Strabomantis aramunha, Cassimiro, José, Verdade, Vanessa K. & Rodrigues, Miguel T., 2008
|
publication ID |
https://doi.org/ 10.5281/zenodo.181933 |
|
DOI |
https://doi.org/10.5281/zenodo.5665674 |
|
persistent identifier |
https://treatment.plazi.org/id/915287B4-0903-FFBD-FF13-FA6101B17E1B |
|
treatment provided by |
Plazi |
|
scientific name |
Strabomantis aramunha |
| status |
sp. nov. |
Strabomantis aramunha sp. nov.
( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 )
Holotype: MZUSP 138693 (field number JC-1212), an adult female, from Serra do Sincorá, Espinhaço range, 13° 04' 07" S and 41° 20' 09" W, 998 m elevation, municipality of Mucugê, State of Bahia, Brazil, collected by J. Cassimiro and F. S. F. Leite on 11 March 2005.
Paratypes (N=6): MZUSP 138687 (JC-1198), an adult female, 13° 00' 33" S and 41° 22' 46" W, elevation 1191 m, on 8 March 2005; MZUSP 138688 (JC-1206), an adult male, 13° 00' 32" S and 41° 22' 48" W, elevation 1207 m, same date; MZUSP 138690 (JC-1214), an adult male, 13° 03' 43" S and 41° 20' 26" W, elevation 947 m, on 11 March 2005; MZUSP 138689 (JC-1213), juvenile female, 13° 04' 07" S and 41° 20' 09" W, elevation 998 m, same date; MZUSP 138691 (JC-1251), juvenile male, on 20 March 2005; MZUSP 138692 (JC- 1277), juvenile female, 13° 01' 20" S and 41° 21' 24" W, elevation 1068 m, on 26 March 2005. All collected by J. Cassimiro and F. S. F. Leite at the surroundings of the type locality.
Variable MZUSP MZUSP MZUSP MZUSP MZUSP MZUSP MZUSP
138693 138687 138688 138690 138691 138689 138692 Etymology: The specific name is from the Tupi language aramunha , meaning giant, in allusion to the large size of the species, and is used as noun in apposition.
Diagnosis: A large broad-headed species (adult male 40.3–41.1 mm SVL, adult female 75.2–79.7 mm; HW respectively 44–46% and 48–49% of SVL), adult females bearing well-developed frontoparietal crests, posterior part of pars fascialis of maxilla deepened, mandibular ramus of the trigeminal nerve passing lateral to the m. levator mandibulae posterior subexternus, eyelid tubercles present posteriorly, not elongated, skin of venter coarsely areolate, leg relatively short (FL 51–53% of SVL) finger discs absent, first finger longer than second, poorly developed discs on toes, first and second toes ridged, fifth toe smaller than third, tarsal fold absent, testes white.
Comparison to other species: The adult Strabomantis aramunha is promptly diagnosed from all other eleutherodactylines by the combination of large size, broad-head, flared maxilla, frontoparietal bearing welldeveloped crests in females, mandibular ramus of the trigeminal nerve passing lateral to the m. levator mandibulae posterior subexternus, posterior eyelid tubercles present and not elongated, skin of venter coarsely areolate, absence of finger discs, first finger longer than second, poorly developed discs on toes, first and second toes ridged, fifth toe smaller than third, and, tarsal fold absent. These characters place squarely the new species in the Eleutherodactylus sulcatus group (Lynch 1997; Lynch & Duellman 1997), currently in the genus Strabomantis ( Hedges et al. 2008) . The only other species of Strabomantis occurring in Brazil is S. sulcatus ( Lynch 1975, 1997; Frost 2007; Hedges et al. 2008). Strabomantis sulcatus differ from S. aramunha by the smaller size (SVL 28–42 mm; Lynch 1975, 1980), concealed surfaces of thigh and shank presenting light blotches, and by the presence of tarsal fold. From the other species of Strabomantis , S. aramunha differ by the larger size (adult female S. cadenai , SVL 40.6 mm; Lynch 1997), skin of venter areolate (smooth in S. cadenai , S. laticorpus ; Lynch 1997), by not presenting elongate conical tubercles on eyelid (present in S. cadenai , S. cerastes , S. ingeri , S. laticorpus , S. necopinus , S. ruizi ; Lynch 1997), large flattened warts absent (present in S. helonotus ; Lynch 1997), tarsal fold absent (present in S. anomalus , S. cornutus , S. ingeri , S. necopinus , and S. ruizi ; Lynch 1997), cranial crests present (absent in S. anomalus , and S. cheiroplethus ; Lynch & Myers 1983; Lynch 1990), reduced basal web only between first and third toes (extensive toe webbing in S. anomalus , S. anatipes and S. zygodactylus ; Lynch & Myers 1983).
Males and the young specimens, by being smaller and presenting narrower heads most resemble the adult Haddadus binotatus (formerly “ Eleutherodactylus ” binotatus, Hedges et al. 2008 ). However, under close examination H. binotatus is promptly differentiated from S. aramunha by the smoother dorsal skin and the presence of tubercles not bearing keratinized tips (present in S. aramunha ). Additionally, the head is narrower and longer than wide (wider than long in S. aramunha ), the body is slender, the legs are longer, and the supernumerary tubercles are more numerous in H. binotatus than in S. aramunha .
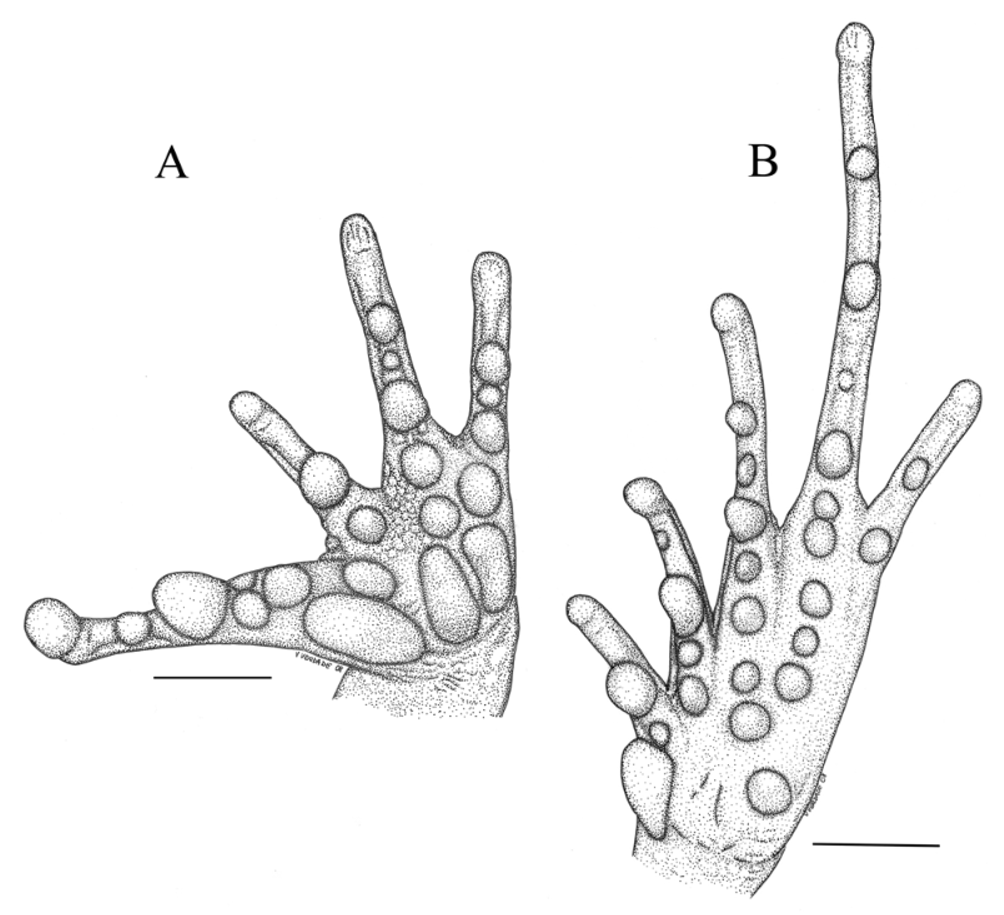
Description of holotype: Head wider than long (HW = 36.4 mm; HL = 32.6 mm); a pair of frontoparietal crests externally visible; eyes dorsolateral, median sized (ED = 9.9 mm), diameter similar to the eye-nostril distance (EN = 10.0 mm); snout sub-elliptical in dorsal view, rounded in lateral view, overhanging lower jaw; nostrils small, slightly protuberant, directed laterally and positioned distally at the canthus rostralis; canthus rostralis sharp, accentuated by bony keel; loreal region deeply concave, tuberculate; flared maxilla, with posterior pars fascialis deepened; upper eyelid bearing small tubercles posteriorly, not elongated; interorbital space highly depressed, bearing small tubercles; tympanic membrane smooth; tympanic annulus prominent, higher than long ( TYD = 4.5 mm; TYH = 5.5 mm), oblique; tympanum-eye distance less than tympanum diameter (TED = 3.6 mm); supratympanic fold evident, extending from posterior corner of eye to axils.
Skin of dorsum spiculate under magnification, with tubercles more concentrated on posterior half; dorsolateral ridges present; skin of venter coarsely areolate; anal opening plicate, surrounded by small tubercles; ulnar ridge slightly evident, evidenced by small and white tubercles; relative finger lengths I> III> II ˜ IV; subarticular tubercles present; finger tips slightly expanded, not bearing discs; fingers free, not fringed or ridged; dorsal surface of thigh and shank tuberculate, tubercles with keratinized tips; hidden parts of thigh and shank smooth; no calcar ornamentation; sole of foot smooth; toe tips slightly expanded, with poorly developed discs; toe relative lengths I <II <V <III <IV; reduced basal web between first and third toes; internal margins of first and second toes ridged.
Color in life: Ground color of dorsum light brown, with two dark dorsolateral stripes extending from posterior corner of the eyelids to groin, medial dorsum with undefinite darker mottling, canthus rostralis dark brown, flanks barred from the dorsolateral stripes to venter, venter irregularly mottled with brown; dorsal surface of forearms, thighs and shanks barred; anterior and posterior surface of thigh uniformly faintly pigmented with brown; iris bronze.
Color in preservative: Similar in life, although slightly faded.
Measurements of holotype: SVL 75.2 mm, HL 32.6 mm, HW 36.4 mm, ED 9.9 mm, EN 10.0 mm, TYD 4.5 mm, TYH 5.5 mm, ID 7.9 mm, HAL 19.3 mm, FL 40.0 mm, TL 38.3 mm, FOL 34.9 mm, IMT 3.7 mm.
Variation (N=6): Adult male and females of S. aramunha differ in size (male SVL 40.3–41.1 mm; female SVL 75.2–79.7 mm), HW/SVL ratio (males 0.44–0.46, females 0.48–0.49), development of flared maxilla, and presence of frontoparietal crests. Females are larger, present more developed flared maxilla, and are unique in presenting cranial crests. Males present the skin of dorsum spiculate with tubercles densely and evenly distributed, while in females, the density of tubercles is more variable, usually more concentrated on the posterior half. There is ontogenetic variation in the development of frontoparietal crests and labial flaring in females. In the smaller female (SVL 40.2 mm) of the type series cranial crests are absent; they are already seen in a slightly enlarged specimen (SVL 46 mm) becoming highly conspicuous in large specimens. Sexual dimorphism and ontogenetic variation observed is congruent with those reported for broad-headed eleutherodactylines ( Lynch 1975, 1997; Lynch et al. 1994; Savage & Myers 2002).
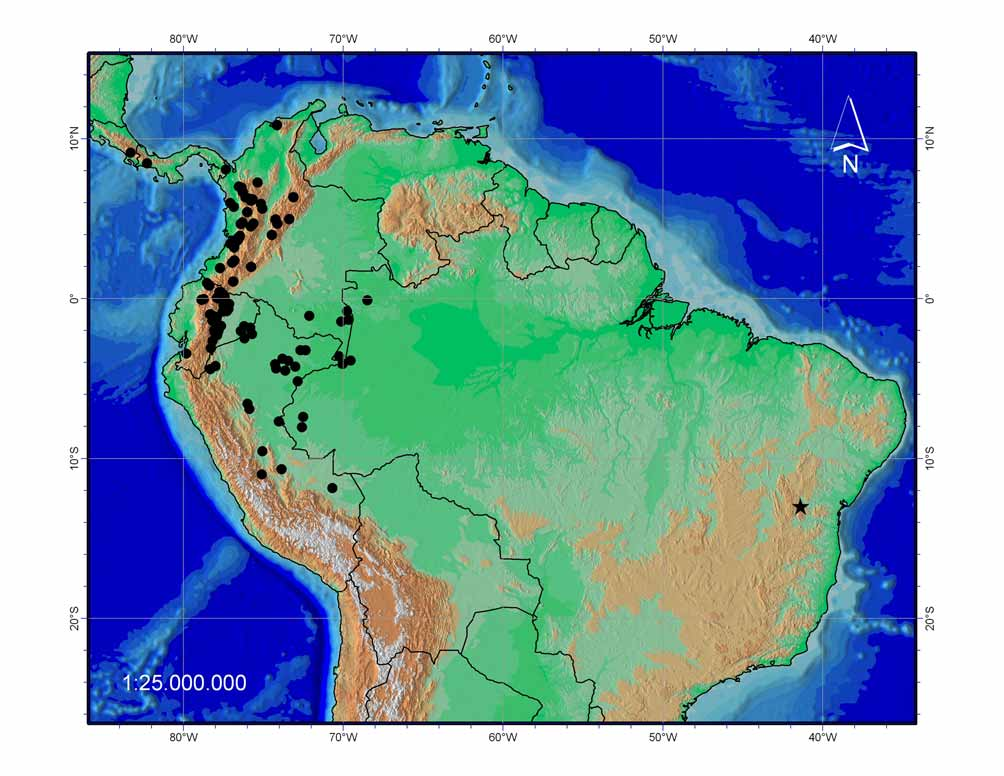
Distribution and ecology: Strabomantis aramunha is presently known only and supposed to be endemic to the “campos rupestres” of the neighborhood of Mucugê municipality, Serra do Sincorá, Espinhaço Range ( Fig. 4 View FIGURE 4 ). The Espinhaço range is well known by the high level of species endemism (e.g. Bokermann 1956, 1964; Cunha 1966; Bokermann & Sazima 1973, 1978; Sazima & Bokermann 1978, 1983; Caramaschi & Sazima 1984, 1985; Vanzolini & Heyer 1988; Pinna 1992; Wege & Long 1995; Stattersfield et al. 1998; Heyer 1999; Lugli & Haddad 2006a, b; Napoli & Juncá 2006; Rodrigues et al. 2006). This mountain complex lies over Eastern Brazil at the transitional limits of the Atlantic forest and the Cerrado biomes with elevations from 700–2000 m a.s.l. ( Stattersfield et al. 1998). The region is characterized by open and rocky areas dominated by shrubs and grasses referred to as “campos rupestres” or “campos de altitude” (for detailed characterization of Brazilian “campos de altitude” see Safford 1999a, b).
All specimens of S. aramunha were found at night, motionless on exposed rock outcrops in the “campos rupestres”. This is also the way broad-headed frogs have been frequently found as reported by Lynch (1997). Despite the rain, we did not hear the frogs calling. Nevertheless, the apparent absence of advertisement call is not infrequent among eleutherodactylines ( Savage 1987; Savage & Myers 2002).
Remarks: This is the first record of an endemic eleutherodactyline from the “campos rupestres” of the Espinhaço range. These frogs are generally leaf-litter inhabitants that deposit their eggs in terrestrial humid habitats ( Duellman 1978; Duellman & Trueb 1994). Presently the only recorded eleutherodactyline frog from these high elevation areas is the widespread Ischnocnema ramagii (MTR unpublished data). In Brazil, eleutherodactyline frogs generally are associated with forested habitats, and, even those associated to non-forested biomes, like the Brazilian Cerrado, occur inside riparian forests. The single exception is Ischnocnema juipoca , known to occur in open areas ( Haddad & Sazima, 1992). In this sense, the discovery of the new species Strabomantis aramunha from “campos rupestres” of the Espinhaço range is highly surprising and may represent a relict lineage evolved along with retraction of forests in this mountain complex ( Giulietti & Pirani 1988).
The discovery of a large broad-headed eleutherodactyline in the high altitude mountains of Espinhaço range in eastern Brazil is enigmatic and reveals how complex is the understanding of the relationships and biogeography of these mountains. In the absence of an exhaustive phylogenetic study including east and southeastern South American Terrarana ( Hedges et al. 2008), we can only rely on comparative examples to study the origin of Strabomantis aramunha . Phylogenetic and biogeographical relationships of endemic species from the Espinhaço range are virtually unexplored but some patterns are evident. Some frogs, like Thoropa megatympanum , Bokermannohyla alvarengai , B. nanuzae , B. oxente , B. saxicola , Rupirana cardosoi , Phasmahyla jandaia , Hylodes otavioi , seem to have their closest relatives in species occurring in the eastern Atlantic forest ( Bokermann 1956, 1964; Bokermann & Sazima 1973, 1978; Sazima & Bokermann 1983; Caramaschi & Sazima 1984; Heyer 1999; Lugli & Haddad 2006a, b). This is also the case of some lizards like the genera Placosoma and Enyalius ( Cunha 1966; Rodrigues et al. 2006). A very distinctive and possibly much older pattern is shown by species revealing relationships with species present in high elevation areas of northern and/or western South America. For example, species of Microlophus , the closest outgroup known for the lizard genus Eurolophosaurus , are Andean or transandean ( Frost et al. 2001). Similarly, among the species of the cisandean Tropidurus of the torquatus group, Tropidurus mucujensis most resemble Tropidurus bogerti , a species from the Ayuantepui in the Tepui region of Venezuela ( Rodrigues 1987). Old Atlantic forest relationships are also disclosed by the relictual presence of the Atlantic forest anguid lizard Diploglossus fasciatus in western Amazon Basin ( Ávila-Pires 1995). Again among lizards, the Rhachisaurinae, an endemic subfamily of gymnophthalmids restricted to these mountains was recently recognized ( Pellegrino et al. 2001). Such examples are indicative of how far we are to understand the faunal relationships of this old, Precambrian ridge of eastern South America.
The new species is placed in the genus Strabomantis based on morphological characters. However, males of S. aramunha are similar to Haddadus binotatus . This could indicate the generic misplacement of the species. Phylogenetic studies including species from a wide range of Eleutherodactylines (e.g. Craugastor , Haddadus , Holoaden , Ischnocnema , Oreobates , Pristimantis , Strabomantis , etc.) would be needed to asses this and other hypothesis of relationships within this group of frogs.
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| MZUSP MZUSP MZUSP MZUSP MZUSP MZUSP MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.