Orthochirus afar Kovařík & Lowe, 2016
|
publication ID |
https://doi.org/ 10.5281/zenodo.7163285 |
|
publication LSID |
lsid:zoobank.org:pub:8F0AFCDB-6F55-4889-97A5-D70DE24591F5 |
|
persistent identifier |
https://treatment.plazi.org/id/9429879C-E96D-FFE5-EE84-FA8DFAF284BB |
|
treatment provided by |
Felipe |
|
scientific name |
Orthochirus afar Kovařík & Lowe, 2016 |
| status |
|
Orthochirus afar Kovařík & Lowe, 2016 View in CoL
( Figures 77–82 View Figures 77–78 View Figures 79–82 , 113 View Figure 113 )
Orthochirus afar Kovařík & Lowe, 2016 , in Kovařík et al., 2016c: 10–18, figs. 41–77, 155, tab 1.
= Orthochirus borrii Rossi, 2017 (2016) : 6–7, figs. 3–4 (type locality: Djibouti, Dihdaoua´ad, MZUF). Syn. n.
Orthochirus aristidis: Lourenço & Leguin, 2011: 1–3 View in CoL (in part), figs. 3–5 (misidentification); Lourenço & Ythier, 2021: 336–337 (in part), figs. 1–2 (misidentification).
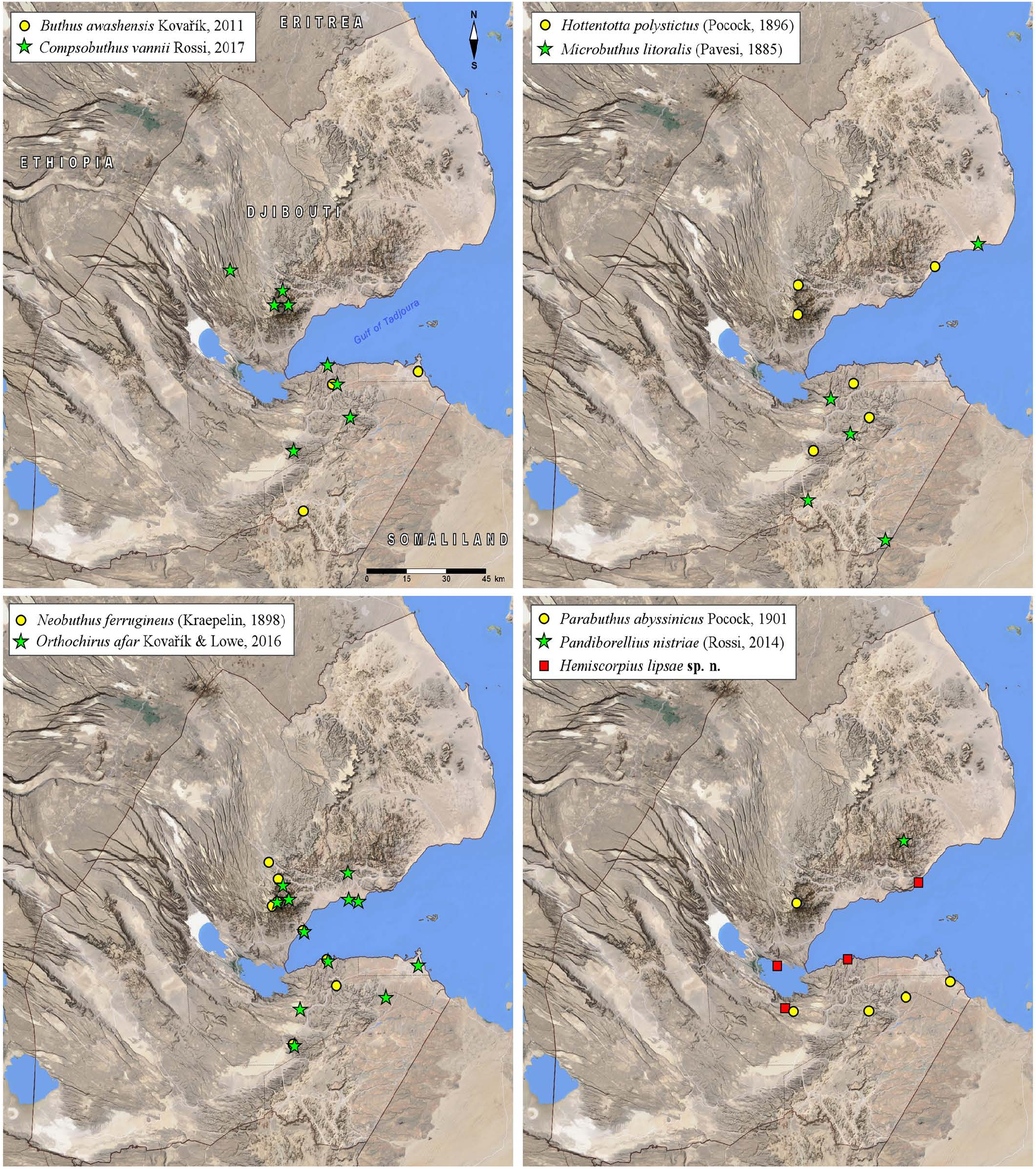
TYPE LOCALITY AND TYPE REPOSITORY. Ethiopia, Afar Region, Gewane , 10°09'38"N 40°39'45"E, 631 m a. s. l. GoogleMaps , FKCP.
TYPE MATERIAL EXAMINED. Eritrea, Assab , 1♂ 2♀ (paratypes of Orthochirus borrii Rossi, 2017 ), 10.I.–15. II.1907, leg. K. Katona, HNHM. Ethiopia, Afar Region, Gewane, 10°09'38"N 40°39'45"E, 631 m a.s.l. (Locality No. 12 EO), 23–24.XI.2012, 1♂ (holotype, figs. 41–42, 45–46, 49, 59–60, 66–68, 74 in Kovařík et al., 2016c) 1♂ 1♀ (paratypes, figs. 43–44, 47–48, 50–58, 61–65, 69–71, 75 in Kovařík et al., 2016c) 1♂ juv. (paratype), leg. F. Kovařík GoogleMaps , FKCP; 11°43'22"N 40°56'52"E, 457 m a.s.l. (Locality No. 12EM), 20.XI.2012, 1♀ juv. (paratype), leg. F. Kovařík GoogleMaps , FKCP.
DJIBOUTI MATERIAL EXAMINED ( FKCP). Djibouti, Arta Province, Arta plage, 11.5857°N 42.8286°E, 22.XII.2013 GoogleMaps , 1♂ (No. 8978), leg. J. Lips; Arta Province, Goubetto , 11.4234°N 42.7327°E, 615 m a. s. l., 29. GoogleMaps V.2014, 2♀ (No. 9528), leg. J. Lips; Djibouti Province, Djibouti, 11.5680°N 43.1461°E, 10 m a. s. l., 15.IX.2013 GoogleMaps , 1♂ (No. 7864), leg. J. Lips; Djibouti Province, Goubetto, 11.4640°N 43.0341°E, 240 m a. s. l., 26.I.2022 GoogleMaps , 2juvs. (No. 25598), leg. J. Lips; Tadjourah Province, Bankouale , 11.8246°N 42.6737°E, 650 m a. s. l., 15. GoogleMaps IV.2011, 1♂ (No. 5071), leg. J. Lips; Tadjourah Province, Tadjourah, 11.7829°N 42.9010°E, 17.10.2013 GoogleMaps , 2juvs. (No. 8347), leg. J. Lips; Tadjourah Province, Raissali , 11.7731°N 42.9355°E, 15 m a. s. l., 10. GoogleMaps IV.2013, 1♂ (No. 10993), leg. J. Lips; Tadjourah Province, Daylmoli, 11.8672°N 42.8982°E, 340 m a. s. l., 16.I.2022 GoogleMaps , 1♂ (No. 25637), leg. J. Lips; Tadjourah Province, 11.7707°N 42.6519°E, 1500 m a. s. l., 12. GoogleMaps VI.2014, 1♀ (No. 11144), leg. J. Lips; Tadjourah Province, Sagalou , 11.6774°N 42.7446°E, 16.I.2022 GoogleMaps , 1♀ (No. 25621), leg. J. Lips; Tadjourah Province, Ditillou , 11.7811°N 42.6934°E, 665 m a. s. l., 27. GoogleMaps VI.2014, 1♂ ( Figs. 77–78 View Figures 77–78 , No. 9897), leg. J. Lips; Barra Yer (Petit Barre), 11.31°N 42.71°E (11°18'33.56"N 42°42'39.17"E), 585 m., I. 2017, 2♂ 2♀ 4juvs, leg. R. Štarha .
DIAGNOSIS. Total length of adults 23–30 mm (♂), 29 mm (♀). Base color a uniform black; sternites III–VI brown with transverse posteromedian yellow patches on IV–VI (♂), or V (♀); pedipalp fingers pale, fixed finger yellow to orangeyellow, movable finger yellow; tarsomeres of legs yellow to white, telson reddish brown. Carapace densely, finely granulate, including preocular triangle, and median ocular tubercle; superciliary carinae smooth, lustrous. Tergites densely, finely granulate; tergites I–III without distinct carinae, IV–VI with single weak median carina. Sternites III–VI without carinae, moderately to sparsely shagreened laterally and anteromedially, smooth medially and posteromedially; sternite VII densely finely granulate or shagreened, with one median pair of carinae; spiracles narrow, slit-like. Metasoma glabrous, widened posteriorly, metasoma V W/ I W 1.13–1.20; dorsal surface sparsely granulate on metasoma I–II, smooth on III–V; metasomal II–V with large punctae on ventral and lateral surfaces, IV with mean puncta diameter 6–8% of segment length, inter-punctal surfaces smooth; metasoma I, II and III with 10, 8 and 6 carinae, respectively, metasoma IV–V with 4 carinae; V with ventrolateral carinae smooth in anterior half, weakly or moderately crenulate in posterior half; metasoma V L/W 1.07–1.18. Telson elongate, but relatively stout for the genus, telson L/ vesicle D 3.00–3.27; vesicle ventrally punctate; aculeus short, thick, about same length as vesicle. Pedipalp patella with dorsointernal, dorsomedian and dorsoexternal carinae present, smooth. Neobothriotaxic type Aβ; pedipalp femur with ‘trichobothrium’ d 2 absent. Pedipalp movable finger with 10–11 rows of median denticles, 9–10 ID, 8–9 OD, 4 subterminal and one terminal denticle. Fixed finger with 9 rows of median denticles, 8 ID, 8 OD. Leg coxae sparsely, finely granulate, margins densely finely granulate; tibial spurs present on legs III and IV; tarsal setation sparse on all legs, basitarsi with short, spiniform macrosetae, without bristlecombs; telotarsi with two series of short spiniform macrosetae on ventral surface. Pectines long in both sexes, extending to (♂), or almost to (♀) distal limit of coxa IV; pectinal tooth counts, 17–20 (♂), 16–19 (♀).
COMMENTS. Rossi (2017) described Orthochirus borrii from Djibouti and Eritrea in a paper titled “Complementi alla fauna del Corno d’Africa: famiglia Buthidae C. L. Koch, 1837 (Scorpiones) , con la descrizione di tre nuove specie” in his selfpublished journal Rivista Aracnologica Italiana. This journal issue was publically accessible (i.e., published) in March 2017, but was falsely pre-dated 14 July 2016 (cf. Kovařík et al., 2019: 19). The description of O. afar was published on 11 October 2016. It is determined here that the types of Orthochirus afar and paratypes of Orthochirus borrii match each other precisely in the following key characters: coloration, trichobothrial pattern, pedipalp finger dentation, pectinal tooth count and lamellar structure, proportions, setation, carination and sculpture of pedipalps, carapace, tergites, sternites, and metasoma, morphometrics of metasomal punctae, shape of the telson, as well as armature of chelicerae and pedipalp fingers. We conclude that Orthochirus borrii Rossi, 2017 is a junior synonym of Orthochirus afar Kovařík & Lowe, 2016 , syn. n.
Kovařík et al. (2020a) synonymized O. aristidis (Simon, 1883) with O. olivaceus (Karsch, 1881) and confirmed its distribution in northern Sudan and southern Egypt. Lourenço & Ythier (2021: 337) reversed this synonymy and restored O. aristidis . They based their decision on differences between the colors of O. olivaceus (“ pale ” or “ partially pale ”) shown in Kovařík et al. (2020a: 2, 4–8, figs. 1–16) and O. aristidis (“ typical very dark to blackish colouration ”) which they assumed was represented by a dark female from Nubia (leg. Brignoli, 1975), the type locality (Lourenço & Leguin, 2011: 3, figs. 3–4; republished in Lourenço & Ythier, 2021: 338, figs. 1–2). However, it is difficult to reach firm conclusions by comparing colors of old museum specimens. Darker pigmentation patterns can fade to a uniform pale brown, orange or yellow color after long storage in fluid preservatives, a fact that these authors themselves acknowledged when referring to the holotype of O. olivaceus (“… pale colouration most certainly associated with its age and faded condition ”). This also appears to be the present condition of the old type material of O. aristidis (Lourenço & Leguin, 2011: 2, figs. 1–2), which precludes any determination of natural color patterns from that material. To ascertain the colors of fresh material of O. aristidis , we consulted the original description by Simon (1882):
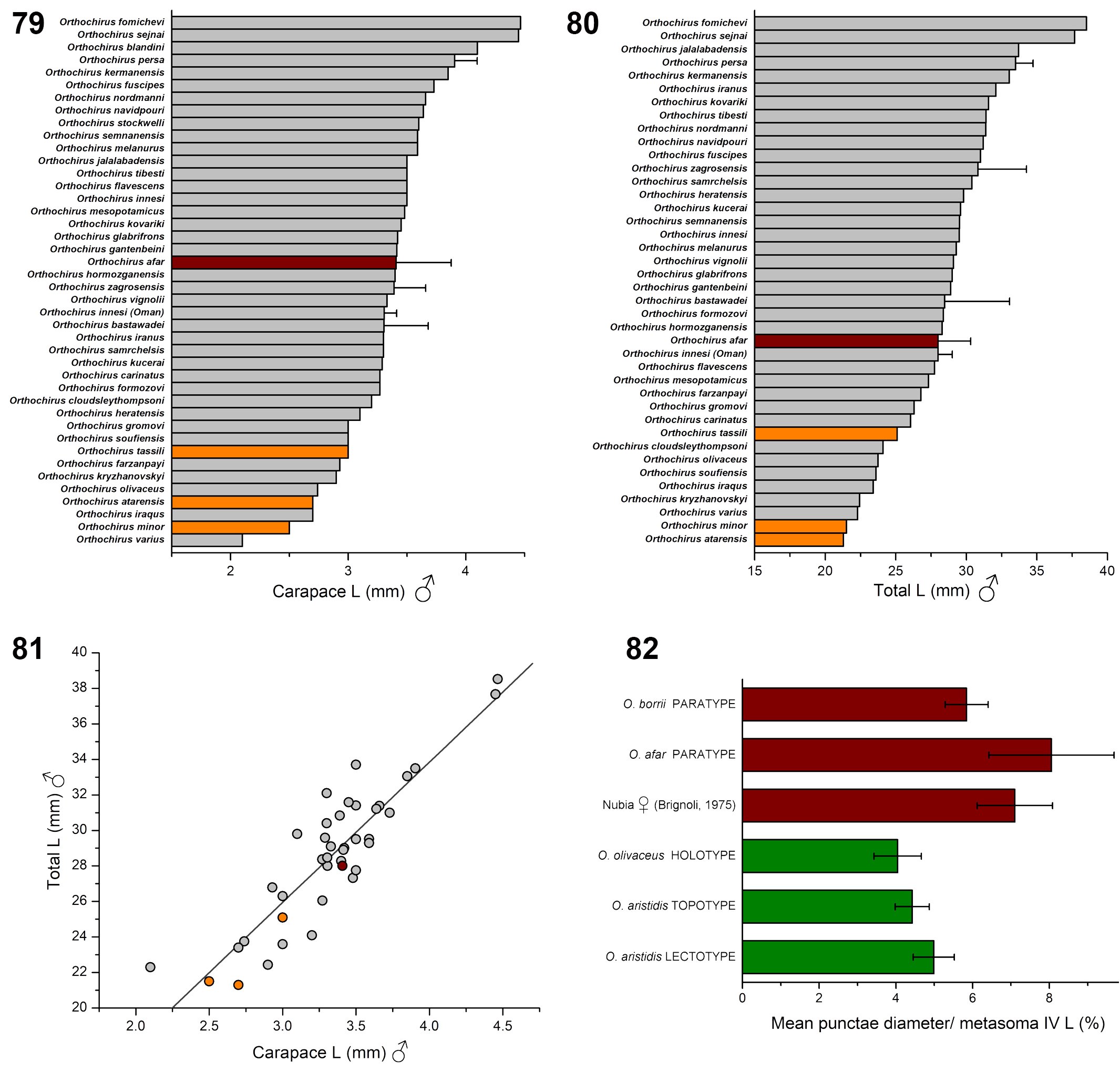
“ Truncus caudaque supra nigro-virescentes, vesica rufeola, segmenta abdominalia subter Testacea, pedes albo-testacei, pedes maxillares trochantero femoreque fusco-virescentibus, tibia testacea fusco costata, manu albo-testacea. ”
(dorsal trunk (= prosoma/ mesosoma) and metasoma blackgreen, telson vesicle rufous, sternites testaceous, legs whitetestaceous, pedipalps with trochanter and femur browngreen, patella testaceous with brown carinae, manus whitetestaceous; N.B.: “testaceous” = brownish-red or brownishyellow, and “white-testaceous” = pale brownish-red or pale brownish-yellow), and the subsequent diagnosis by Simon (1910):
“… patte-mâchoire jaune pàle avec le trochanter et le femur noirs, le tibia légèrement rembruni, pattes entièremert jaune pale. ”
(pedipalp pale yellow with black trochanter and femur, patella slightly darkened, entire legs pale yellow). Thus, according Simon (1882, 1910), O. aristidis is indeed “ partially pale ”. This color pattern matches the color pattern of O. olivaceus as originally described by Karsch (1881):
“… die Kämme, Beine, Mandibeln und Palpen gelb, nur der Humerus der letzteren braun angedunkelt. ”
(pectines, legs, chelicerae and pedipalps yellow; only the femur of the latter is dark brown). The adjectives “ nigrovirescentes ” and “ fusco-virescentibus ” (black-green and brownish-green), applied to O. aristidis , are significant because Karsch (1881) also characterized the base color of O. olivaceus as “ dunkel-olivengrün ” (dark olive green), and named the species accordingly. The agreement between the color patterns and dark greenish hues in the original descriptions of O. aristidis and O. olivaceus supports their synonymy by Kovařík et al. (2020). These color patterns also match that of a recently collected male from northern Sudan, consistent with its identification as O. olivaceus (Kovařík et al., 2020a: 7, figs. 13–14). The dark female from Nubia (leg. Brignoli, 1975) that was assumed to be a “ topotype ” of O. aristidis (Lourenço & Leguin, 2011: 2–3, figs. 3–4) has a black pedipalp patella and chela manus, both of which are as dark as the femur, and the legs are black except for lighter distal tibial and tarsal segments. This is most likely the natural color because the specimen is “… well preserved and presents … typical very dark to blackish colouration …” (Lourenço & Ythier, 2021: 337), and a photograph of a very similar live female from Djibouti, identified as conspecific, has identical coloration (Lourenço & Leguin, 2011: 4, fig. 5). This coloration differs markedly from the originally described color pattern of O. aristidis . The dark female from Nubia also differs from O. aristidis in having larger punctae on the posterior metasomal segments. Image analysis of the photographs yields a puncta diameter on metasoma IV ventral and lateral surfaces of 7.10 ± 0.98 % (n = 37) (mean ± SD, expressed as a percentage of metasoma IV L), as opposed to 4.99 ± 0.54 % (n = 19) for the lectotype female of O. aristidis . These conspicuous differences in both color and morphometrics lead us to conclude that the dark female from Nubia (leg. Brignoli, 1975) cannot be O. aristidis . On the other hand, the puncta diameters of the lectotype of O. aristidis are statistically matched to those of the O. olivaceus holotype female (4.05 ± 0.61 %; n = 37), and a female from Wadi Halfa, Nubia (4.43 ± 0.44 %; n = 36) that was proposed to be a topotype of O. aristidis (Kovařík et al., 2020a: 6, figs. 11–12) ( Fig. 82 View Figures 79–82 ). We therefore reject the restoration of O. aristidis by Lourenço & Ythier (2021: 337), an act based on a misidentified topotype, and we return O. aristidis (Simon, 1882) syn. res. to synonymy with O. olivaceus (Karsch, 1881) . The dark female from Nubia (leg. Brignoli, 1975) (misidentified topotype) exhibits habitus and coloration identical to that of O. afar (Kovařík et al., 2016c: 10, figs. 41–44), and the morphometrics of its metasoma IV punctae are statistically matched to those of O. afar (♀ 8.06 ± 1.63 %; n = 55) ( Fig. 82 View Figures 79–82 ). It is either O. afar , or a closely similar species. The statement by Lourenço & Ythier (2021: 336) that “ Orthochirus afar is most certainly a synonym of Orthochirus aristidis ” is incorrect, being based on their misidentified topotype. The photographed live dark female from Djibouti (Lourenço & Leguin, 2011: 4, fig. 5) is probably also O. afar , as the presence of O. afar in Djibouti is confirmed here by examination of new materials. Rossi (2017) independently concluded that this photographed live female had been misidentified as O. aristidis , and regarded it as a synonym of O. borrii (= O. afar ). Most of the distinctions between O.
afar and “ O. aristidis ” previously offered by Kovařík et al. (2016a: 13, under ‘AFFINITIES’) were based on the incorrect assumption that the misidentified topotype of Lourenço & Leguin (2011) represented O. aristidis . In hindsight, these differences may represent either intraspecific variation in O. afar , or morphological differences between O. afar and a closely similar species in Nubia. It appears that at least two species of Orthochrius occur sympatrically in the area of Nubia, O. olivaceus and O. afar or a closely similar species.
Kovařík et al. (2020a) viewed the type locality ‘Sicilien’ of O. olivaceus as a labeling error, as there are no confirmed records of Orthochirus in Sicily, or elsewhere in Europe. They reasoned that the specimen most likely originated from Egypt, along with other scorpion materials acquired by the same collector (Schneider). This viewpoint was supported by the precise match in morphology between the holotype and a female from Nubia. It gains further credence from the synonymy of O. aristidis (from Nubia) with O. olivaceus , a conclusion independently supported by exact matches of color patterns and morphometrics, as shown above. Lourenço & Ythier (2021: 337) took the opposing position that Sicily can be the type locality, arguing that: “… Sicily does have habitats which could be in adequation with Orthochirus species’ biology. The original population could have vanished since the 19th century, as it happened for other populations (Lourenço & Rossi, 2013). ” Their hypothesis poses a perplexing biogeographic puzzle: how to explain the existence two widely disjunct populations of O. olivaceus , one in the Nubian desert around the Nile of southern Egypt / northern Sudan, and the other isolated on the island of Sicily, separated from each other by ~ 2,500 km of intervening Sahara Desert and Mediterranean Sea? Sicily was connected to North Africa in the past, during the Messinian Salinity Crisis at the end of the Miocene. However, this connection was through Tunisia, not the Nile basin. During this period, and during later Pleistocene glacial regressions, faunal interchange could occur between Sicily and Tunisia ( Schmitt et al., 2021). If any Orthochirus were once present in Sicily, they were most likely to have been O. innesi , which is broadly distributed in Tunisia, northern Libya and northern Egypt (Lourenço & Leguin, 2011: 15, fig. 39). This puzzle can be solved most simply by assuming a labeling error.
ADDITIONAL COMMENTS. Lourenço & Ythier (2021: 340–345) diagnosed and described another species of Orthochirus : O. arenicola from coastal Somalia. Their diagnosis suffers from many problems. First and foremost, it is based on a single type specimen that is an early instar juvenile male. Descriptions of new species from single specimens are only defensible when there are unique qualitative characters, or distinct quantitative characters without overlapping variation in other species. Description of new species from early instar juveniles is problematic because many scorpion taxonomic characters (e.g., morphometrics, setation, carination, granulation, and secondary sexual characters) exhibit ontogenetic variation between juveniles and adults. Diagnoses based on juveniles require systematic comparisons with same stage juveniles of other species, which is usually difficult or impossible because most species were described from adults, and many are only known from adults. Even if such comparisons can be made, they may not yield useful diagnostic information because many species-specific characters are only expressed in later instars or upon reaching sexual maturity. This only exacerbates the problem of determining whether the characters of a single type specimen are unique or overlap with those of other species. One of the differential diagnostic characters is: “ carapace and tergites less granulated ”. Granulation is known to exhibit ontogenetic variation. It can be weak or absent in early instars of species that develop strong granulation in adults. In the absence of adults, it is impossible to know if this is a speciesspecific character, or a juvenile-specific character. Similarly, the diagnostic character: “ Ventral aspect of metasomal segment V without any granulations posteriorly ” could be restricted to juveniles, with granulation developing in later instars or adults.
Another problem is the use of size as a diagnostic character: “… probably small to moderate in size compared to other known species of the genus, reaching a total length of 21 to 25 mm for males.” The cited adult size is an assumption, not an observed character state. It is impossible to know the size of adults, because only an early instar juvenile is available. Diagnoses should not include unknown assumed characters unless there is evidence to support the assumptions. The authors’ rationale for assuming this size range is that it is: “ … the average total length (21 to 25 mm) of moderate-size Orthochirus species such as O. tassili …, O. atarensis … or O. minor … ”. However, there is no independent evidence (e.g., animal tracks in the sand, photographs of adults, observer reports) to support an assumption of “ moderate -size ”; it could be a smaller or larger species of Orthochirus . Indeed, the authors’ proposed size is a nebulous, shifting character. The diagnosis specifies “ small to moderate ” size, instead of “ moderate -size ”. In the absence of data, the value of an unknown variable could be estimated statistically by taking the mean value (“ average total length ”) for the taxonomic group of interest, i.e., the genus Orthochirus . Figs. 79–80 View Figures 79–82 show the size distribution of male Orthochirus , as measured by carapace length (CL) and total length (TL). Carapace length is included here because total length measurements may be distorted by mesosomal expansion or contraction. It is evident that the three species, O. tassili , O. atarensis and O. minor are not of “ moderate ” (i.e., “ average ” for the genus) size. Their sizes are skewed towards the extreme lower end of the distribution, and two of them are among the smallest members of the genus. Mean values and ranges for these species are: CL, 2.73 ± 0.25, 2.5–3.0 mm; TL, 22.63 ± 2.14 mm, 21.30–25.10 mm (n = 3). The last values correspond to the diagnostic character “ 21 to 25 mm ”. In contrast, the overall mean values and ranges for male Orthochirus are: CL, 3.35 ± 0.46, 2.50–4.47 mm (n = 42); TL, 28.60 ± 3.99, 21.30– 38.52 mm (n = 40). Why would the authors assume that adults of O. arenicola are among the smallest members of the genus, and not of “ moderate ” or “ average ” size?
The apparently arbitrary assumption of 21–25 mm adult size was used to extrapolate the developmental stage of the specimen as third instar, using a morphometric progression factor (“… it is possible to suggest that the described specimen is a third instar juvenile ”). The theoretical progression factor of length measurements for arthropod molts is 1.26 (the cube root of 2, assuming mass and volume doubling in each molt). The holotype measurements are (mm): CL, 1.80; TL, 14.55. Predicted lengths for subsequent instars are: CL × (1.26) n, or 2.27, 2.86, 3.60; TL × (1.26) n, or 18.33, 23.10, 29.11 (where n = 1, 2, 3 are the number of molts). Therefore, only two molts are required to attain a mean adult size of 21–25 mm. Shulov & Amitai (1960) analyzed the growth of O. innesi negebensis and proposed that males matured at the fifth instar. Assuming that adult males of O. arenicola are also fifth instars, an adult size of 21–25 mm predicts that the holotype is third instar. However, if the adult size is assumed instead to be the mean size for Orthochirus (CL 3.35 mm, TL 28.60 mm), three molts would be required to attain adult size, and the holotype is predicted to be second instar. By avoiding the true “ average ” or “ moderate ” adult size of Orthochirus (a statistically unbiased assumption), and choosing the lower end of the size range (a statistically biased assumption), the authors obtained the result of third instar, not second instar. Although inferred from a biased assumption, the authors boldly claimed that their specimen is “ most certainly a third instar juvenile ”. Certainty comes from observation, not from biased assumption and extrapolation. The illusory morphing of confidence levels of instar prediction from “ possible to suggest ” to “ probably ” to “ certainly ” has the bogus ring of propaganda, not science.
One of the key diagnostic characters proposed for O. arenicola is the absence of femur trichothrium i 2.As the authors noted, absence of i 2 is a known developmental trait of second instar juveniles of species belonging to diverse buthid genera (e.g., Androctonus , Buthacus , Buthiscus , Buthus , Hottentotta , Heteroctenus and Tityus ). In those species, i 2 is normally present in later instars or adults. If the holotype is second instar, the absence of i 2 is more likely to be a general buthid condition without diagnostic value at the species level. If it is third instar, it may be more plausible as a diagnostic character. This is a motivation for the biased assumption that adults are only 21–25 mm in size, i.e., to achieve third instar status and obtain a diagnostic character. However, even if it were third instar, the value of this character for species recognition is unknown, because early ontogeny of i 2 in other Orthochirus has not been characterized. The authors themselves admitted as much: “… not enough elements are available to suggest a particular ontogenetic variation in Orthochirus species. ” It should also be noted that the absence of a single trichobothrium in a single specimen is sometimes observed as an irregularity in one pedipalp segment, but the authors did not rule this out by showing bilateral loss of i 2. Consistent neobothriotaxic loss of i 2 in adults is rare in buthids. It occurs in the very small genera Femtobuthus and Picobuthus ( Lowe, 2010) , but has not been found in most other buthids.
Another questionable diagnostic character is: “ General colouration brownish-yellow ”, along with the differential diagnostic character: “ a distinct and paler pigmentation pattern, even supposing that the adults may be darker ”. This does not allow for the likelihood that the specimen may have faded after long museum storage (46 years). Also, if it is supposed that “ adults may be darker ”, then this is a juvenilespecific character and its validation requires systematic comparison of pigmentation in same instar juveniles of other Orthochirus species. No such comparison was provided. Fading is suggested by their fig. 21, which shows a lack of UV fluorescence. However, they considered this lack of fluorescence to be another taxonomic character for their diagnosis (“… no reaction to UV light was observed …”), rather than an artifact of fading. Variable loss of scorpion fluorescence occurs during long storage in fluid preservatives, and an isolated observation of reduced fluorescence in a single old museum specimen is insufficient for constructing a diagnosis (Lowe & Kovařík, 2019, 2022).
According to the differential diagnosis: “ Orthochirus arenicola sp. n. can be distinguished from the other African species of Orthochirus , and in particular from Orthochirus aristidis Simon , which presents the most close geographic distribution, by the following main features: (i) a smaller size in adults ….”. The authors’ concept of O. aristidis is based in part on their misidentified female topotype, which, as shown above, is actually O. afar or a closely similar species. The populations they referenced as having the “ most close geographic distribution ” to O. arenicola would correspond to O. afar (in Djibouti, Eritrea, Ethiopia and Somaliland). Adult males of O. afar can range in size from from 26 mm (Kovařík et al., 2016c: 18, tab. 1; O. borrii male paratype, examined) to 30 mm ( Figs. 77–78 View Figures 77–78 ). This is around the average size for the genus Orthochirus ( Figs. 79–81 View Figures 79–82 ), and is certainly larger than the size range of 21–25 mm given in the diagnosis of O. arenicola . However, since the latter size range was arbitrarily chosen (or perhaps biased to force third instar status), it has no validity as a diagnostic character. If the holotype of O. arenicola is a juvenile O. afar , the adult size range of 26–30 mm would imply second instar status, and the absence of i 2 would be a character shared with many other second instar buthids. Several ontogenetically invariant characters are either shared or overlap between O. afar and O. arenicola : pectinal tooth count 17–20 in males (vs. 17 in O. arenicola ); pedipalp chela fingers with 9–11 subrows of median denticles (vs. 8–9 in O. arenicola ); femur petite ‘trichobothrium’ d 2 absent (vs. d 2 absent in O. arenicola ). O. afar has contrasting pale pedipalp chela fingers and tarsi, and the same contrasting pattern is seen in O. arenicola (chela fingers and tarsi more pale than proximal segments and body). This suggests that the body and proximal pedipalp and leg segments of O. arenicola were originally darker, but faded to a lighter shade of brown. A pattern of contrasting pale leg tarsi and pedipalp chela fingers is found in many Orthochirus with dark bodies (Kovařík et al., 2019, 2020c; Kovařík & Navidpour, 2020). It does not occur in known species with naturally pale bodies: e.g., O. kryzhanovskyi Kovařík, Fet & Yağmur, 2020 (cf. Kovařík et al., 2020b: 23, figs. 106–107), O. pallidus (Pocock, 1897) and O. flavescens (Pocock, 1897) (cf. Zambre et al., 2011: 11, figs. 18A, 18B), O. minor and O. tibesti ( Lourenço et al., 2012: 328–329; verbal descriptions). The more parsimonious hypothesis is that the holotype of O. arenicola is a faded second instar juvenile of O. afar , the geographically most proximate Orthochirus species in the region, rather than an exotic, small new species of the genus, lacking both femoral trichobothrium i 2 and UV fluorescence.
The diagnosis of O. arenicola contains many uncertainties and assumptions, being based on a single early instar juvenile. It is not possible to know if O. arenicola is a distinct species, or a juvenile of a previously described species. The juvenile characters are taxonomically uninformative, and may be symptomatic of juvenile status, rather than species identity or phylogenetic position. The species cannot be reliably compared to other Orthochrius diagnosed by adult characters. It is premature to name a new species on the basis of such tenuous information. The diagnosis seems to be a product of wishful thinking, with fictitious characters conjured to differentiate imaginary adults from other Orthochirus . We have no choice but to consider O. arenicola Lourenço & Ythier, 2021 a nomen dubium.
Lourenço & Ythier (2021: 339) synonymized Orthochiroides Kovařík, 1998 with Orthochirus Karsch, 1891 . We already showed that this synonymy was unjustified, and restored the genus (Kovařík & Lowe, 2022). Hence, all of their taxonomic acts are invalidated. Do they offer anything other than misguided attempts to negate our results? The last section of their paper, reviewing the ecology of the Somali coast, could be an original contribution. However, that entire section is duplicated verbatim (86% text, the rest cosmetic wording changes), without citation, from the copyrighted online work of other authors (Magin & Burdette, 2013–2020).
DISTRIBUTION. Djibouti, Eritrea, Ethiopia, Somaliland,? Sudan ( Fig. 113 View Figure 113 , fig. 155 in Kovařík et al., 2016b: 34).
| HNHM |
Hungarian Natural History Museum (Termeszettudomanyi Muzeum) |
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Orthochirus afar Kovařík & Lowe, 2016
| Kovařík, František & Lowe, Graeme 2022 |
Orthochirus borrii
| Rossi 2017 |
Orthochirus aristidis: Lourenço & Leguin, 2011: 1–3
| Lourenco & Leguin 2011: 1 - 3 |