Dobrodesmus mirabilis Shear, Ferreira & Iniesta
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4178.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:CBAD9B97-AD9A-41F2-A341-64F899861247 |
|
DOI |
https://doi.org/10.5281/zenodo.6073631 |
|
persistent identifier |
https://treatment.plazi.org/id/946F87AA-FF8E-FFCE-FF7C-21ABFAE4BA81 |
|
treatment provided by |
Plazi |
|
scientific name |
Dobrodesmus mirabilis Shear, Ferreira & Iniesta |
| status |
sp. nov. |
Dobrodesmus mirabilis Shear, Ferreira & Iniesta , n. sp.
Figs 1–14, 16, 18–21 View FIGURES 17, 18 View FIGURES 19 – 22
Types. Male holotype ( ISLA 3637) male paratype, and numerous juvenile paratypes from Gruta de Mangabeira, Ituaçu Municipality, Bahia State , Brazil ( Figs 23–28 View FIGURES 23 – 27 View FIGURE 28 ), collected by R. Ferreira, 30 December 2006, deposited in the Zoology Collection, Seção de Invertebrados Subterrâneos (ISLA) at the Universidade Federal de Lavras, Campus Universitário de Lavras, Minas Gerais , Brazil. The male paratype remains mounted on a scanning electron microscope stub.
Etymology. The species epithet “ mirabilis ” means amazing in Latin and refers to the characteristics observed in the species.
Diagnosis. As for the genus, see above.
Description. Male ( Fig 1; now fragmentary) about 21–22 mm long, 1.0 mm wide.
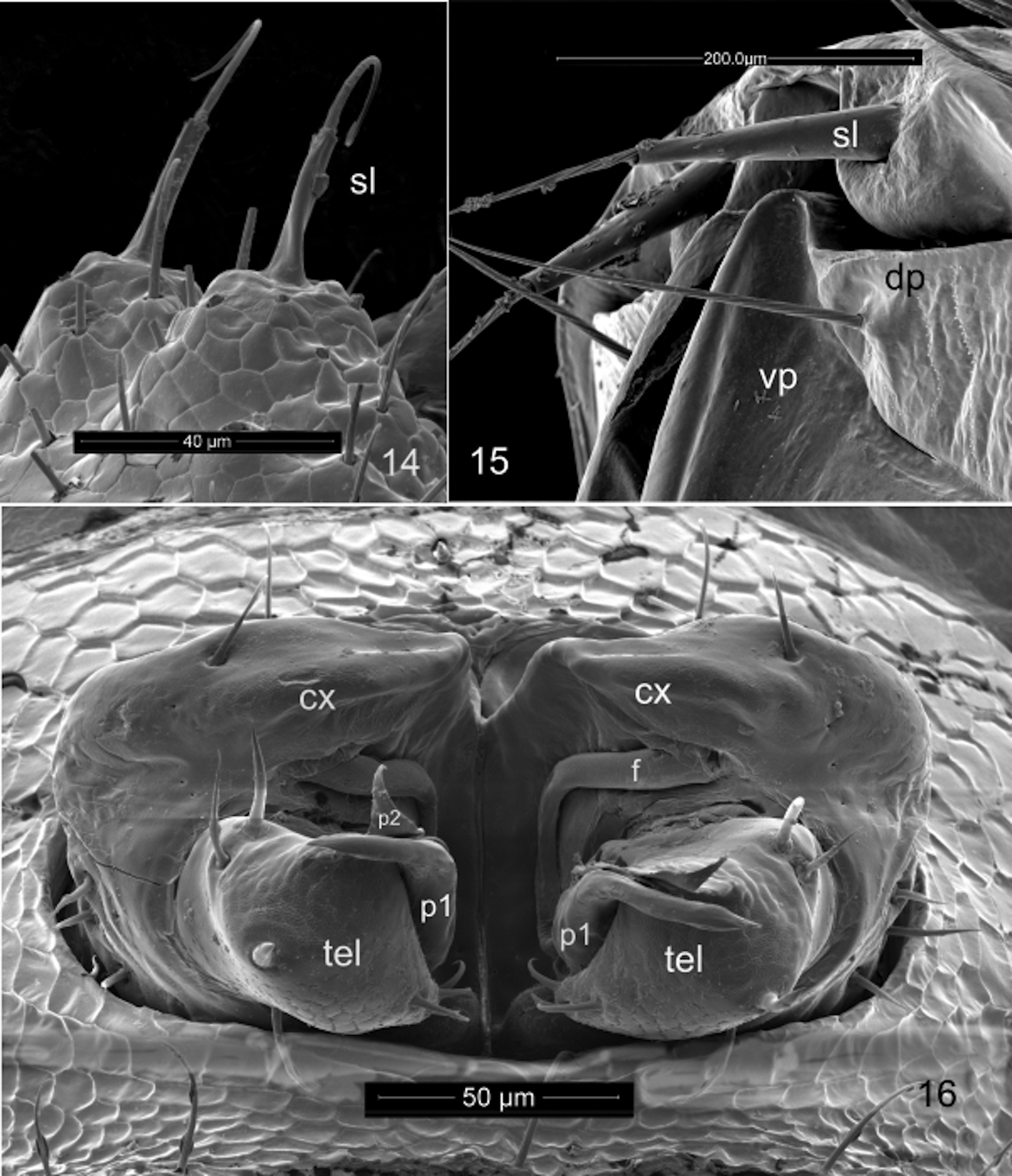
Head subglobular, setose. Antennae 1.6–1.7 mm long, if extended posteriad, reaching to posterior margin of fifth ring. Penultimate antennal segment with cluster of club-shaped setae lining outer apex ( Fig 3 View FIGURES 2 – 7 ).
With troglobiotic character states; colorless, cuticle thin, poorly sclerotized, brittle, legs long and thin.
Collum ( Fig 2 View FIGURES 2 – 7 ) densely setose, subelliptical, anteriorly rounded, posterior margin nearly straight, posteriolateral corners rounded.
Typical ring ( Figs 4, 5 View FIGURES 2 – 7 ) with dense coat of fine, short setae, 12 longer, stouter setae with prominent apertures in two transverse rows on each metazonite. Midbody rings 1.0 mm wide across paranota, paranota level, projecting directly laterad, squarish, anteriolateral corners ( Fig 6 View FIGURES 2 – 7 ) strongly angular, with numerous stout triangular teeth, posteriorlateral corners ( Fig 7 View FIGURES 2 – 7 ) acutely produced, lateral edges finely and irregularly serrate in anterior half.
Metazonite cuticle with numerous intercalary microscutes, ( Figs 9, 10 View FIGURES 8 – 10 ) limbus unmodified, completely smooth. Prozonite sculpture regular rows of polygonal cells.
Ozopores ( Fig 8 View FIGURES 8 – 10 ) on rings 5, 7, 9, 10, 12, 13, 15–38, located on produced posteriolateral corner of paranotum.
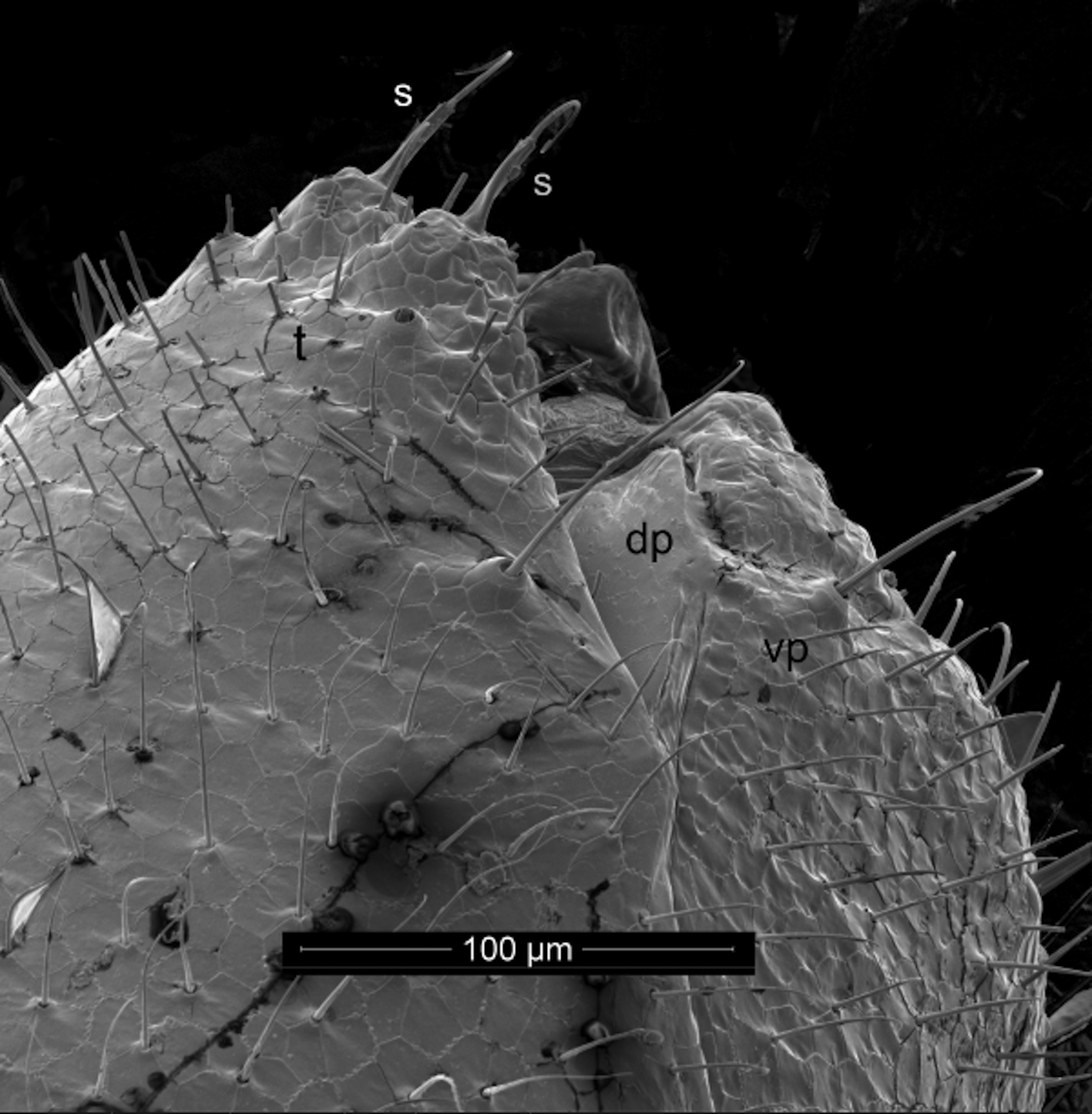
Epiproct ( Fig 11 View FIGURE 11 ) twice as long as wide, densely setose, terminal process appears set off by groove, deeply divided into two subtriangular processes, each bearing spinneret for complement of two spinnerets. Paraprocts strongly margined, with oblique transverse sulcus dividing paraprocts into smaller dorsal and larger ventral portions ( Fig 11 View FIGURE 11 ). Paraprocts densely setose, without evident marginal or submarginal macrosetae, but with large, distinct depression or pore at distal angle. Hypoproct entire, semicircular.
Ventral surfaces of rings with transverse depression between insertions of legpairs ( Fig 5 View FIGURES 2 – 7 ).
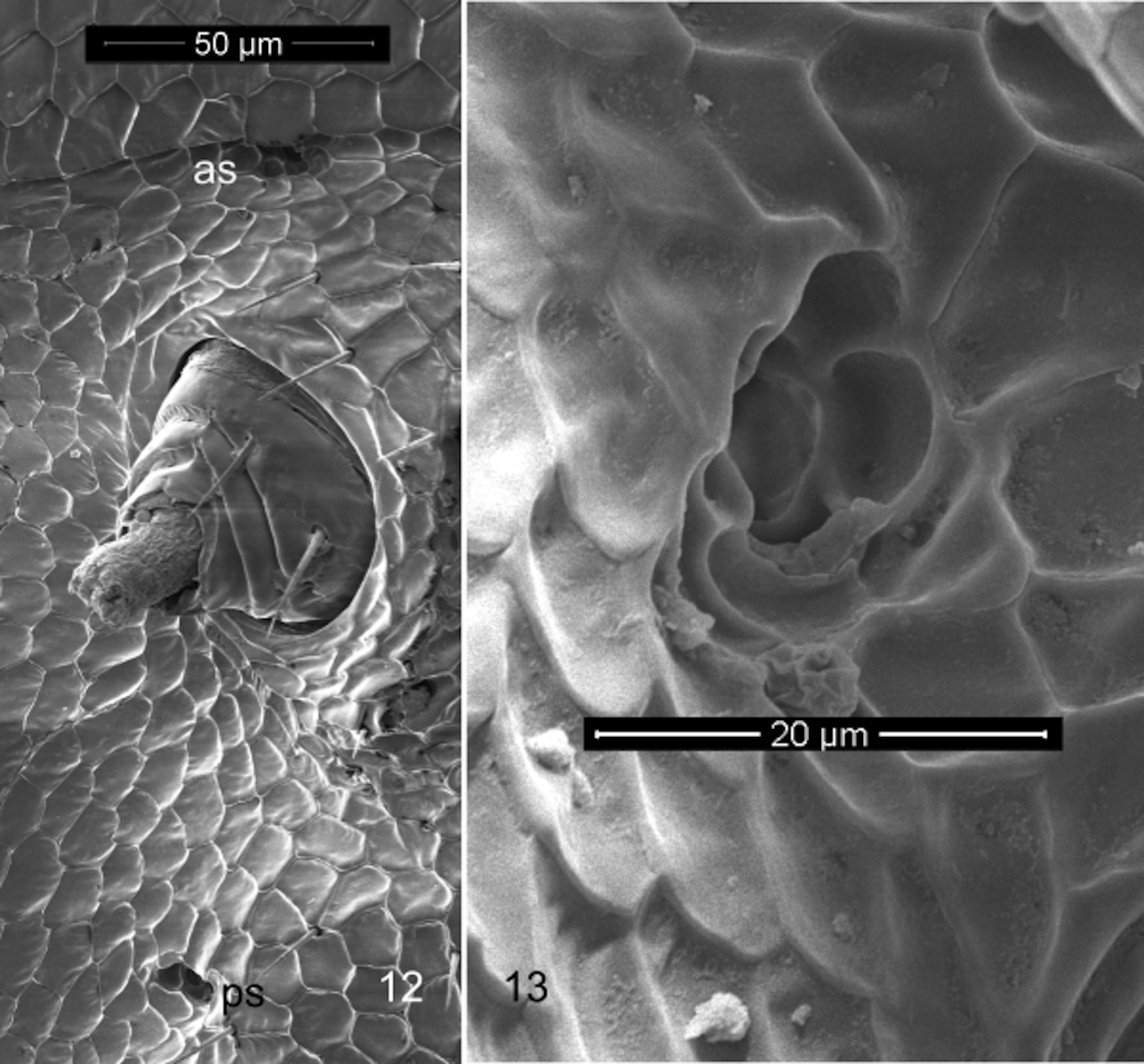
Spiracles small ( Fig 13 View FIGURES 12, 13 ), strongly reduced, advanced directly anterior of each leg coxa ( Fig 12 View FIGURES 12, 13 ).
Legs long, thin; midbody legs about 1.2 mm long.
Sternum of second legpair not incorporated in second ring, freely articulating; second leg coxae quadrate, with opening of vas deferens mesal on each. No anterior legpairs incrassate.
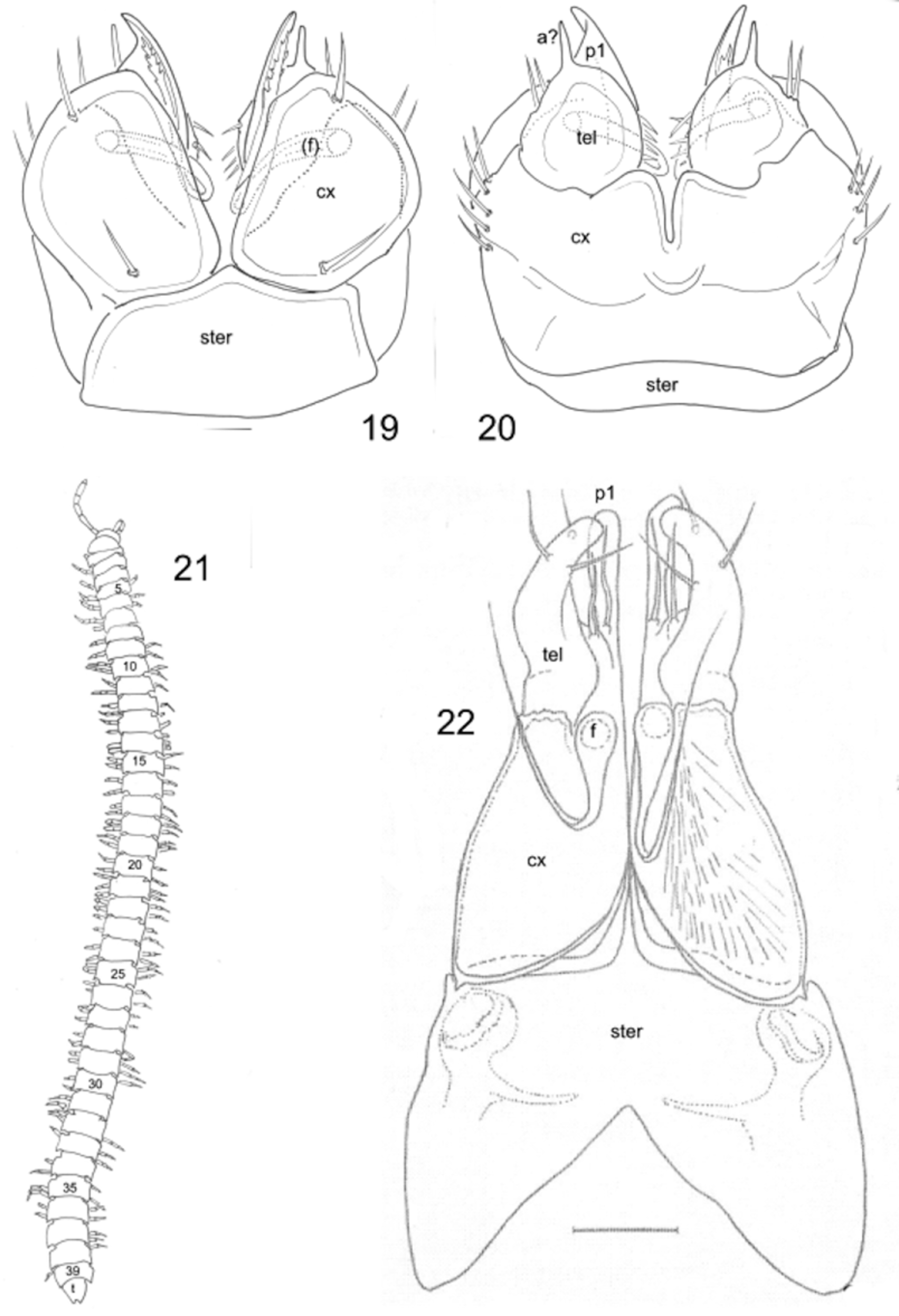
Gonopods ( Figs 16 View FIGURES 14 – 16 , 18 View FIGURES 17, 18 , 19, 20 View FIGURES 19 – 22 ) in tightly constricting oval aperture, coxae entirely exposed, constricted by margin of aperture. Sternum complete, articulated with, rather than partly or entirely fused to, coxae. Coxae large, egg-shaped, closely appressed, perhaps fused in midline, with clusters of 5–7 strong setae on lateral side, mesally with distinct angular flange; mesal coxal surface behind flange either fimbricate or with dense, fine setae. Coxae evidently immovable with respect to one another. Cannula not detected, but long, distally multihastate flagellum articulated with posterior apical coxal lobe sheathed in broad, distally acuminate coxal process. Telopodite singlearticled, subglobular with thin, triangular process guiding coxal sheathing process; small, distal, acute process (possibly representing acropodite) subtended by two setae, one of which has many small setulae, mesal group of three stout setae. No seminal (prostatic) groove or channel detected. Telopodite not divided into prefemoral and acropodal regions.
Females not collected.
Notes. Observations using optical microscopy on the anatomy of the male holotype were made difficult by the transparency (intra- and inter-ring muscles and digestive tract are clearly visible) and brittleness of the cuticle, so subsequent observations may alter some details. Certain features were clarified using the paratype male for scanning electron microscopy. It was particularly difficult to detect the ozopores and while their distribution appeared to fit the “normal” polydesmidan pattern (except for their continuation beyond ring 19) we are not entirely sure of this; on one specimen of 24 rings, ozopores appeared not to begin until ring seven. It may be that the chemical defense system has been reduced due to relaxed predator pressure in the subterranean environment. The spiracles are so small as to be undetectable with light microscopy ( Fig 13 View FIGURES 12, 13 ); their position was only determined by careful searching using scanning electron microscopy. The positions of the spiracles on each ring are advanced anteriorly so that the posterior spiracles are between the legpairs of each side, and the anterior spiracles are near the posterior margin of the prozonite ( Fig 12 View FIGURES 12, 13 ). Reduced or apparently absent spiracles occur elsewhere in the Polydesmida . Mesibov (2009) at first thought the Australian haplodesmid Agathodesmus Silvestri lacked spiracles, but later examination with scanning electron microscopy revealed them as very reduced ( Mesibov 2013).
Spiracles could not be detected in species of the haplodesmid genus Prosopodesmus Silvestri ( Mesibov 2012) , and Golovatch & VandenSpiegel (2014, 2015) failed to find spiracles in Koponenius Golovatch & VandenSpiegel species, also haplodesmids. It seems doubtful that openings to the tracheal system could be entirely lacking in a millipede of any size, but their position may be anomalous or they may be so small and misshapen (as in the case of D. mirabilis ) as to be overlooked even with scanning electron microscopy.
Spinnerets in the Polydesmida are typically four in number, arranged in a quadrangle ( Shear 2008). In contrast, two spinnerets are typical of the orders Callipodida and Chordeumatida, and at least one species in the order Stemmiulida ( Shear 2008; Mauriès et al. 2010). The spinnerets of D. mirabilis do not resemble typical polydesmidan spinnerets ( Fig 14 View FIGURES 14 – 16 ), but their long basal sleeves and setal shafts are very similar to the spinnerets of callipodidans ( Fig 15 View FIGURES 14 – 16 ; further illustrations in Shear 2008). Eostemmiulus caecus Mauriès, Golovatch & Geoffroy , arguably the most basal member of the Stemmiulida , also has two spinnerets, in contrast to other members of its order in which the number ranges from four to eight, and those spinnerets resemble polydesmidan spinnerets in having a short, basal sleeve instead of arising from unsocketed mounds as is usual in Stemmiulida .
The dividing ridge on the paraprocts ( Fig 11 View FIGURE 11 ) seems to be unique in the Polydesmida and may represent either an existing division of the paraproct into two sclerites, or a recent fusion of an ancestral two sclerites. The dividing ridge can only be seen in specimens with distended anal regions; in other specimens the ridge is tightly appressed to the margin of the epiproct. In scanning electron micrographs, the ridge appears as a possible suture with flexible cuticle between the sclerites. Divided paraprocts are characteristic of at least some callipodidans. A pair of setae on each of the paraprocts appears to be part of the polydesmidan groundplan, but these setae are absent in D. mirabilis , and instead, as in nematophorans, the paraprocts bear numerous fine setae without any regular arrangement.
Akkari & Enghoff (2011) illustrated intercalary microscutes for a number of polydesmidans. The microscutes of D. mirabilis differ from those illustrated in their regularly wrinkled surface ( Figs 9, 10 View FIGURES 8 – 10 ) rather than being smooth and flat; the wrinkling suggests a thin cuticle or membranous surface. Akkari & Enghoff (2011) found the scutes in members of the families Polydesmidae , Macrosternodesmidae , Trichopolydesmidae , Fuhrmannodesmidae , Opisotretidae , Nearctodesmidae and Dalodesmidae , but not in species of Ammodesmidae , Cryptodesmidae , Cyrtodesmidae , Haplodesmidae , Oniscodesmidae and Pyrgodesmidae . Intercalary microscutes are of no known functional significance, though it is worth noting that many of the members of the families that lack them cover themselves with debris and soil in life. Reboleira & Enghoff (2015) illustrate, but do not discuss, intercalary microscutes in the pleurotergal cuticle of the dorypetalid callipodidan Lusitanipus alternans (Verhoeff) . Microscutes have also been detected in two spirostreptidan families (Enghoff 2014, 2016; Enghoff & Fredriksen 2015).
Anamorphosis of D. mirabilis . Of the 24 specimens of D. mirabilis so far collected, only two, the holotype and paratype, are mature, and one other (ISLA 3638) was a juvenile male, with gonopod primordia on ring seven. The other juvenile specimens are either females or are at a stadium too early to show gonopod primordia.
The weak sclerotization and transparency of the juvenile specimens made it difficult to count rings, and some of our data do not correlate with the anamorphosis tables for other Polydesmida . Nevertheless, since the anamorphosis of D. mirabilis is probably atypical in any case, we present our data in the table below as we obtained it. In this table, ring numbers do not include the telson.
If the anamorphosis of this species proceeds as for Euryurus leachii (Gray) ( Miley 1927) , specimens with eight or nine rings represent stadium II. Specimens with 11 rings are likely to be in stadium III, those with 14–16 rings in either stadium IV or V, and those with 17 rings in stadium VI. Polydesmidans with 19+1 (20) rings mature in stadium VIII, but the presence of juveniles with 24–27 rings indicates that molting continues in D. mirabilis .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.