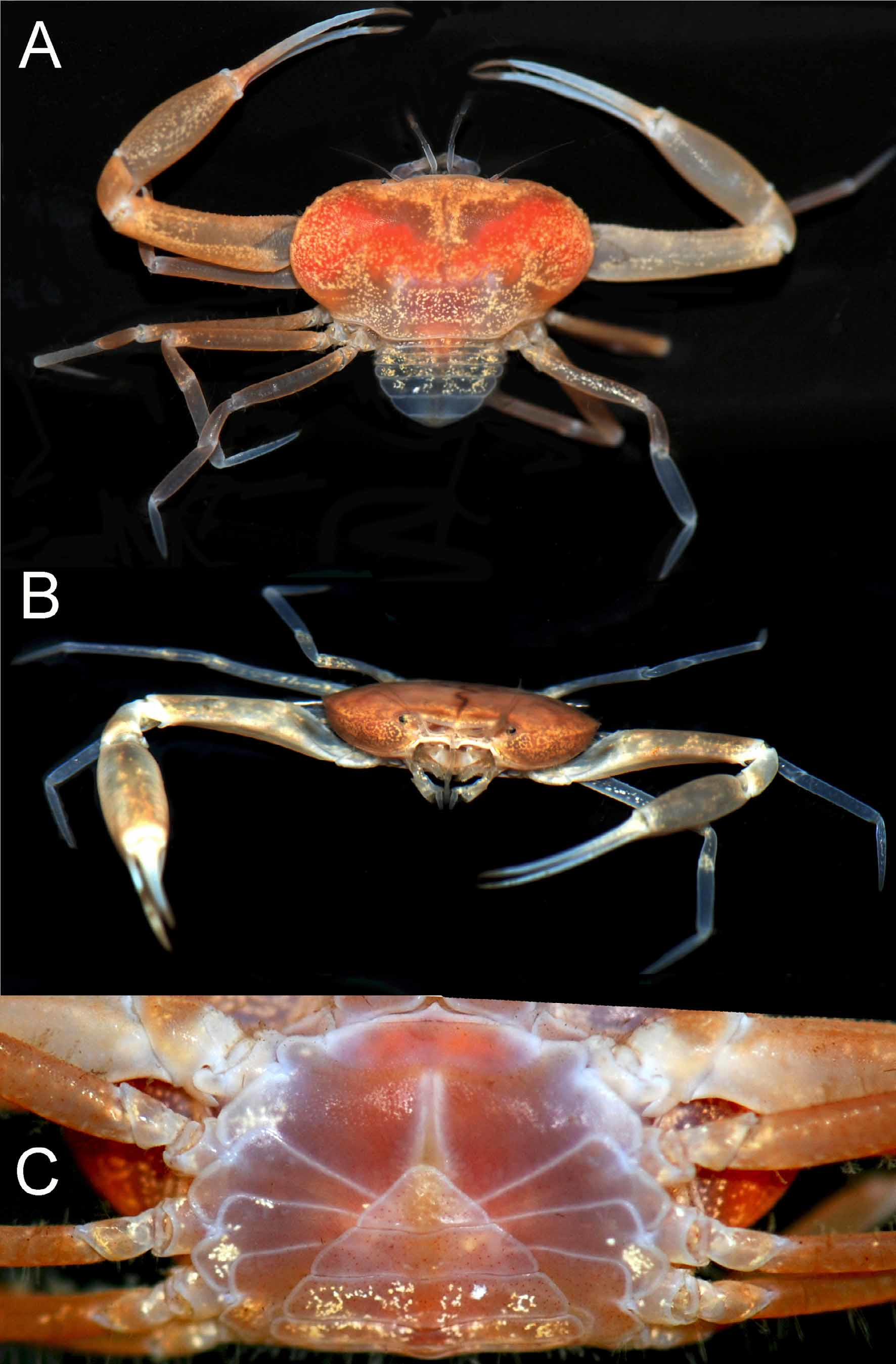
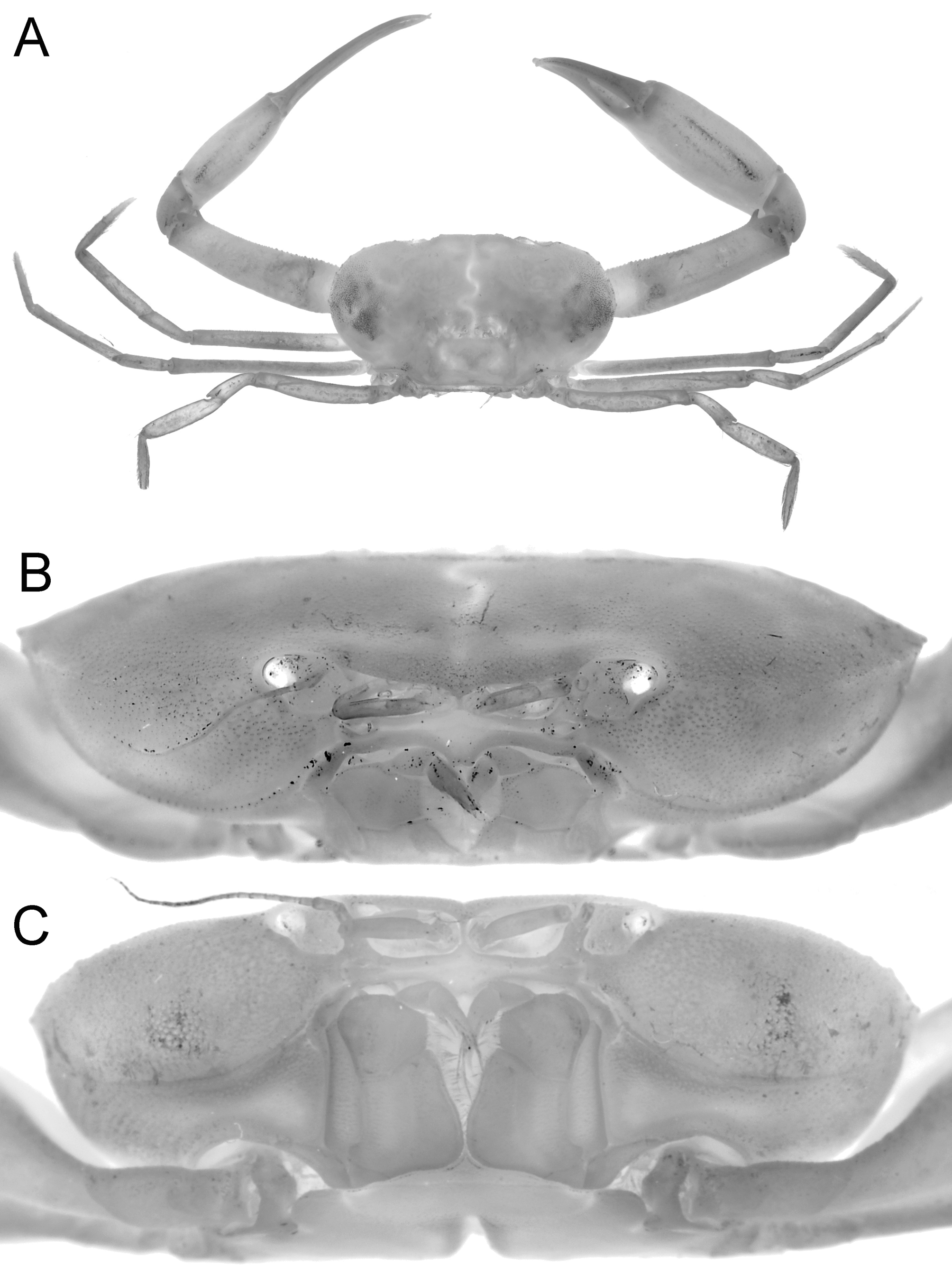
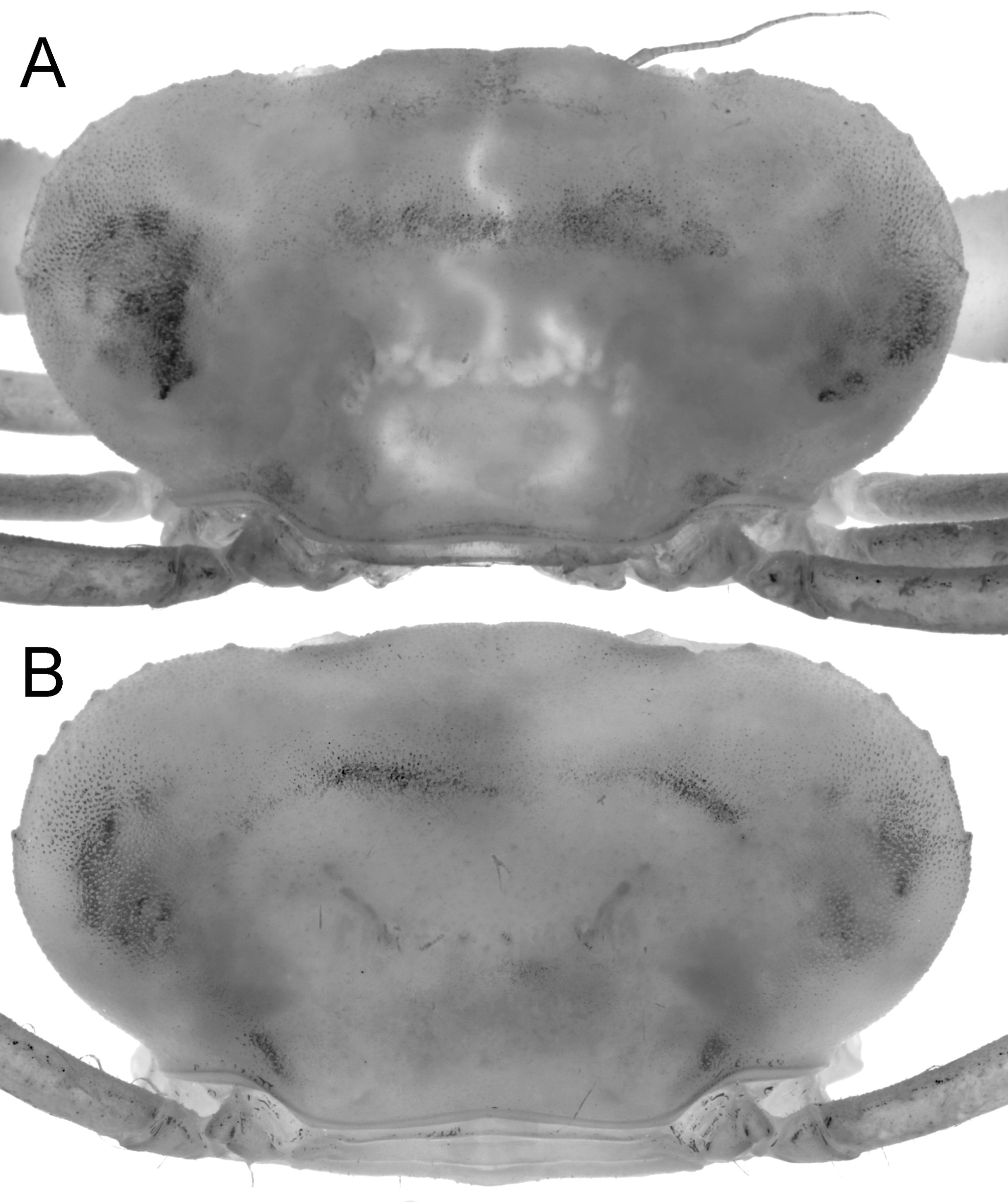
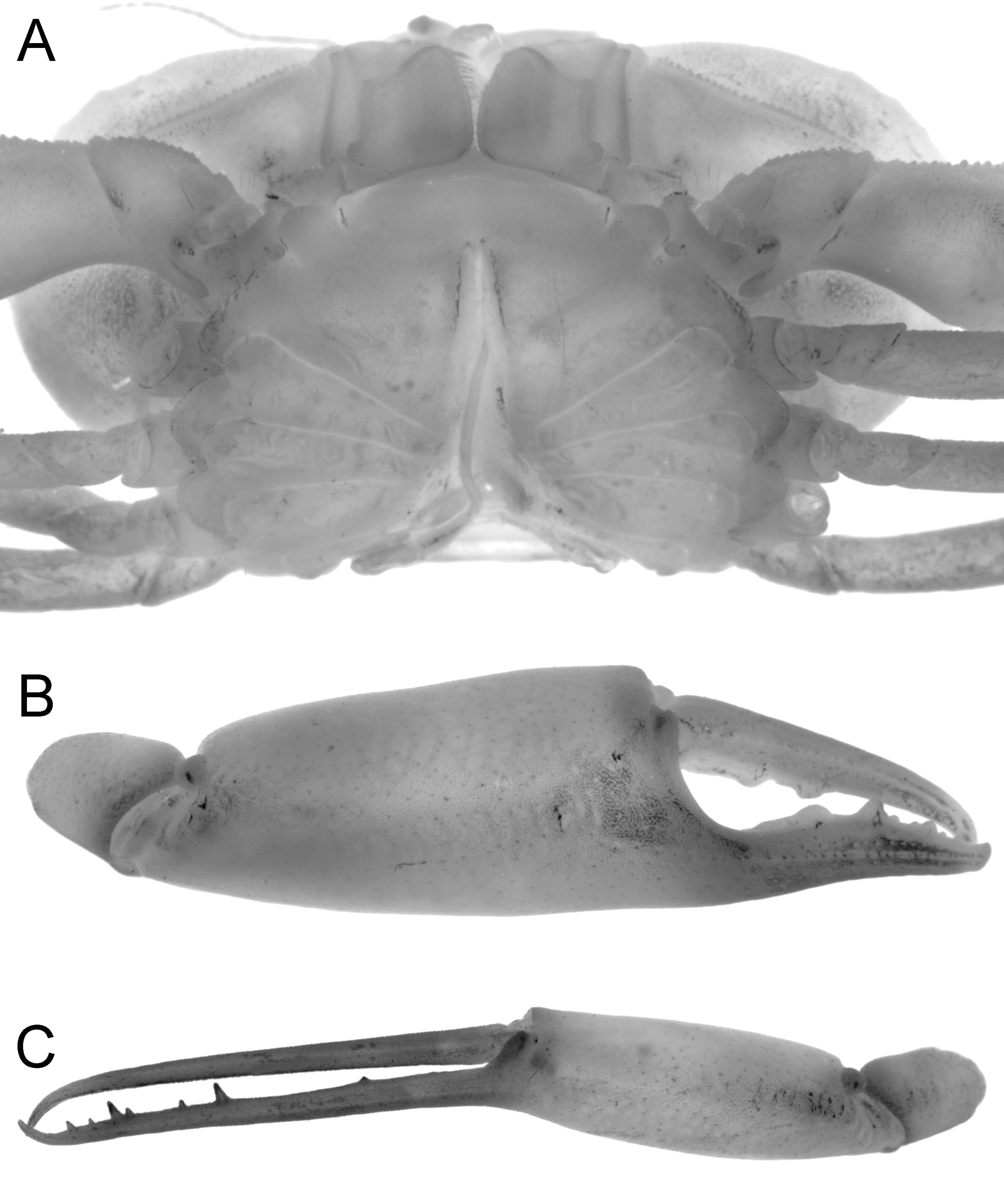
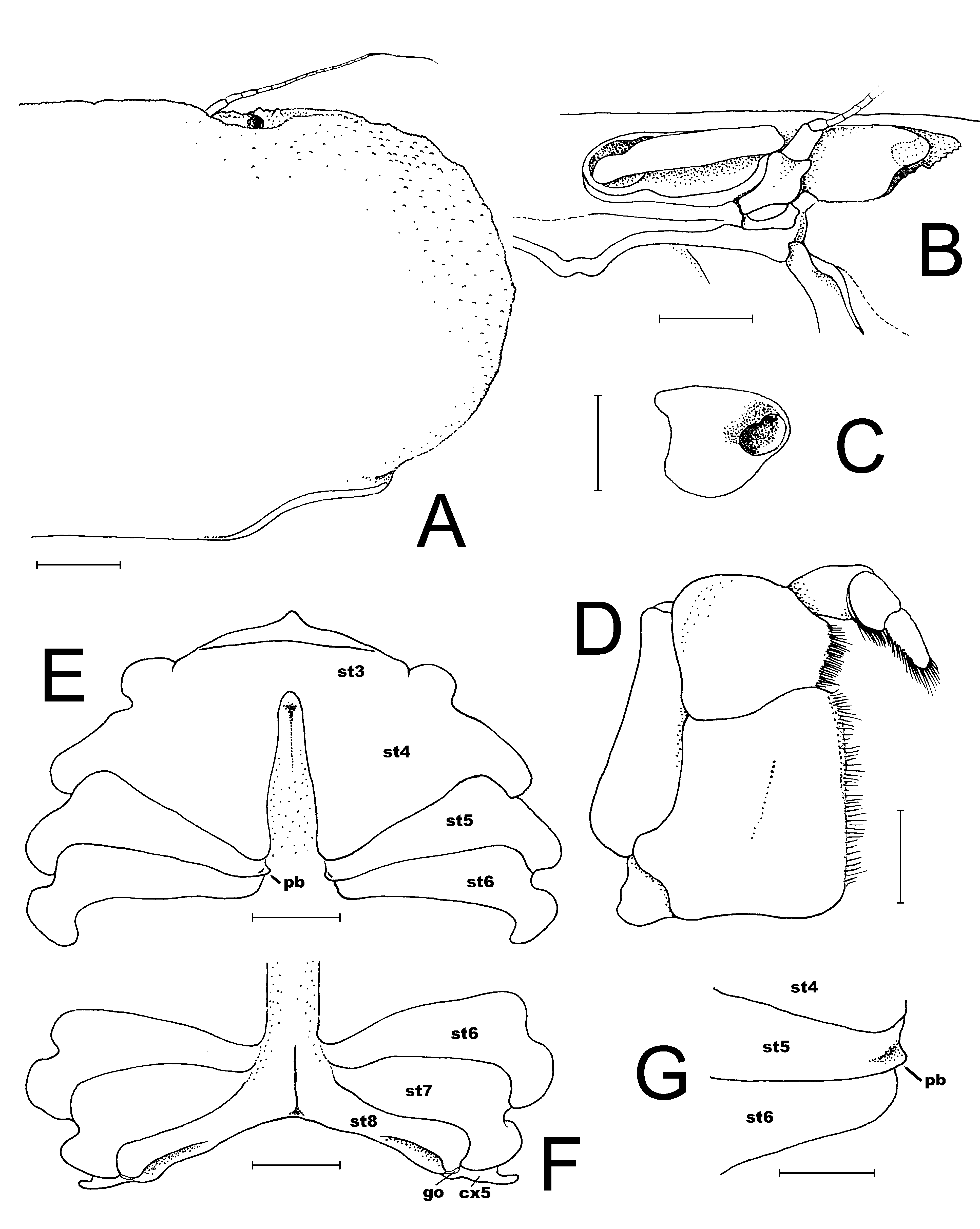
Description of male. Carapace transversely ovate, width 1.87 times length; dorsal surface smooth, without ridges, regions poorly defined ( Figs. 2 View FIGURE 2 C, 3A, 4A, 5, 7A). Front transversely broad but relatively short, not protruding anteriorly, gently deflexed downwards; gently bilobed with shallow median sinus; margin gently convex with small granules ( Figs. 5 View FIGURE 5 , 7 View FIGURE 7 A). Anterolateral margin strongly convex, lined with small granules, with 5 or 6 small tubercles, the last demarcating junction between antero-, posterolateral margins; adjacent dorsal surfaces lined with small granules ( Figs. 5 View FIGURE 5 , 7 View FIGURE 7 A). Posterolateral margin convex, converging towards almost gently concave posterior carapace margin ( Figs. 3 View FIGURE 3 A, 4A, 5, 7A). Sub-orbital, pterygostomial, subhepatic surfaces covered with small, low granules ( Figs. 3 View FIGURE 3 B, 4B, C). Orbits small, subovate in general shape from frontal view, margins complete; supraorbital margin gently granulated; external orbital angle low, barely discernible ( Figs. 3 View FIGURE 3 B, 4B, C, 7B, C); suborbital margin with a low proximal tooth, with 3 or 4 small tubercles on outer margin ( Figs. 4 View FIGURE 4 B, C, 7B). Eyes not filling orbit; peduncle short, tapering, slightly mobile but unable to articulate freely ( Figs. 4 View FIGURE 4 B, C, 7B, C); surface lined with small granules, 1 or 2 larger ones visible from dorsal view ( Figs. 2 View FIGURE 2 C, 4A, 7A); cornea partially recessed into peduncle, small, weakly pigmented ( Fig. 7 View FIGURE 7 C). Basal antennal segment (second article) subquadrate, surface unarmed, inner margin closing orbital hiatus; flagellum long, excluded from orbits ( Figs. 4 View FIGURE 4 B, C, 7B). Antennular fossa rectangular; antennules folding transversely ( Figs. 4 View FIGURE 4 B, C, 7B). Epistome longitudinally narrow; posterior margin convex, with bilobed triangular median projection ( Figs. 4 View FIGURE 4 B, 7B). Endostomial ridges distinct ( Fig. 7 View FIGURE 7 B). Third maxilliped relatively short, outer surfaces faintly rugose; ischium almost squarish, median oblique sulcus very shallow, almost undiscernible; merus with anterolateral margin produced, rounded, but not prominently auriculiform; exopod relatively stout, outer margin gently concave, with distinct subdistal tooth, long flagellum reaching to just beyond width of merus ( Figs. 4 View FIGURE 4 C, 7D).
Ambulatory legs long, slender; second leg longest ( Figs. 3 View FIGURE 3 A, 4A, 8B); surfaces smooth, without spines; merus with scattered setae, ventral margins of propodus, dactylus prominently setose ( Figs. 4 View FIGURE 4 A, 8B). Basis-ischium short; posterior margin granulated; anterior margin with low subdistal tubercle ( Fig. 8 View FIGURE 8 B). Merus laterally flattened; posterior margin on legs 1–3 gently granulated, anterior margin more strongly granulated. Dactylus of legs 1–3 gently tapering to sharp tip, dactylus of last leg gently upcurved ( Figs. 3 View FIGURE 3 A, 4A, 8B).
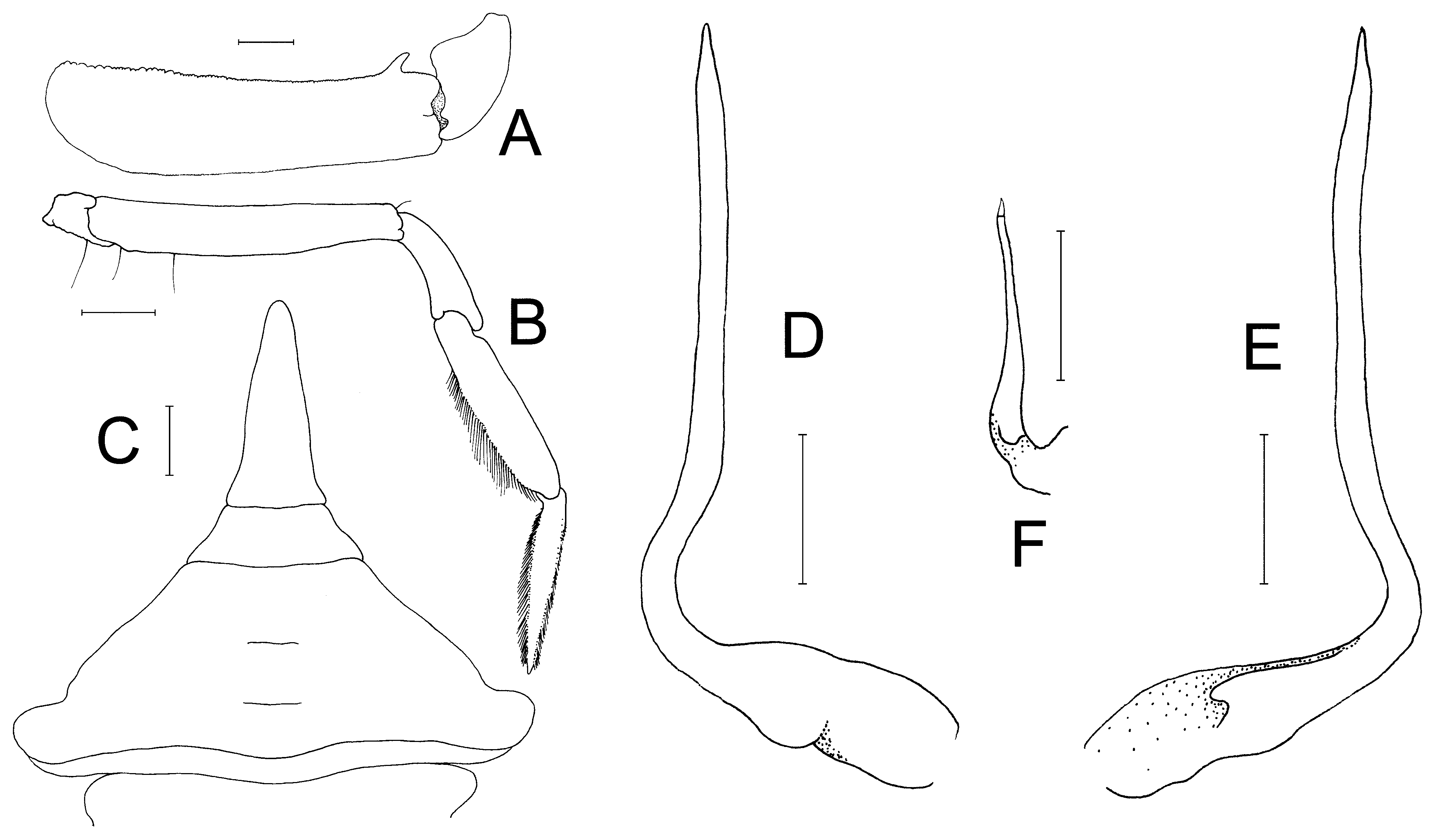
Male chelipeds long, asymmetrical; outer surfaces of palm gently rugose with scattered pits ( Figs. 3 View FIGURE 3 B, 4A, 6B, C). Basis-ischium short, with posteriorly directed spur-like process which articulates with coxa, posterior margin finely granulated. Merus subcylindical, elongated, ca. 4 times as long as wide; posterior margin smooth; anterior margin lined with small granules, with prominent subdistal spine ( Figs. 3 View FIGURE 3 A, 4A, 8A). Carpus short, triangular, without distinct spines or granules, smooth ( Figs. 3 View FIGURE 3 A, 4A, 8A). Major chela relatively stout, fingers about ½ length of palm, with median longitudinal groove on outer surface; tips of fingers hooked, light-brown; cutting margins of dactylus with 2 distinct proximal teeth, propodus with broad subproximal tooth, median tooth, several denticles along distal half ( Fig. 6 View FIGURE 6 B). Minor chela slender with long slender forceps-like fingers much longer than palm; with shallow median longitudinal groove on outer surface; tips of fingers hooked, light-brown; cutting margins with 7 sharp, spiniform teeth on propodus, dactylus unarmed ( Fig. 6 View FIGURE 6 C).
Thoracic sternum relatively broad, surfaces smooth ( Figs. 6 View FIGURE 6 A, 7E, F). Sternites 1, 2 completely fused, longitudinally narrow; suture between sternites 2, 3 distinct, gently convex anteriorly; sternites 3, 4 fused with only lateral clefts visible; sutures between sternites 4–8 all medially interrupted; shallow longitudinal groove present between sternites 6–8 ( Figs. 6 View FIGURE 6 A, 7E, F). press-button of male for abdominal locking peg-like, on posterior margin of sternite 5 ( Fig. 7 View FIGURE 7 E, G). Penis coxal, long, sits in submedian transverse groove on sternite 8 ( Figs. 6 View FIGURE 6 A, 7F). Sternoabdominal cavity deep, reaching to sternite 3 ( Figs. 6 View FIGURE 6 A, 7E).
Male abdomen distinctly T-shaped, with 5 mobile somites; somite 1 partially hidden under posterior carapace margin; somite 2 transversely very narrow, broader than somite 1; somites 3–5 completely fused, forming one subtrapezoidal structure, only faint short submedian groove indicating positions of somites, lateral margins of somite 3 convex, lateral margins of somites 4, 5 sinuous; somite 6 longitudinally short, lateral margins sinuous; telson longitudinally elongated, linguiform, 2 times longer than broad, lateral margins sinuous, tip rounded ( Fig. 8 View FIGURE 8 C).
G1 slender; basal part relatively stouter, narrowing medially, turning sinuously upwards to form slender, straight distal half; tip tapering sharply, distal surfaces almost without setae ( Figs. 6 View FIGURE 6 A, 8D, E). G2 short, approximately one-third G1 length, distal segment very short ( Fig. 8 View FIGURE 8 F).
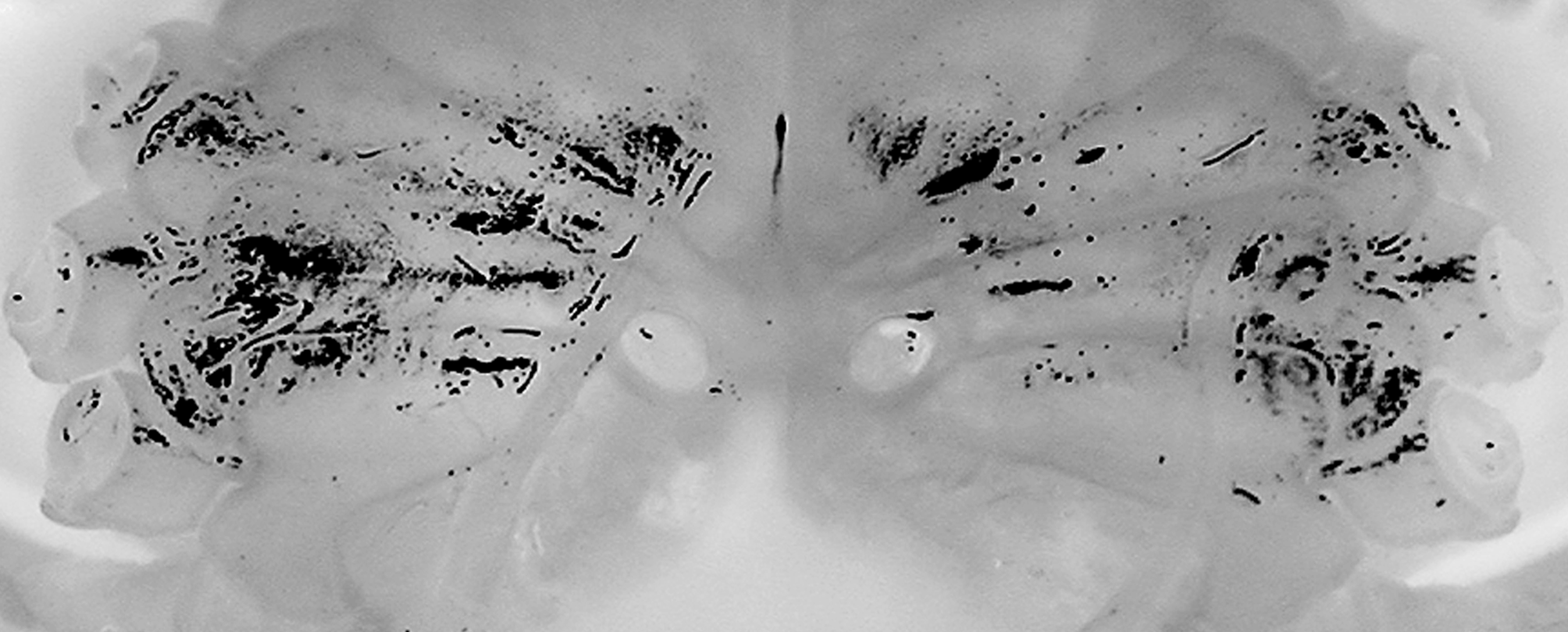
Female. Compared to the male, the female carapace is slightly broader (length to width ratio 1.88) ( Figs. 2 View FIGURE 2 A, B, 5B). The tubercles on the anterolateral margin and suborbital tooth are also relatively larger and more prominent ( Fig. 5 View FIGURE 5 B), and the posterior carapace margin is more deeply concave ( Fig. 5 View FIGURE 5 B). The female chelipeds are symmetrical; both resembling the minor chelae of the male ( Figs. 2 View FIGURE 2 C, 3A, 6C), but the spiniform teeth are also restricted to the pollex (6 on one finger, 7 on other), with proximal one low, blunt. The margins of the ambulatory legs are also relatively more prominently granular. The female is an adult, with the abdomen broadly triangular, all somites and telson free, and covering about a third of the thoracic sternum ( Fig. 3 View FIGURE 3 C). The pleopods are fully developed and setose, and the internal ovaries are well developed. The longitudinal groove on the thoracic sternum is only present on sternite 8, reaching the complete suture between sternites 7 and 8. The vulvae are large, positioned on the posterior half of sternite 6 obliquely subovate and covered by a thin membrane ( Fig. 9 View FIGURE 9 ).
Etymology. The author takes great pleasure to name the species after Matt Ramsey, the collector of the species. His perseverance in the field finally resulted in obtaining specimens of this elusive species.
Colour. In life, the carapace is orange to orange-red in adult females; that of males more beige and less reddish ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 ). The chelipeds meri, carpi and palms are dirty white to orange, most of the fingers are bluish-white with the tips light brown ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 ). The meri and carpi of the ambulatory legs are orange, the propodus is usually bluish-white to orange, while the dactylus is bluish-white, and the joints are bluish-white ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 ).
Ecology. The type locality of Ahihi-Kina‘u is located on the southwest end of Maui first to be designated as a Natural Area Reserve in 1973 ( Fig. 1 View FIGURE 1 ). Covering a total area of 1,238 acres, the reserve includes 807 acres of submerged ecosystems as well as important anchialine habitats and lava fields from the last eruption of Haleakalā some 200 to 500 years ago. It is a key conservation site for anchialine pools in Hawaii and this habitat is not open to the public.
According to Matt Ramsey, although there are many anchilaine pools throughout Ahihi-Kina‘u Natural Area Reserve and the state, the crabs were only observed from three locations, the farthest being about 2.5 km apart from where the present specimens were collected. Little is known about the ecology of the new species. The collector, Matt Ramsey provided this account of the species: “As for trapping the little critter, the crab is extremely difficult to catch. I've been trying for the last 5 years without success until last week. The only reason I was able to catch it was pure luck. Each crab was sitting on a ledge when I saw it ( Fig. 2 View FIGURE 2 A, B), so I was able to put one net under the ledge and chase it over the ledge and into the net with another net. It has been extremely difficult to lure the crab into a trap of any kind. I've tried bottle traps and traps of all baits, shapes and sizes without success. The difficulty lies in the shy behavior of the crab. If there is bait of any kind in the anchialine pool, the more aggressive species are usually quick to approach and disturb or set off the traps. Larger Grapsus crabs are usually the first to charge in as well as any Palaemon debilis . Even if there are only anchialine shrimps in the pools with the crab, the crab will not go near the bait if Calliasmata , Procaris , or Metabetaeus are near it. I have even made traps that had extremely thin entry holes, but caught nothing but Halocaridina rubra or Metabetaeus . The pools that the crabs are found in are shallow (~1 to 2 meters at the deepest) with lava substrate. For most of the pools, there is no sand, silt, or vegetative matter on the bottom. In one of the pools, there is some algae and a very small, thin patch of fine silt, but this location is the exception. The pools are close to the shoreline (~20 meters) and the salinity has been around 30 to 33 ppm. Unlike some of the other pools, the pools with the crabs often have marine species such as Parhippolyte mistica and small bright pink crabs that I see in tidepools along the coastline. I have conducted both day and night surveys and have seen the crab during both occasions. I seem to remember seeing them more often during the night surveys but this may be due to the fact that they are more obvious at night due to their light color contrasting against the black rocks. I can definitely say that I have seen them only during times of high tide. I do not recall ever seeing them when the tide was low. The behavior - they are usually found just at the edge of a crack or hole in the rocks. They usually sit facing outwards with their pinchers and arms extended out ( Fig. 2 View FIGURE 2 B). At first I thought that this was to reach out and snap up some shrimps passing by, but I have not observed any actions like that. The only behavior that I have observed is one of defense or panic. When the large shrimps crawl or swim by, the crab opens its pinchers and stretches them out even further. Not surprisingly, it did this when I tried to corner it with the net. The usual reaction that occurs when it is disturbed is that it runs ... very quickly. Because they are usually situated next to a crack, it is easy for them to disappear into the rocks. When they are in the open or moving, they fold their pinchers against their bodies. I cannot say whether they can see well or not, but it seems like they are at least sensitive to light and movement in the water. During the day, if I hover over the pond and watch the crab, they do not seem to notice me, but if my shadow covers the crab, it runs. Same goes for the night. If the beam from the flashlight goes over the crab for more than a second or so, it will run. The crabs are usually solitary with one or rarely two in a pool. And if there were two in the pool, they were no where near the other. There was only one occasion where I saw two crabs near each other, but they quickly dispersed when I tried to catch them.”
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
SubFamily |
Carupinae |
|
Genus |