Myiopharus neilli, O’Hara, James E., 2007
|
publication ID |
https://doi.org/10.5281/zenodo.177452 |
|
DOI |
https://doi.org/10.5281/zenodo.6244034 |
|
persistent identifier |
https://treatment.plazi.org/id/9527878A-2766-AA2E-68AD-FEC8CDF5FD6C |
|
treatment provided by |
Plazi |
|
scientific name |
Myiopharus neilli |
| status |
sp. nov. |
Myiopharus neilli View in CoL sp. nov.
Figs. 1–3, 6–14 View FIGURES 1 – 6. 1 View FIGURES 7 – 11 View FIGURES 12 – 14 .
Cited as “ Myiopharus sp.” in Neill (1982), Charlet (1992), Charlet (1999), Knodel et al. (2000) and Brewer and Charlet (2004).
Holotype. Male, labelled: “ Canada Manitoba/ Lowe Farm/ 49°21'N 97°35'W / G.B. Neill”, “ex. adult of/ Zygogramma / exclamationis / [host] coll. 9.viii.1977 ”, “ HOLOTYPE / Myiopharus / neilli / O'Hara [red label]” ( CNC). Puparium pinned below specimen.
Allotype. Female, labelled: “ Canada Manitoba/ Rosenfeld/ 49°12'N 97°33'W / G.B. Neill”, “ex. adult of/ Zygogramma / exclamationis / [host] coll. viii.1977 ”, “ALLOTYPE/ Myiopharus / neilli / O'Hara [red label]” ( CNC).
Paratypes. 22ɗɗ, 23ΨΨ, all in CNC except as otherwise noted. CANADA. Manitoba: Lowe Farm, 49°21'N 97°35'W, G.B. Neill, ex. adult Z. exclamationis , host coll. 9.viii.1977, 6ɗɗ (3 with puparia), 2ΨΨ; same data except 12.viii.1977, 3ɗɗ (all with puparia); Rosenfeld, 49°12'N 97°33'W, G.B. Neill, ex. adult Z. exclamationis , host coll. viii.1975, 1 Ψ (with puparium and host remains); same data except 5.ix.1975, 8ɗɗ (3 with puparia including 1 with host remains), 6ΨΨ (2 with puparia and host remains); same data except 20.viii.1976, 1Ψ (with puparium and host remains); same data except viii.1977, 1 ɗ, 3ΨΨ (1 with puparium); St. Jean, 49°16'N 97°21'W, G.B. Neill, ex. adult Z. exclamationis , host coll. 22.vii.1977, 1ɗ (with puparium); same data except 5.viii.1977, 3ɗɗ (all with puparia, 1 with host remains), 1Ψ (with puparium).
USA. Colorado: Gunnison Co., Gothie, 9500', 30.vii.1961, J.G. Chillcott, 1Ψ. Minnesota: Clay Co., Hawley Plots, 28.vii.1987, V. Beregovoy, taken from sunflower emergence trap, 1Ψ (NDSU); Marshall Co., Warren, L. Charlet, T. Gross & J. Barker, ex. Zygogramma exclamationis (Fabricius) on Helianthus annuus L., host coll. 14.vi.1985, parasitoid emerged i.1986 (in lab), 1ɗ, 1Ψ (NDSU). North Dakota: Cass Co., Prosper Sunflower Plot, 27.vii.1995, L. Charlet & T. Gross, taken on head of H. annuus , 1Ψ (NDSU); Cass Co., east of Amenia, 47°00.1'N 97°06.8'W, 20.vii.2004, J.E. O’Hara, on H. annuus , 1Ψ; same data except 21.vii.2004, 4ΨΨ; same data except 22.vii.2004, 2ΨΨ. South Dakota: Tripp Co., Winner, 3.vii.1924, 1Ψ.
Etymology
Named for Garnet B. Neill, who elucidated the biology of this species in his Ph.D. dissertation ( Neill 1982) and reared many of the specimens in the type series.
Recognition
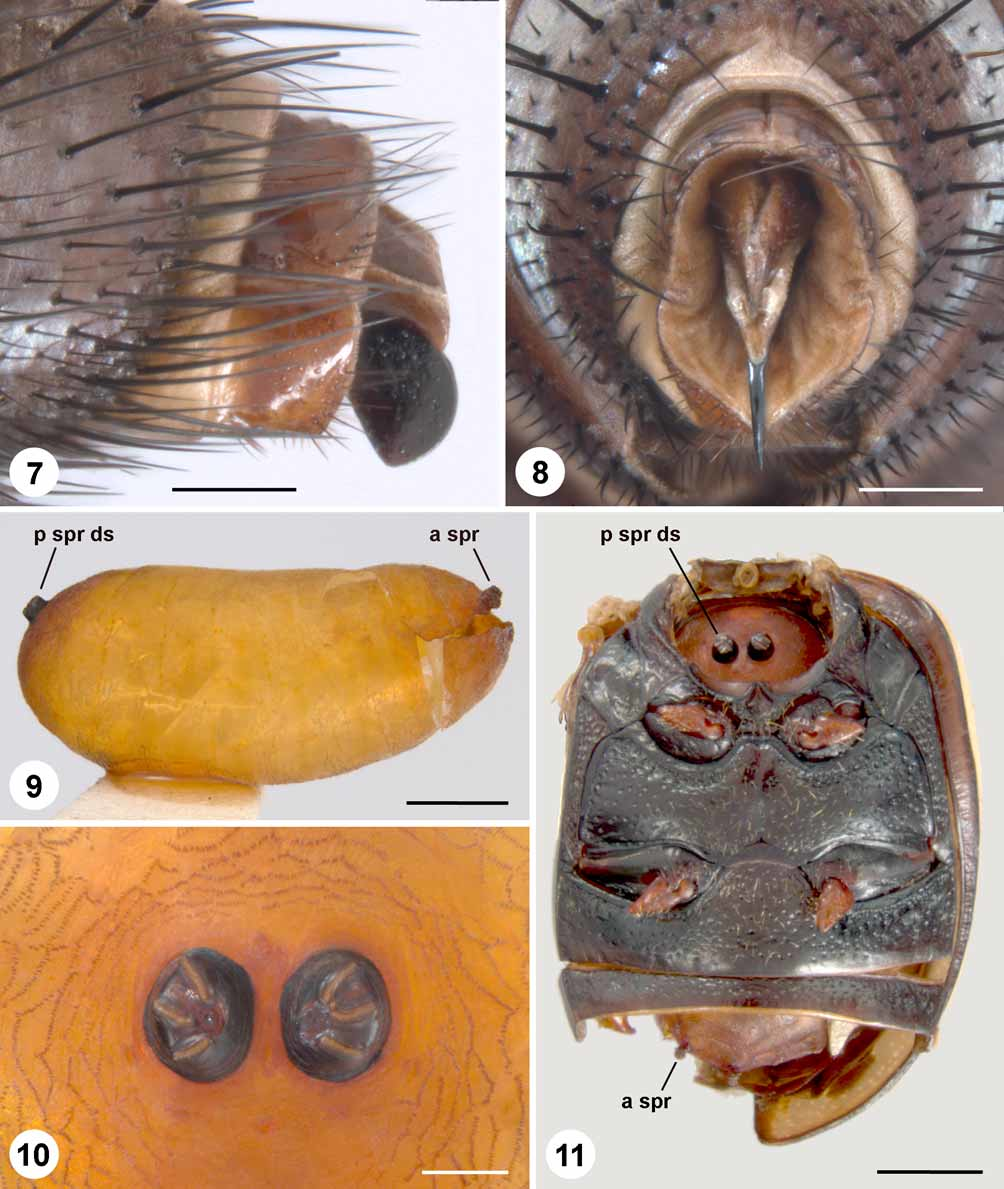
This species is easily recognized in the female sex as a member of the M. dorsalis group by the presence of two distinctive features: a dense tuft of closely appressed setae on the ventral portion of the katepisternum ( Fig. 6 View FIGURES 1 – 6. 1 ), and a laterally flattened and apically rounded ovipositor ( Figs. 7–8 View FIGURES 7 – 11 ) (see discussion under Myiopharus dorsalis species group). The female is readily separated from females of the other known species of the group by its broader parafacial and vertex. The widths of the parafacial and vertex are about equal in the females of M. canadensis , M. dorsalis , and M. securis (these comprising the other members of the M. dorsalis group in America north of Mexico) ( Fig. 4 View FIGURES 1 – 6. 1 , M. dorsalis ), and noticeably narrower than in M. neilli ( Fig. 2 View FIGURES 1 – 6. 1 ). Females of M. neilli and M. dorsalis have a black scutellum, in contrast to a more yellowish scutellum (at least apically) in the females of M. canadensis and M. securis .
Abbreviations: cx 2, mid coxa; kepst, katepisternum; pc orb s, proclinate orbital setae; sbvb s, subvibrissal setae.
Abbreviations: a spr, anterior spiracle; p spr ds, posterior spiracular disc.
The male of M. neilli is similar in coloration to the female, unlike in other members of the M. dorsalis group in which the parafacial and frons of males are silvery and the thorax is black ( Fig. 5 View FIGURES 1 – 6. 1 , M. canadensis ). This sexual dimorphism is common among Myiopharus species. The M. dorsalis group cannot be recognized in males, although the male of M. neilli has a denser group of setae on the ventral portion of the katepisternum than males of most other Myiopharus species. The following combination of character states will serve to distinguish the male of M. neilli from males of all other Myiopharus species in America north of Mexico except for M. aberrans (Townsend) and M. trifurca (van der Wulp): thoracic dorsum viewed from above gray with four thin black vittae (dorsum not solidly black), facial ridge with setae and decumbent hairs on less than lower half (not with semi-erect setae on more than lower half), median discal setae present on abdominal tergites 3 and 4 (not lacking median discals on one or both tergites), three katepisternal setae (not two, with lower seta missing), and no sex patch on abdominal tergites 4 and/or 5. Males of M. neilli and the widespread M. aberrans do not bear much resemblance to one another despite sharing the aforementioned characteristics. The male of M. neilli can be separated from that of M. aberrans as follows: abdominal tergites 3 and 4 with white pruinosity on anterior two-thirds to three-quarters (slightly yellowish and uniformly pruinose over all of tergites 3 and 4 in M. aberrans ), vertex broader (approximately one-third head width in M. neilli [see description below] and one-quarter head width in M. aberrans [0.24–0.28 head width, m=0.26, n=10]), and ocellar setae thin but well developed (scarcely differentiated from ocellar hairs in M. aberrans ). The male of M. neilli is similar in appearance to that of M. trifurca , but differs from it in having a slightly broader parafacial and vertex (vertex 0.26–0.31 head width, m=0.29, n= 11 in M. trifurca ), and a denser group of setae on the ventral portion of the katepisternum (sparsely setose in M. trifurca ). Myiopharus trifurca is presently known from Mexico, Arizona and New Mexico, which is south of the known range of M. neilli .
Description
Male habitus, Fig. 1 View FIGURES 1 – 6. 1 . Female habitus, Fig. 3 View FIGURES 1 – 6. 1 . Length 5.1–6.9mm.
Head. Not sexually dimorphic. Parafacial silvery white (male without the silvery reflective sheen found in the males of some Myiopharus species). Fronto-orbital plate concolorous with parafacial or with golden tinge. Genal groove yellowish orange when viewed from certain angles. Aristomere 3 mostly yellowish brown, rest of antenna mostly black. Maxillary palpus yellow apically, darker basally. Eye moderately to densely haired, 0.74–0.80 head height (m=0.77, n= 10 in male; m=0.77, n= 10 in female). Flagellomere 1 slender, ending well above vibrissa, 3 times longer than wide. Vertex broad in both sexes, at narrowest point 0.32–0.38 head width (m=0.35, n= 10 in male; m=0.35, n= 10 in female). Aristomere 1 short. Aristomere 2 scarcely longer than wide. Aristomere 3 slightly longer than flagellomere 1, evenly tapered to tip. Fronto-orbital plate with 5–8 frontal setae and 1 strong reclinate inner orbital seta that is subequal in size to outer orbital setae; lowermost frontal seta about level with apex of pedicel. Two strong proclinate outer orbital setae in both sexes. Ocellar seta somewhat thin. Outer vertical seta varied from slightly to well developed, larger than setae of postocular row. Inner vertical setae strong, parallel to one another or slightly convergent. Parafacial bare, noticeably wider than width of flagellomere 1. Facial ridge with setae and decumbent hairs decreasing in size dorsally, haired on less than half of its length. Vibrissa situated above lower margin of head. Lower facial margin not protruding beyond vibrissal angle when viewed in profile. Maxillary palpus clavate. Prementum and labella about as long as flagellomere 1.
Thorax. Black in ground color, dorsum not sexually dimorphic and with moderate white pruinosity (appearing gray against black ground color) except for 4 black vittae, inner pair of vittae thin and continuous across transverse suture, outer pair broad and interrupted anterior to suture. Legs black in fresh specimens, faded to reddish black in older museum specimens. Prosternum usually sparsely haired, rarely bare (ca. 10% of specimens). Postpronotum with 3 setae arranged in a triangle. Three postsutural acrostichal setae. Three postsutural dorsocentral setae. First postsutural supra-alar weak. Katepisternum with 2 strong setae and usually a weak third seta just below and behind anteriormost seta; in male densely setose medially in front of mid coxa, female with an even denser tuft of closely appressed, parallel-sided, and somewhat blunt-tipped setae in this area ( Fig. 6 View FIGURES 1 – 6. 1 ). Mid coxa without modified setae. Mid tibia with 1 strong anterodorsal seta. Hind tibia with anterodorsal setae uneven in length and not closely spaced. Tarsal claws not longer than 5th tarsomere. Upper and lower calypters white and often with slight yellowish tinge. Wing vein R 4+5 dorsally with 1 to several hairs at base. Vein M smoothly curved at bend and ending anterior to wing tip. Scutellum with 1 pair of widely spaced discal setae, a pair each of well developed basal, lateral and divergent subapical setae (subapicals strongest followed by basals and then laterals), and a pair of crossed apical setae that are subequal to discal setae.
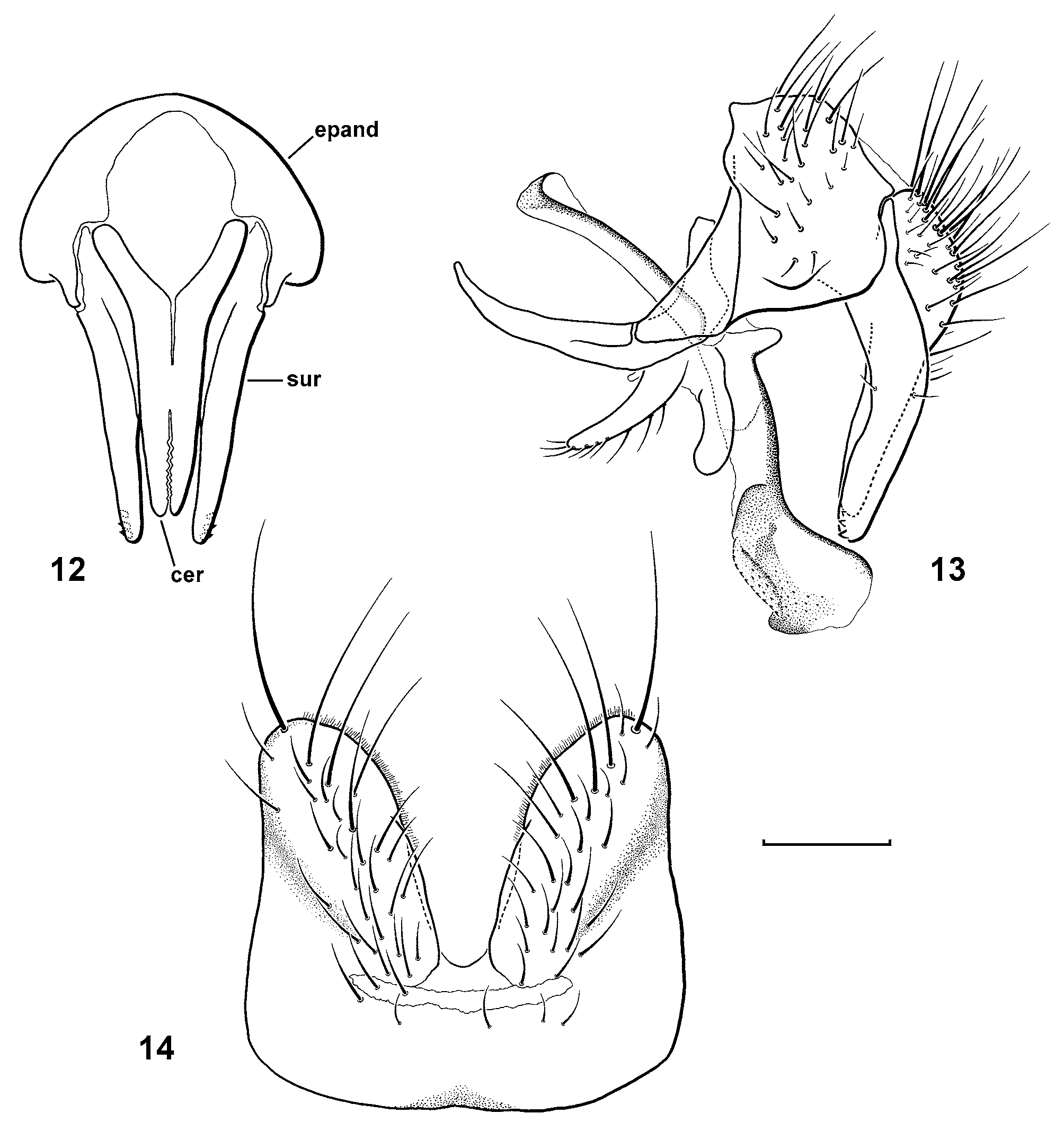
Abbreviations: cer, cercus; epand, epandrium; sur, surstylus.
Abdomen. Black in ground color, with bands of moderate and uniform white pruinosity (appearing gray against black background) on anterior two-thirds to three-quarters of tergites 3 and 4 and about anterior onehalf of tergite 5. Apex deflected slightly downward in female. Middorsal depression on syntergite 1+2 almost reaching median marginal setae. Narrow black vitta medially on tergites 3–5. Syntergite 1+2 with 1 pair of median marginal setae and at least 1 pair of lateral marginal setae. Tergite 3 with 1 pair of median marginal setae, 1 pair of median discal setae (weaker than median marginals), and at least 1 pair of lateral marginal setae. Tergite 4 with row of marginal setae, 1 pair of median discal setae (weaker than median marginals), and at least 1 pair of lateral marginal setae. Tergite 5 with row of weak marginal setae and scattered discal setae. Male without sex patch of tiny appressed hairs on underside of tergite 4 or 5. Sternites 2–3 in male and sternites 2–4 in female each typically with 1 pair of strong setae; sternite 4 in male and sternite 5 in female typically with several moderately strong setae posteriorly; sternites partly overlapped by tergites.
Male terminalia ( Figs. 12–14 View FIGURES 12 – 14 ). Sternite 5 with median cleft smoothly V-shaped, inner margin with fine hairs, posterior lobe rounded apically. Epandrium with slight bulge laterally on lower portion. Pregonite smoothly curved and tapered to a rounded tip, setose along posterior margin. Epiphallus present. Distiphallus divided at base into long, thin sclerite posteriorly and broader winged and sclerotized portion anteriorly. Postgonite parallel-sided with rounded tip. Surstylus slender, gently curved at middle in lateral view, with several small spines at tip. Cerci in lateral view straight along posterior surface, shorter than surstylus, in posterior view tapered only slightly from midpoint to apex, tips separate in apical one-third.
Female terminalia ( Figs. 7–8 View FIGURES 7 – 11 ). Sternite 7 highly modified, forming a black, laterally flattened, apically rounded sclerite below the genital opening.
Puparium ( Figs. 9–10 View FIGURES 7 – 11 ). Slightly larger at posterior end than anterior end, surface nearly smooth. Anterior spiracles raised and rosette-like. Posterior spiracular discs shiny black, narrowly separated and positioned high above midline of puparium, each disc raised above puparium and bearing three nearly straight spiracular slits.
Biology
The biology of the sunflower beetle, Z. exclamationis , has been well documented in the literature (e.g., Westdal 1975; Neill 1982; Charlet 1992; Knodel et al. 2000; Brewer and Charlet 2004). The beetle has one generation per year in the northern plains of North America. The sexually immature adults overwinter in the soil, emerging as sexually mature adults the following May to early June. Mating takes place within a couple of days of emergence, and within a week the females begin laying eggs on the stems and leaves of sunflower plants. Oviposition can continue for nearly two months, during which time up to 2000 eggs can be laid. Beetle larvae appear about mid June and enter the soil to pupate when mature. Prehibernation adults of this summer generation emerge in late July to early September and feed for one to three weeks before entering the soil to overwinter.
The life history of M. neilli , as Myiopharus sp., in south-central Manitoba was detailed in the Ph.D. dissertation of Neill (1982). The following life history account is a summary from that study. Adult females of M. neilli oviposit on prehibernation adults of Z. exclamationis . The parasitoid passes the winter as a first instar free in the haemocoel of the thorax or abdomen of its host. As diapause in the beetle is ending, the first instar of M. neilli moves to the head and enters the supraoesophageal ganglion of the brain. Parasitized beetles do not leave the soil, and larval development of the parasitoid continues underground in its host. The parasitoid leaves the brain late in the first or early in the second instar and enters the buccal cavity, where it makes an opening to the exterior for breathing. With the posterior spiracles positioned against the opening, a respiratory funnel forms around the posterior portion of the maggot. The anterior end is kept free, and the maggot feeds on the host’s tissues throughout its second and third (final) instars. Though the host is immobile during this period, it does not die until the maggot is almost fully grown and occupies most of its thorax and abdomen. Just before pupariation, the parasitoid creates two openings, one along the postero-lateral margin of the abdomen (through which the adult fly will later emerge) and one in the membrane between the prothorax and mesothorax (often decapitating the beetle) ( Fig. 11 View FIGURES 7 – 11 ). Larval development takes about three weeks from the time the first instar becomes active until pupariation, and the pupal stage lasts about two weeks. Adult M. neilli emerge from the soil in June, about one month after the emergence of non-parasitized beetles.
Neill (1982) conjectured that M. neilli has two generations per year. Adult flies emerge in June, when posthibernation adults of Z. exclamationis are available for parasitization. A generation of M. neilli is likely passed in these posthibernation beetles, producing a second emergence of adult flies in late summer when prehibernation adults of Z. exclamationis are present. Larvae of this generation of M. neilli overwinter as first instars in their beetle hosts.
Myiopharus neilli View in CoL is a solitary endoparasitoid of adult Z. exclamationis and has not been recorded from other host species. Neill (1982) recorded a parasitism rate of 0.1 to 17.1% in prehibernation adults of Z. exclamationis at various locations in south-central Manitoba during 1975–1977. Charlet (1992) reported 1.7% parasitism in a collection of prehibernation adults of Z. exclamationis in North Dakota in 1987.
| CNC |
Canadian National Collection of Insects, Arachnids, and Nematodes |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |