Pseudophacopteron longicaudatum, Malenovský & Burckhardt & Queiroz & Isaias & Oliveira, 2015
|
publication ID |
https://doi.org/ 10.5281/zenodo.5303773 |
|
publication LSID |
lsid:zoobank.org:pub:1451DAF4-3F24-496C-86B5-3EEF4B9C5261 |
|
DOI |
https://doi.org/10.5281/zenodo.6401502 |
|
persistent identifier |
https://treatment.plazi.org/id/961D87B4-6C3E-7B2B-3002-A9346BD1FCB7 |
|
treatment provided by |
Marcus |
|
scientific name |
Pseudophacopteron longicaudatum |
| status |
sp. nov. |
Pseudophacopteron longicaudatum View in CoL sp. nov.
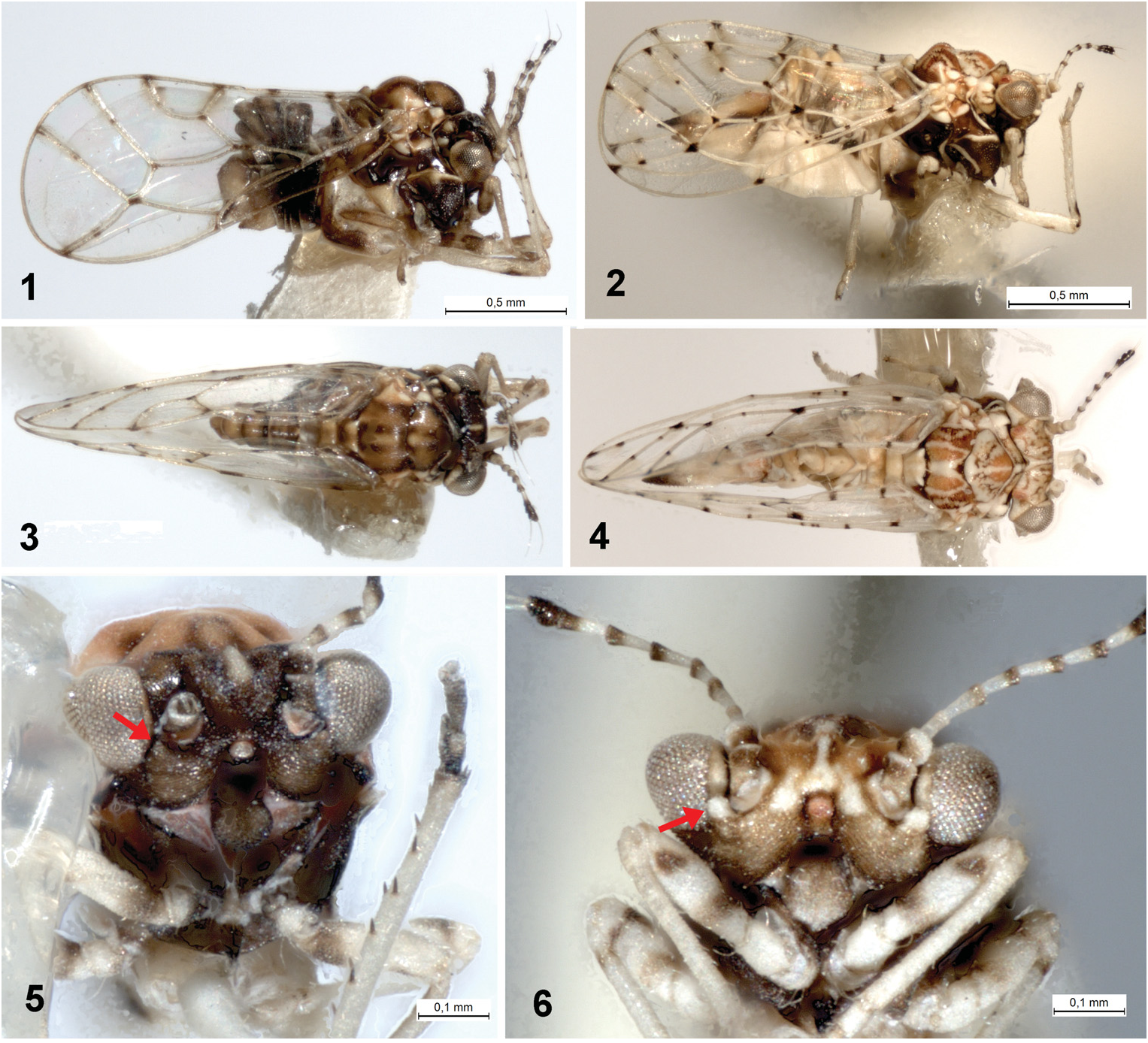
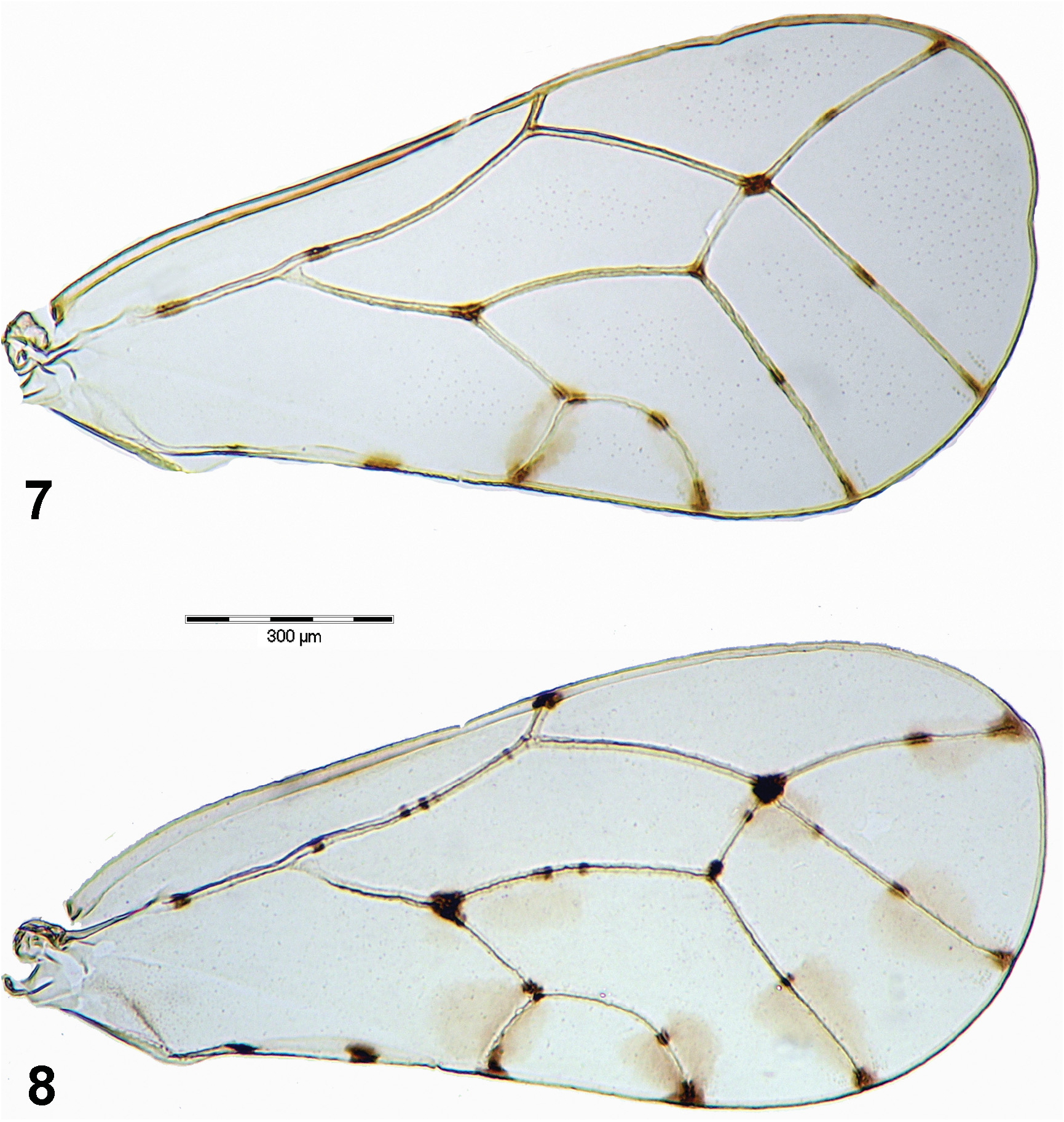
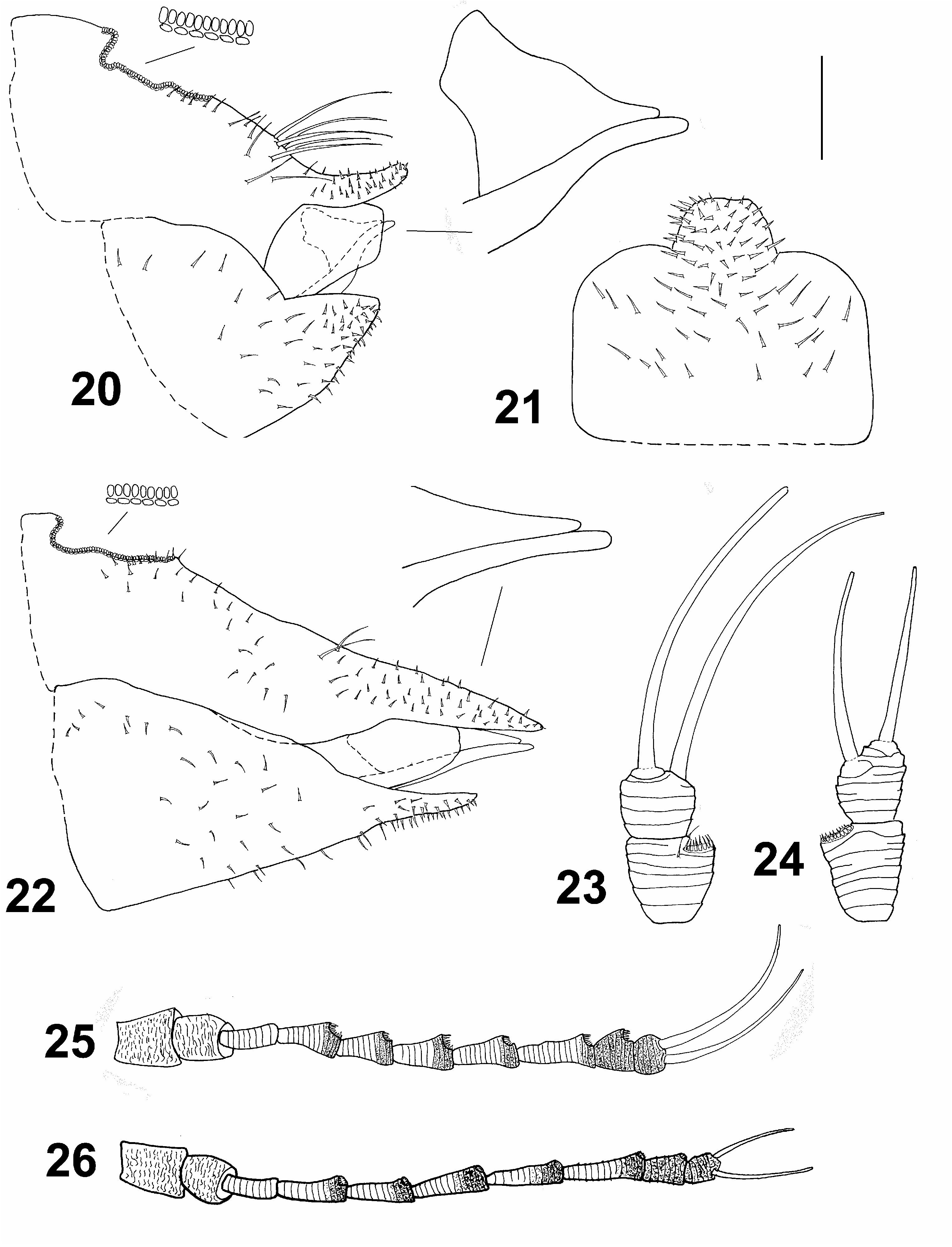
( Figs 2, 4, 6 View Figs 1–6 , 8 View Figs 7–8 , 11 View Figs 9–13 , 17–19 View Figs 14–19 , 22, 24, 26 View Figs 20–26 , 28 View Fig , 36–40 View Figs 36–40 )
Pseudophacopteron sp. : BURCKHARDT & QUEIROZ (2012), CASTRO et al. (2013).
Type locality. Brazil, Minas Gerais: Vazante, Fazenda Bainha, 17°53.4′S 46°55.5′W, 662 m a.s.l.
Type material. HOLOTYPE: ♂, BRAZIL: MINAS GERAIS: ‘ Vazante, Fazenda Bainha, around the house and in eucalypt plantation , S17°53.4′ W46°55.5′, 662 m, 11 September 2014, Aspidosperma tomentosum Apocynaceae , remnants of cerrado forest and eucalypt plantation, sweeping vegetation, D. Burckhardt & D. L. de Queiroz leg., #147(5)ʼ ( MZSP, dry mounted). GoogleMaps PARATYPES: BRAZIL: DISTRITO FEDERAL: 12 ♀♀, 1 adult without abdomen, Brasilia, 3–11 August 1991, Aspidosperma tomentosum , emerged ex galls, VFE 19288 [ V. F. Eastop leg.] ; 2 skins of fifth instar immatures, same data but 27 July 1991 ( BMNH, dry- and slide-mounted). GOIÁS: 2 ♂♂ 3 ♀♀, Serra Dourada, March 2010, Aspidosperma tomentosum, T. M. Silva leg. ; 1 ♀, same data but Aspidosperma macrocarpon ( NHMB, preserved in 70% ethanol). MINAS GERAIS: 4♂♂ 12 ♀♀, same data as holotype ( NHMB, dry-mounted and preserved in 70% ethanol; 1 ♂ 1 ♀ in LEEF, dry-mounted; 1 ♂ 1 ♀ in MZSP, dry-mounted); 2 ♀♀, same but Fazenda Bainha , 17°53.1′S 46°55.0′W, 660 m, 23 September 2011, #19 ( NHMB, no.00002949 , preserved in 70% ethanol); GoogleMaps 1♀, same but Vazante, near Fazenda Maria Olivia , 17°53.8′S 46°56.9′W, 650 m, 14 July 2012, disturbed forest along river , #42 ( NHMB, preserved in 70% ethanol); GoogleMaps 2 ♀♀, same but Três Marias, BR 040 , km 280 , Casa do Caminhoneiro , 18°13.7′S 45°13.2′W, 740 m, 10 July 2012, scrub along road , #36(5) ( NHMB, preserved in 70% ethanol); GoogleMaps 16 ♂♂ 10 ♀♀ 26 skins of fifth instar immatures, Uberlândia, 8 km S, Reserva Ecológica do Clube Caça et Pesca Itororó ( CCPIU), approx. 18°59′S, 48°18′W, 855 m, July 2013, on Aspidosperma tomentosum, D. C. Oliveira leg.; GoogleMaps 9 fifth and 6 fourth instar immatures, same data but March 2013 ( MMBC, dry- and slide-mounted and preserved in 70% ethanol; GoogleMaps 1 ♂ 1 ♀ in NMPC, dry-mounted). GoogleMaps MATO GROSSO DO SUL: 1 ♂ 2 ♀♀, Municipio Campo Grande, near BR 163 , 20°53.7′S 54°34.3′W, 440–450 m, 16 September 2012, cerrado edge along unpaved road , D. Burckhardt & D. L. Queiroz leg., #74(8) ( NHMB, no. 00002948 , preserved in 70% ethanol). GoogleMaps PARANÁ: 1 ♀, Jaguariaíva, Parque do Cerrado , 24°09.878′S 49°39.610′W, 807 m, 10 July 2013, cerrado vegetation , D. L. Queiroz leg., #530 ( NHMB, dry-mounted). GoogleMaps SÃO PAULO: 4 ♂♂ 4 ♀♀, Mogi Guaçu, 1 October 1981, Aspidosperma tomentosum, M. Cytrynowicz leg. ( BMNH, dry- and slide-mounted). GoogleMaps
Description. Adult. Coloration. Vertex orange brown to dark brown with pale yellow markings on median ridge, lateral bulges in front and near posterior margin of vertex pale yellow with orange brown and dark brown markings; genae, frons and clypeus brown to dark brown, tips of genal tubercles below toruli pale yellow ( Figs 4, 6 View Figs 1–6 ). Pronotum and mesopraescutum brown to dark brown with midline and lateral corners pale yellow; mesoscutum brown to dark brown with four pale yellow stripes; mesoscutellum pale yellow; pleural sclerites dark brown with pale yellow markings ( Figs 2, 4 View Figs 1–6 ). Antenna off-white, segments 1–2 basally, segments 4–8 apically, and segments 9–10 entirely dark brown ( Figs 6 View Figs 1–6 , 26 View Figs 20–26 ). Legs pale yellow with two dark brown transverse streaks on pro- and mesofemora, and small dark brown patches on all tibiae basally; metafemur with a small dark brown patch on dorsal side subapically; metacoxa extensively dark brown ventrally ( Figs 2, 6 View Figs 1–6 , 11 View Figs 9–13 ). Fore wing membrane transparent, clear with oval light brown infuscations around apices of veins Rs, M 1+2, M 3+4, Cu 1a and Cu 1b, around touching point of Rs and M 1+2 and across M+Cu fork. Veins off-white with well-delimited dark brown to black spots on apices and forks of all veins except R 1 fork, one medial spot on each R+M+Cu 1, apical portion of Rs, M 3+4 and Cu 1a, respectively, and one or two medial spots on M, two medial spots on anal vein, and two or three small spots on R and M 1+2 ( Fig. 8 View Figs 7–8 ). Hind wing membrane clear, veins off-white. Abdomen uniformly pale yellow or tergites laterally brownish. Male and female terminalia pale yellow, apex of female proctiger dark brown ( Figs 2, 4 View Figs 1–6 ).
Morphology. Body with microsculpture, matt. Head, in frontal view, about twice wider than high ( Fig. 6 View Figs 1–6 ). Vertex dorsally with raised median ridge and two lateral bulges on either side anteriorly; median epicranial suture completely reduced; lateral ocelli lying on small tubercles slightly above the plane of vertex. Eyes hemispherical; occiput and anteoccipital sclerite narrow. Genae small, weakly swollen, tubercle below torulus distinct and acute ( Fig. 6 View Figs 1–6 ). Antenna moderately long, robust, slightly serrate, segments 4–9 distinctly widening to apex ( Fig. 26 View Figs 20–26 ); one large elliptic rhinarium subapically on each of segments 4–9; rhinaria bearing wreath of long cuticular spines; terminal setae subequal, approximately 1.5 times longer than segments 9 and 10 together ( Fig. 24 View Figs 20–26 ). Fore wing pyriform, apex broadly rounded; costal break situated in apical fifth of vein C+Sc ( Fig. 8 View Figs 7–8 ); membrane with fields of sparse surface spinulation in all cells except c+sc, leaving wide spinule-free bands along veins. Mesotibia with subapical comb on outer side reduced to 1–2 stout setae. Metafemur relatively long and slender, medially distinctly constricted, with a row of several evenly short setae along ventral margin ( Fig. 11 View Figs 9–13 ). Metatibia bearing open crown of 7–8 unsclerotised spurs apically and two rows of 6 and 4–5 similar spurs laterally ( Fig. 11 View Figs 9–13 ). Metabasitarsus about twice longer than broad, conical, lacking sclerotised lateral spurs ( Fig. 11 View Figs 9–13 ). Abdominal tergites 2–4 with distinct dorsal tubercles. Male subgenital plate with dorsal margin slightly convex. Male proctiger relatively slender, very long, cylindrical ( Fig. 17 View Figs 14–19 ). Paramere long but slightly shorter than proctiger; in lateral view, nearly straight and parallel-sided in basal four fifths, apex slightly turned posteriorly, blunt; inner side covered with fine setae ( Fig. 18 View Figs 14–19 ). Distal segment of aedeagus with long shaft, apical dilation relatively short and narrow with apex broadly rounded; sclerotised end tube of ductus ejaculatorius relatively long and sinuate ( Fig. 19 View Figs 14–19 ). Female proctiger and subgenital plate with very long apical extensions covered with short and stout setae; dorsal margin of proctiger posterior to circumanal ring, in lateral view, almost straight; circumanal ring with two rows of pores, pores of outer row contiguous; subgenital plate, in lateral view, with ventral margin straight, apex pointed ( Fig. 22 View Figs 20–26 ); in ventral view, narrowly triangular and gradually narrowing to subacute apex. Dorsal and ventral valvulae lacking distinct teeth laterally ( Fig. 22 View Figs 20–26 ).
Measurements and ratios in Table 1 View Table 1 .
Fifth instar immature ( Fig. 28 View Fig ). Yellowish, specimens preserved in alcohol pale off-white, tergites hardly darker pale brown, eyes red. Body elongate, more or less parallel-sided. Dorsal surface with coarse microsculpture: cephaloprothorax, fore and hind wing pads medially and abdominal tergites (including caudal plate) entirely covered with small irregular (mostly round) granules, anterior margins of free abdominal tergites and caudal plate (including anterior margins of fused segments in some specimens) each with a row of larger, irregularly quadrate cuticular scales; lateral portions of cephaloprothorax and wing pads smooth or at least with a less pronounced microsculpture. Whole body margin with pointed lanceolate setae in following numbers (one side only): head in front of insertion of antenna: 3–5, cephaloprothorax behind eye: 8–10, fore wing pad: 14–19, hind wing pad: 3–5, abdomen: (4–6) + (7–9) + (34–42); a few isolated lanceolate setae also in submarginal region and on dorsum of free abdominal tergites and caudal plate. Antenna inserted on ventral side, oriented obliquely outwards and backwards over the body, short and robust, not extending beyond eye posterior margin, lacking distinct divisions and bearing two rhinaria posteriorly. Tarsal arolium membranous, hardly visible in slide-mounted specimens, sessile, broadly fan-shaped, as large as claws. Abdomen dorsally with four large free sclerites and caudal plate (incompletely fused in some specimens); caudal plate margin broadly rounded. Anus small, rhomboid, in ventral position. Circumanal ring small, with fore and hind margins close together; outer ring composed of a single row of pores, hardly sinuate laterally. Measurements and ratios in Table 2 View Table 2 .
Diagnosis. Pseudophacopteron longicaudatum sp. nov. is probably closely related to P. aspidospermi sp. nov. (see the comments under the latter). Adult resembles P. nervosum Brown & Hodkinson, 1988 , P. punctinervis Brown & Hodkinson, 1988 , and P. vitivenis Brown & Hodkinson, 1988 in the fore wing pattern with light brown infuscations on the membrane on apices of most veins and several dark dots on veins, it is, however, generally larger (see BROWN & HODKINSON 1988). From the known Neotropical Pseudophacopteron spp. , it can be reliably differentiated by the very long male and female terminalia. The adults also differ in the reduced mesotibial comb of setae.
The fifth instar immatures of P. longicaudatum differ from P. aspidospermi (and partly also from the immatures of other similar species discussed in the Diagnosis section under P. aspidospermi ) in the more elongate and parallel-sided body shape, the microsculpture of dorsal surface (more or less smooth cuticle of lateral portions of cephaloprothorax and wing pads, presence of rows of quadrate scale-like projections of cuticle near anterior margins of abdominal tergites), the shorter and more robust antenna, the smaller number of lanceolate setae on the wing pad margins, the presence of several isolated lanceolate setae on the abdominal dorsum, the smaller circumanal pore ring, and the larger tarsal arolium.
Etymology. Adjective derived from Latin longus = long and cauda = tail, referring to the long female terminalia.
Host plant and biology. Gall-inducer on leaf lamina of Aspidosperma macrocarpon Mart. and A. tomentosum Mart. (Apocynaceae) ( Figs 36, 37 View Figs 36–40 ). The intralaminar lenticular galls (sensu ISAIAS et al. 2013) on A. macrocarpon were described by CASTRO et al. (2013): they are induced on the abaxial surface of the leaf by the feeding action of the first-instar immatures of P. longicaudatum ; mature galls are green, 5.3 mm large in diameter and 1.3 mm high, slightly protruding to the adaxial leaf surface with a higher prominence to the abaxial surface, with a single inner central chamber opening to the abaxial surface through a narrow ostiole ( Fig. 40 View Figs 36–40 ). They occur on the leaves of A. macrocarpon in large numbers (in average, there are 18.1 galls per leaf; SD = 14.6, n = 40). The period of induction coincides with the appearance of young tissues, with the highest numbers of galls recorded in January (62.8 % leaves infested) and July (65.2 %) following two peaks of leaf flushing and highest nitrogen content of the host in October and June. In Brazil: Goiás, gall senescence takes place in the dry season from March to September, together with that of the host leaves. The beginning of A. macrocarpon leaf falling in March causes the interruption of P. longicaudatum life cycle by premature abscission of immature galls ( CASTRO et al. 2013). The galls on A. tomentosum have the same structure, being 3.8 mm large in diameter and 1.6 mm high ( Figs 38, 39 View Figs 36–40 ), and occur on the leaves with an infestation index of 75 %. The gall induction occurs exclusively on young leaves (unpublished data). On both host plant species P. longicaudatum sp. nov. presents a univoltine life cycle.
Distribution. Brazil (Distrito Federal, Goiás, Minas Gerais, Mato Grosso do Sul, Paraná and São Paulo). Psyllid galls on Aspidosperma tomentosum which involve this species were also recorded from Brazil: Goiás by ARAÚJO et al. (2007) and SANTOS et al. (2012). The host plants, A. macrocarpon and A. tomentosum , are widely distributed in all regions of Brazil (Acre, Tocantins, Bahia, Maranhão, Pernambuco, Piauí, Distrito Federal, Goiás, Mato Grosso do Sul, Mato Grosso, Espírito Santo, Minas Gerais, Rio de Janeiro, São Paulo, Paraná, Santa Catarina), Bolivia, and Paraguay; A. macrocarpon is also present in Peru and Venezuela ( WOODSON 1951, HASSLER 2014).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Psylloidea |
|
Family |
|
|
Genus |
Pseudophacopteron longicaudatum
| Malenovský, Igor, Burckhardt, Daniel, Queiroz, Dalva L., Isaias, Rosy M. S. & Oliveira, Denis C. 2015 |
Pseudophacopteron sp.
| CASTRO A. C., OLIVEIRA D. C., MOREIRA A. S. F. P. & ISAIAS R. M. S. 2013: 524 |
| BURCKHARDT D. & QUEIROZ D. L. 2012: 524 |