Cicinnus packardii ( Grote, 1865 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4084.4.6 |
|
publication LSID |
lsid:zoobank.org:pub:B1923A1E-E9FA-421E-87C9-2151D9CD1921 |
|
DOI |
https://doi.org/10.5281/zenodo.6055063 |
|
persistent identifier |
https://treatment.plazi.org/id/9B3C87C1-FF8A-FFAD-D4A6-FA8AFCCCFB6D |
|
treatment provided by |
Plazi |
|
scientific name |
Cicinnus packardii ( Grote, 1865 ) |
| status |
|
Cicinnus packardii ( Grote, 1865)
( Figs 1–5 View FIGURES 1 – 5 , 18 View FIGURE 18 , 19 View FIGURES 19 – 21 , 22 View FIGURES 22 – 23 )
Perophora packardii Grote, 1865 , Plate 4, Fig. 6 View FIGURES 6 – 11 female Perophora packardii ; Grote 1867
Perophora packardi View in CoL ; Foetterele 1902, misspelling Perophora packardi View in CoL ; Lima 1922 *, misspelling Perophora packardi View in CoL ; Lima 1927 *, misspelling Cicinnus packardi View in CoL ; Schaus 1928, Fig. 87c male, misspelling Cicinnus packardi View in CoL ; Monte 1934 *, misspelling
Cicinnus packardi View in CoL ; Bondar 1950 *, misspelling Cicinnus packardii ; Silva et al. 1968 *
Cicinnus packardi View in CoL ; Biezanko 1986 *, misspelling Cicinnus packardi View in CoL ; Becker 1996, misspelling
Cicinnus packardi View in CoL ; Pastrana 2004 *, misspelling Cicinnus packardii ; Herbin & Monzón 2015
*References that almost certainly refer to Cicinnus despecta ( Walker, 1855) View in CoL or a related South American species.
Type material. Holotype, ♀: CUBA: Cuba., Poey, Collection/ 612/ Type No. 7749, Perophora PACKARDII, A.R. Grote / [photo examined, ANSP]. No paratypes .
Additional specimens examined. ( 14 ♂, 2 ♀ total) CUBA: 4 ♂, 1 ♀, Matazanas: W. Schaus coll., St . Laurent diss.: 10-31-15:2 ( AMNH); VII, XI, USNM-Mimal: 1314, 1 316, 1317 ( USNM). 1 ♂, Central Soledad : 29.VIII.1932, B.B. Leavitt ( MCZ). 3 ♂, 1 ♀, Soledad , Santa Clara : 3.VIII.1932, 5.IX.1932, Bates and Fairchild , St. Laurent diss.: 11-6-15:1, 11-12-15:1, 11-20-15:1 ( MCZ). 2 ♂, Santiago : Collection Wm Schaus , USNM-Mimal: 1319, 1320 ( USNM). 1 ♂, Sierra Maestra, East Cuba, 1000 ft: 28.I.1930, O. Querci, 47 I. C.M., USNM-Mimal: 1315 ( USNM). 3 ♂, No additional collecting data: Dognin Collection, USNM-Mimal: 1321, 1322, 1324, St. Laurent diss.: 12-1-15:1 ( USNM).
Additional specimen photographs examined. ( 2 ♂, 1 ♀ total) CUBA: 1 ♂, Santiago de las Vegas: USNM- Mimal: 2736 ( USNM) . 1 ♂, Matazanas Province, Cienga Zapata, nr. Playa Larga , 3 m: 10–11.II.1981, D.R. Davis, USNM-Mimal: 2734 ( USNM) . 1 ♀, Cienfuegos Prov., nr. Pasa Caballos , 6 km S . Cienfuegos, 10 m: 13– 14.II.1981, D.R. Davis, USNM-Mimal: 2735 ( USNM) .
Diagnosis. Both sexes of this species can be recognized by the presence of a B-shaped hyaline patch on the forewings and strongly contrasting postmedial lines on both fore and hindwings. Postmedial lines are accented by brown shading on the outer edges, especially on the hindwings. The most similar species not treated in the present work, C. felderia Schaus, 1928 and C. hanseni Herbin & Monzón, 2015 , display more heavily contrasting markings and deeper, redder shading on the outer edges of the postmedial lines, and are found in Mexico and Central America. Additionally, C. hanseni is smaller and has narrower wings than C. packardii . The light brown ground color, presence of dark shading in the postmedial region of all wings, especially along the outer edge of the postmedial line, and the usually larger hyaline discal mark differentiates C. packardii from the following two species. The hindwing discal spot of the female of C. packardii is somewhat darker than that of female C. bahamensis sp. n., described below.
Description. Male. Head: Small, scales on frons swept ventrad, pale off-white to gray, eyes very large comprising roughly half of head area, eyes bordered posteriorly by dark brown collar of scales reaching labial palpi, labial palpi small, segments weakly defined ventrally because of the presence of ventral tufts, segments smaller distally, dorsally with darker scales contrasting with overall straw coloration. Antenna bipectinate to tip, scape and pedicel weakly tufted.
Thorax: Gray to very light brown, densely covered in scales of varying widths, generously sprinkled with darker petiolate scales.
Legs: Vestiture thick, scales long, coloration as for thorax, darker petiolate scales present. Tibial spurs short, indistinct.
Forewing dorsum: Forewing length: 19–24 mm, avg.: 21.4 mm, n=14. Triangular, apical quarter of outer margin concave, convex mesally, apex falcate. Ground color pale gray, overall generously speckled by dark petiolate scales. Discal spot marked by small, fused B-shaped hyaline area, bisected by M2, outlined by darker gray scales. Postmedial line pale brown, nearly straight, sometimes with slight undulations near posterior wing margin, postmedial line angled sharply towards costa immediately after passing R5. Antemedial line very faint or absent, brownish, bulging outward. Postmedial area always darker than medial area, usually brown, but sometimes darker gray, shading darkest along postmedial line, shaded region widening approaching posterior wing margin.
Forewing venter: As in forewing dorsum but more heavily speckled, pinkish suffusion sometimes present near discal mark, postmedial and antemedial lines absent, postmedial line replaced by faint gray, curved streak extending from costa to M3.
Hindwing dorsum: Rounded, similar coloration and patterning as forewings but postmedial line terminates before reaching anterior wing margin, brown postmedial suffusions concentrated near anal angle of wing, hyaline discal mark absent, but replaced by faint gray mark.
Hindwing venter: Following similar pattern as forewing venter but lines usually absent, faint remnant of postmedial line may be present, frenulum absent or highly reduced.
Abdomen: Short, vaguely triangular, reaching just barely beyond anal margin of hindwing, depth equal to that of thorax, truncated to slightly upturned posterior tip, coloration a continuation of gray thoracic color. Venter as for dorsum.
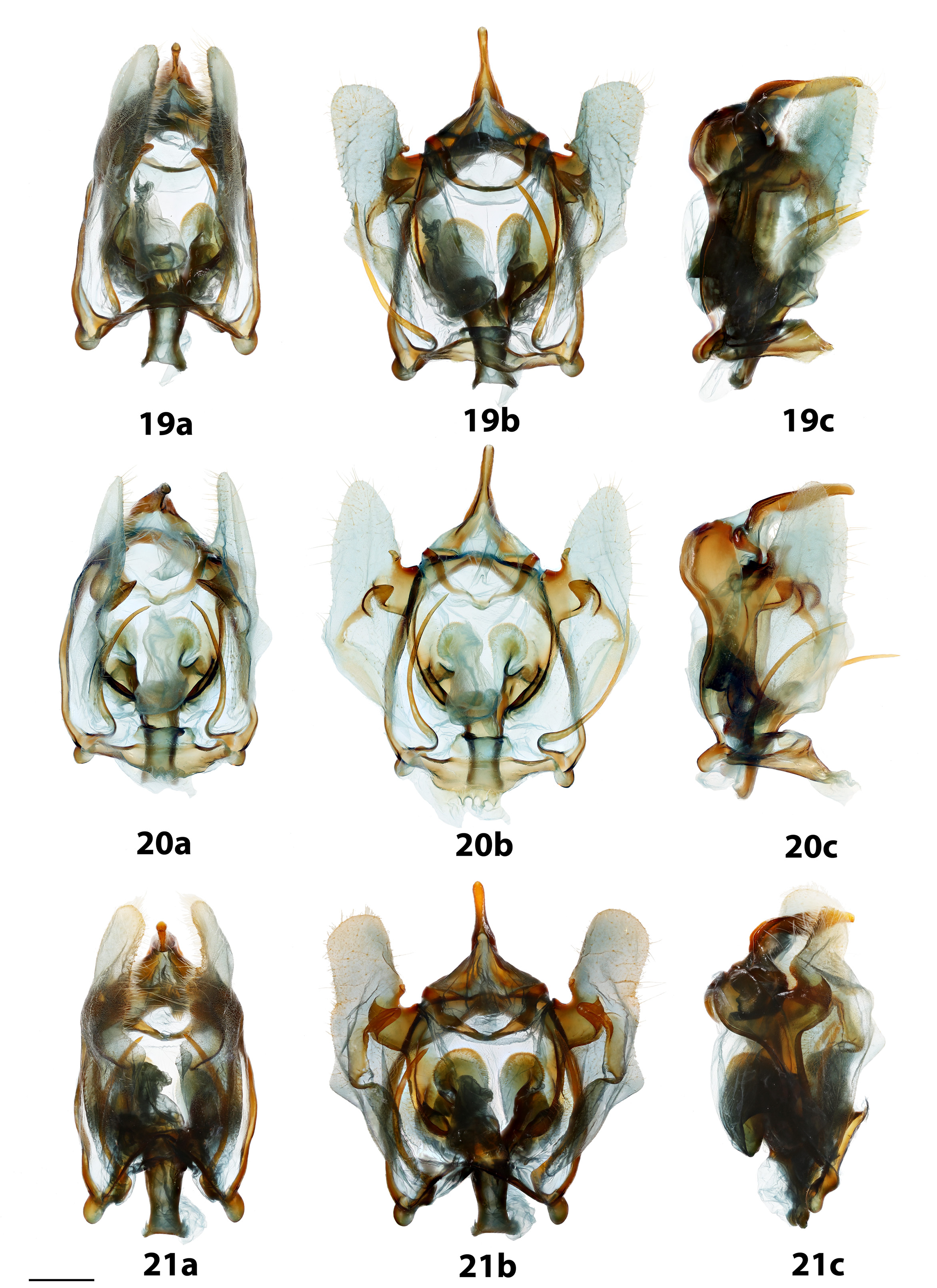
Genitalia: ( Fig. 19 View FIGURES 19 – 21 ) n=4. Very complex; uncus elongate, very narrow, tubular, apex rounded. Tegumen somewhat box-like or ovoid, indented or smooth mesally. Gnathos absent. Valves mostly membranous, relatively short, bent upwards at base of uncus, sclerotized mesally as two robust, somewhat pointed processes, thin band of sclerotization from processes to ventral valve edge curved to nearly straight. Vinculum box-like, ventral corners of vinculum accentuated as relatively large, rounded knobs. Saccus quadrate, variable, smooth or wrinkled, weakly sclerotized distally. Elongated, inward curving tusks originating at base of vinculum reach just below tegumen. Juxta fused to both phallus and vinculum; pair of complicated, invaginated structures extend from juxta dorsally over phallus, forming connection with vinculum. Phallus short, broad, somewhat rectangular when viewed ventrally, cannot be excised from genitalia capsule without damaging juxta-vinculum complex. Vesica roughly phallus-length, bag-like, without cornuti.
Female. Head: As in male but smaller, antenna smaller overall.
Thorax: As in male.
Legs: As in male.
Forewing dorsum: Forewing length: 26 mm, avg. 26, n=2. As in male but slightly broader, less falcate, postmedial and antemedial lines fainter or absent, postmedial shading fainter but present. B-shaped hyaline discal spot with dark gray scales along M2 separating hyaline patch into two distinct halves.
Forewing venter: As in forewing dorsum but more heavily speckled, pale pink-brown suffusion present.
Hindwing dorsum: As in male but broader, postmedial shading fainter but present, discal mark darker and larger.
Hindwing venter: Following similar pattern as forewing venter.
Abdomen: As in male but stouter, venter of VIII segment with pair of small, longitudinal sclerotized bands.
Genitalia: ( Fig. 22 View FIGURES 22 – 23 ) n=1. Simple; papillae anales somewhat widened distally, trapezoidal, covered in fine setae, setae shorter basally. Apophyses anteriores longer and more curved than apophyses posteriores. Apophyses widened distally. Ductus bursae short, wide, opening immediately into elongate corpus bursae. Corpus bursae poorly preserved. Dorsal sclerotization of VIII tergite doubled, accordion-like, forming posteriorly directed subtriangle with internal, bulbous mass apically. Lamella antevaginalis small, concave, with small, thin, amorphous masses covered in short, thick setae located on either side of ostium.
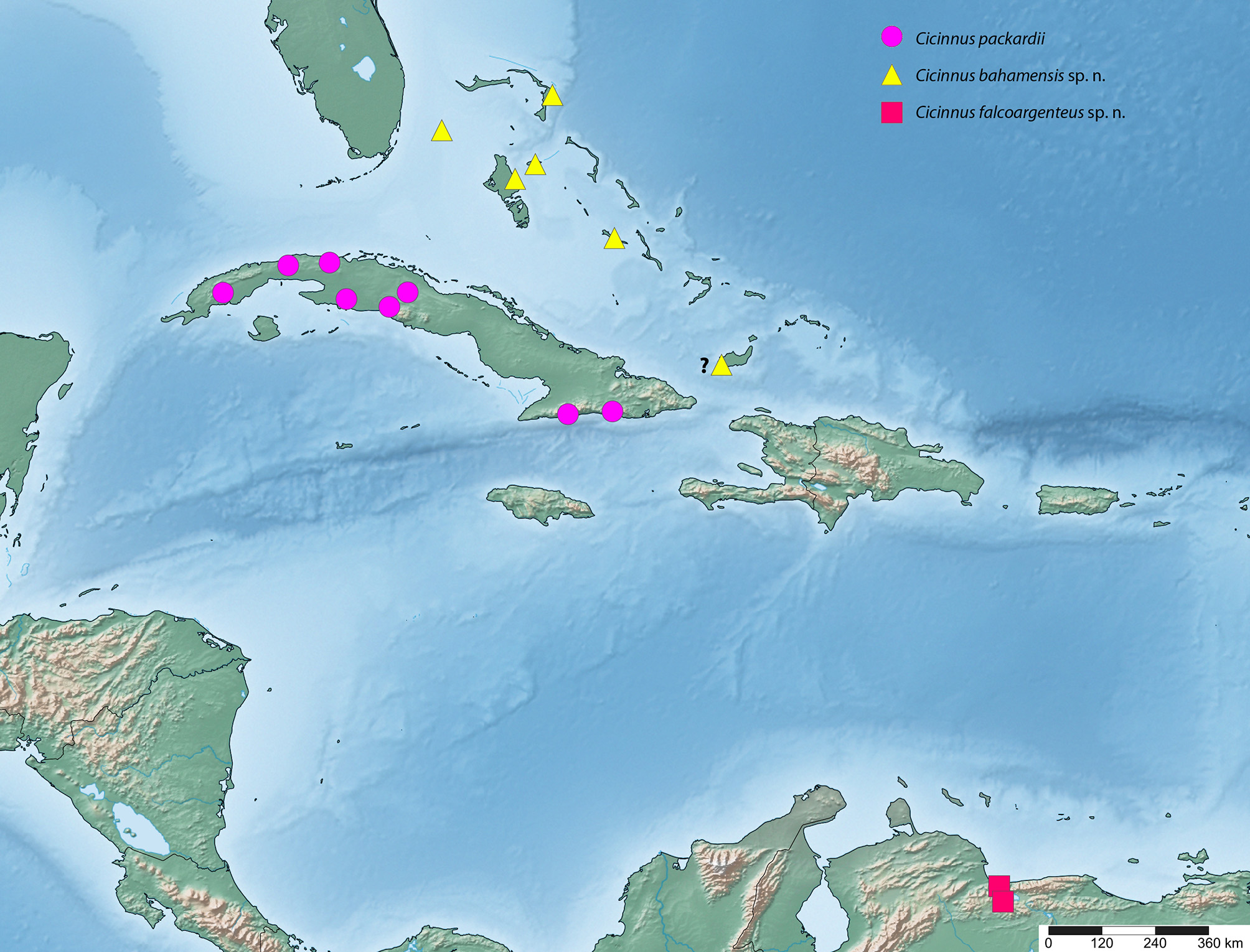
Distribution ( Fig. 18 View FIGURE 18 ). This species is endemic to Cuba, with most records from the western and west-central parts of the island. Eastern records are restricted to the southern coast.
Natural history. While there is nothing published on the biology of C. packardii in Cuba, the CUIC has three larval sacks collected in Pinar del Rio, Cuba on 29.III.1939. There are no adults accompanying these sacks, therefore it is not possible to verify their identity, though the locality certainly suggests C. packardii since no other Mimallonidae are known from the island. The sack structure is exactly like that of C. bahamensis sp. n. ( Figs 9, 10 View FIGURES 6 – 11 ). Unfortunately no host records accompany these specimens, except the word “Artemisia” which is probably a misspelling of Artemisa, a municipality near Pinar del Rio, and not the plant genus belonging to Asteraceae .
Remarks. Cicinnus packardii is frequently misspelled as “ C. packardi ” in the literature. Confusingly, C. packardii is numerously referenced in Brazilian literature ( Lima 1922, 1927, Monte 1934, Bondar 1950, Silva 1968, Biezanko 1986) as occurring in Brazil, often in agricultural publications listing larval host plants. However, this species is known only from Cuba, and all South American references to this species almost certainly can be attributed to the somewhat similar C. despecta or a related species such as C. conlani Herbin & Mielke, 2014 . Pastrana (2004) later repeated this mistake, listing this species for Argentina as well as Brazil. Cicinnus despecta , identified as C. packardii , was commonly seen in many of the visited collections (R. A. St. Laurent pers. obs.).
Cicinnus bahamensis St. Laurent & McCabe , sp. n. ( Figs 6–15 View FIGURES 6 – 11 View FIGURES 12 – 15 , 18 View FIGURE 18 , 20 View FIGURES 19 – 21 , 23 View FIGURES 22 – 23 )
Type material. Holotype, ♂: BAHAMAS: Great Exuma: BAHAMAS-Great, Exuma-Simons Pt, 23.31.50 – 75.47. 30, 23 January 1980, Tim L. McCabe/ St. Laurent diss. : 10-15-15:5/ HOLOTYPE male Cicinnus bahamensis St Laurent and McCabe, 2016 [handwritten red label]/ (CUIC).
Paratypes, 84 ♂, 16 ♀ total: BAHAMAS: Abaco: 4 ♂, J.L. Bonhote, St. Laurent diss. : 10-15-15:3 ( NHMUK). Great Exuma: 53 ♂, 13 ♀, Simon’s Point, 23.31.50 – 75.47.30, T. McCabe : 10.I.1980 [1♂, CUIC]; 11.I.1980 [3♂, 2♀, AMNH, TM]; 12.I.1980 [3♂, TM], McCabe slide 5305; 13.I.1980 [ 2♂, 2♀, CUIC, TM], St. Laurent diss. : 11-10-15:1; 14.I.1980 [2♂, 1♀, TM]; 18.I.1980 [4♂, 1♀, TM]; 20.I.1980 [1♂, TM]; 21.I.1980 [1♂, 1♀, RAS]; 22.I.1980 [4♂, TM, NYSM]; 23.I.1980 [5♂, 2♀, CUIC, USNM, NYSM, TM], McCabe slide 5302; 26.I.1980 [1♀, NYSM]; 22.XII.1981 [1♂, CMNH]; 20.XII.1981 [1♂, TM]; 26.XII.1981 [1♂, RAS]; 27.XII.1981 [1♂, MGCL]; 8.I.1982 [1♂, TM]; 13.I.1982 [1♂, TM]; 17.I.1982 [2♂, TM]; 21.I.1982 [1♂, TM]; 23.I.1982 [1♂, TM]; 28.I.1982 [1♂, TM], McCabe slide 5015; 10.IV.1986 [3♂, USNM, TM]; 11.IV.1986 [5♂, TM, donated to research collection of Daniel Herbin, France]; 12.IV.1986 [2♂, TM]; 13.IV.1986 [1♀, TM], McCabe slide 5030; 15.IV.1986 [2♂, TM]; 16.IV.1986 [3♂, TM]; 21.IV.1986 [1♂, 2♀, TM]. New Providence: 7 ♂, Nassau: 16.II1902, J.L. Bonhote, St. Laurent diss. : 10-15-15:2 ( NHMUK). North Andros: 1 ♂, Fresh Creek : no additional data ( AMNH). South Bimini: 17 ♂, 1 ♀, F.H. Rindge , 8.VI.1950 [3♂]; 9.VI.1950 [6♂]; 10.VI.1950 [ 4♂], St. Laurent diss. : 10-31-15:1; 12.VI.1950 [4♂]; V.1951 [1♀] (AMNH). 2 ♂, 2 ♀, No collector: V.1950 [1♀]; V.1951 [2♂]; 14– 22.VIII.1951 [1♀] (AMNH). All paratypes with the following yellow label: PARATYPE male/female Cicinnus bahamensis St Laurent and McCabe, 2016 .
Additional specimens examined [not to be included in type series.] ( 5 ♂, 1 ♀ total) BAHAMAS: 1 ♂, “ Bahamas,” no specific island data: L. Bonhote ( NHMUK) . Abaco: 1 ♀, near Snake Creek , N 26.417915°, W 77.041898°: 9.IX.2014, coll. L. Yang, ex. larva (BME) GoogleMaps . Great Inagua: 2 ♂, 5 km. S of Salt Works : 30.IX.1986, M. Simon & L. Miller, UV, Allyn Museum, Acc. 1986–19, St. Laurent diss.: 10-15-15:1 [possibly representing a second, undescribed, species from the Bahamas] ( MGCL). Little Abaco: 2 ♂, emerged 8.VII.1922, 10.XI.1922, LRG? [initials illegible] ( NHMUK).
Diagnosis. Both sexes of this species can be distinguished from the similar C. packardii by the lighter gray ground color, fainter markings on all wings, and the reduction of brown shading on the outer margin of the postmedial lines, which is usually entirely absent. Additionally, the postmedial region in C. packardii is always much darker than that of the medial region, whereas these regions are nearly always concolorous in C. bahamensis sp. n. Genitalia differences also reliably separate C. bahamensis sp. n. from C. packardii : in C. bahamensis sp. n. the uncus is slightly broader, the sclerotized mesal processes of the valves are smaller, less robust, and usually sharper, and the ventral knobs of the vinculum are smaller.
Description. Male. Head: Small, scales on frons swept ventrad, pale off-white to gray, eyes very large comprising roughly half of head area, eyes bordered posteriorly by dark brown collar of scales reaching labial palpi, labial palpi small, segments weakly defined ventrally due to ventral tufts, segments smaller distally, dorsally with darker scales contrasting with overall straw coloration. Antenna bipectinate to tip, scape and pedicel weakly tufted.
Thorax: Gray, densely covered in scales of varying widths, generously sprinkled with darker petiolate scales.
Legs: Vestiture thick, scales long, coloration as for thorax, except tarsus off-white, darker petiolate scales present. Tibial spurs short, triangular.
Forewing dorsum: Forewing length: 19.5– 21 mm, avg.: 20 mm, n=14. Triangular, apical quarter of outer margin concave, convex mesally, apex falcate. Ground color pale gray, overall generously speckled by dark petiolate scales. Discal spot marked by small, fused B-shaped hyaline area, bisected by M2, outlined by darker gray scales. Postmedial line dark gray or pale brown, nearly straight with slight undulations along length or somewhat curved after M2, postmedial line angled sharply toward costa immediately after passing R5. Antemedial line very faint or absent. Postmedial area occasionally with tan suffusion near wing margin, overall barely darker than medial area.
Forewing venter: As in forewing dorsum but more heavily speckled, pinkish suffusion sometimes present near discal mark, postmedial and antemedial lines absent, postmedial line replaced by faint gray, curved streak from costa to M3.
Hindwing dorsum: Rounded, similar coloration and patterning as forewings but postmedial line terminates before reaching anterior wing margin, hyaline discal mark absent, but replaced by faint gray mark.
Hindwing venter: Following similar pattern as forewing venter but lines absent, frenulum absent or highly reduced.
Abdomen: Short, vaguely triangular, reaching just barely beyond anal margin of hindwing, depth equal to that of thorax, truncated to slightly upturned posterior tip, coloration a continuation of gray thoracic color. Venter as for dorsum.
Genitalia: ( Fig. 20 View FIGURES 19 – 21 ) n=7. Very complex; uncus elongate, tubular, apex rounded. Tegumen circular or ovoid, indented mesally, width of indent variable. Gnathos absent. Valves mostly membranous, relatively short, bent upwards at base of uncus, sclerotized mesally as two somewhat pointed processes, thin band of sclerotization from processes to ventral valve edge steeply curved, nearly C-shaped. Vinculum box-like, ventral corners of vinculum accentuated as rounded knobs. Saccus quadrate, variable, smooth or wrinkled. Elongated, inward curving tusks originating at base of vinculum reach just below tegumen. Juxta fused to both phallus and vinculum; pair of complicated, invaginated structures extend from juxta dorsally over phallus, forming connection with vinculum. Phallus short, broad, somewhat rectangular when viewed ventrally, cannot be excised from genitalia capsule without damaging juxta-vinculum complex. Vesica roughly phallus-length, bag-like, without cornuti.
Female. Head: As in male.
Thorax: As in male.
Legs: As in male.
Forewing dorsum: Forewing length: 25–26 mm, avg.: 25.5 mm, n=3. As in male but slightly broader, less falcate, postmedial and antemedial lines fainter or absent. B-shaped hyaline discal spot with dark gray scales along M2 separating hyaline patch into two distinct halves.
Forewing venter: As in forewing dorsum but more heavily speckled.
Hindwing dorsum: As in male but broader.
Hindwing venter: Following similar pattern as forewing venter but more heavily speckled.
Abdomen: As in male but stouter, venter of VIII segment with pair of small, longitudinal sclerotized bands.
Genitalia: ( Fig. 23 View FIGURES 22 – 23 ) n=3. Simple; papillae anales somewhat widened distally, trapezoidal, covered in fine setae, setae shorter basally. Apophyses anteriores roughly same size as apophyses posteriores, but thinner. Apophyses widened distally. Ductus bursae short, wide, opening immediately into elongate corpus bursae. Corpus bursae cylindrical, without any sclerotized structures. Small, bag-like appendix bursae present. Dorsal sclerotization of VIII tergite forms posteriorly directed subtriangle. Lamella antevaginalis small, concave, with amorphous masses covered in short, thick setae located on either side of ostium, left mass (when viewed ventrally) larger than right.
Distribution ( Fig. 18 View FIGURE 18 ). This species is endemic to the Bahamas, with records from the following islands: Great Exuma, the Abacos, New Providence, South Bimini, and North Andros. This species may also be present on Great Inagua of the lower Bahamas, but see remarks.
Habitat. The plant community at the type locality of C. bahamensis sp. n., Simon’s Point, Great Exuma Island, can be considered a “Coastal Coppice” ( Correll & Correll 1982). Annual average rainfall is about 114 cm per year on Exuma, but typically only about 15 cm for January through April. The mid-winter xeric conditions are reflected by the plant community on the island, whereby the following species are among the most common: Chrysobalanus icaco L. ( Rosaceae ), Erithalis fruticosa L. ( Rubiaceae ), Bourreria ovata Miers (Boraginaceae) , Caesalpinia bahamensis Lam. , Leucaena leucocephala (Lam.) de Wit (Fabaceae) , Malphigia polytricha A. Juss. (Malphigiaceae), and Metopium toxiferum (L.) Krug & Urb. ( Anacardiaceae ). Simon’s Point is on the windward side of the island, consequently mangrove species were virtually absent. The leeward side of the island (a mangrove community) was subjected to infrequent sampling. Cicinnus bahamensis sp. n. was common at light on the windward side but was not observed on the leeward side of the island. Constant offshore winds made it necessary to collect in a recessed, wind-protected carport on Simon’s Point.
According to Eric LoPresti and Louie Yang (BME) (pers. comm.), C. bahamensis sp. n. larvae were frequently encountered on Psidium L. ( Myrtaceae ) on coppices of small islands on the eastern side of Abaco Island. Psidium was also present on Great Exuma but not in the immediate vicinity of the light traps, suggesting either polyphagy or dispersal abilities.
Natural history. Eric LoPresti and Louie Yang (pers. comm.) provide evidence that C. bahamensis sp. n. feeds on Psidium longipes Berg (McVaugh) as per encounters with larvae and larval sacks on this plant on Abaco Island. Feeding damage in close proximity to the affixed larval sacks suggests that P. longipes is indeed being utilized as the host plant. An adult reared from collected larvae (in BME) matches the habitus of C. bahamensis sp. n. and the first author examined several specimens of C. bahamensis from the Abaco Islands (NHMUK). Therefore, we reasonably determine that the natural history information that has been made available to us pertains to this new species. Furthermore, Psidium has been reported as hosts for several Mimallonidae and thus the association of C. bahamensis sp. n. with this host plant genus was not unexpected ( Silva et al. 1968).
Larval vouchers are not available for our detailed examination, thus a larval description cannot be provided at this time. We do however recognize the distinct similarity in larval morphology to that of C. melsheimeri ( Harris, 1841) , which the first author has reared. The sack structure of the probable final instar larvae of C. bahamensis sp. n. differs from that of C. melsheimeri in that it is formed from larval frass covered in silk (see Figs 9, 10 View FIGURES 6 – 11 ), and apparently does not incorporate leaf tissue, as is the case in the latter species. We note, however, that the smaller sacks of earlier instars ( Fig. 7 View FIGURES 6 – 11 ) collected on Psidium do incorporate some leaf material, and in this way greatly resemble the sacks of C. melsheimeri .
Etymology. This new species is named for the Bahamas, the country to which it is endemic.
Remarks. Material of C. bahamensis sp. n. has existed in major collections (AMNH, NHMUK) for decades but has avoided formal description. Only one other species has been described from the Caribbean Islands until now (with the exception of Trinidad), and thus the description of C. bahamensis sp. n. effectively doubles the known diversity of this family in the region. We note that no Mimallonidae , including this new species, have been recorded from Hispaniola.
The Cuban species C. packardii appears to be very closely related to C. bahamensis sp. n. based on external and genitalia morphology, and this previously described species was thus included in the present work for comparison purposes. Morphological differences suggest that these two species are not conspecific, despite their general similarity. Although we note only minor differences in genitalia, the degree of similarity between these two species is not unique. Both C. bahamensis sp. n. and C. packardii belong to a larger group of Cicinnus characterized by hyaline patches on the forewings and genitalia characters, whereby members of this group have some of the most homogenous male genitalia across species (R. A. St. Laurent pers. obs.). Another member of this group, the recently described C. conlani , displays male genitalia highly reminiscent of the Caribbean species ( Herbin & Mielke 2014, Fig. 15 View FIGURES 12 – 15 [incorrectly oriented and labeled in print]). However, C. conlani , of the Brazilian Cerrado, is broadly allopatric with the two Caribbean island species ( Herbin & Mielke 2014). Furthermore, C. falcoargenteus sp. n., to be described below, also belongs to this closely related group of Cicinnus , and shows very similar genitalia characters, despite distinct external characters and allopatry. We also examined C. felderia from Mexico (St. Laurent diss.: 10-31-15:3, AMNH) and the published figures of the Guatemalan species C. hanseni , and noted great similarity in male genitalia. These species clearly belong to a group with very close affinities, and future work may prove this group of Cicinnus to be more aptly placed in a distinct genus or at least a well-defined species-group.
Two extremely worn, nearly destroyed, specimens from Great Inagua, Bahamas (in MGCL) are smaller, have shorter wings, and somewhat browner coloration when compared to specimens from the rest of the Bahamas. These specimens also show some differences in the genitalia, namely in the more widely flailed proximal end of the phallus. Given the relative isolation of Great Inagua from more northern Bahamas islands and minor differences in genitalia and external characters, we consider this population as possibly belonging to an additional undescribed species, if not, at least a geographic variation. We do not describe this population as new for lack of a good series of quality specimens on which to base a description and designate type specimens. Pending additional material from Great Inagua or nearby, we will merely acknowledge the presence of this population and bring awareness of it into the scientific record for potential future work.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Mimallonoidea |
|
Family |
|
|
Genus |
Cicinnus packardii ( Grote, 1865 )
| Laurent, Ryan A. St. & Mccabe, Timothy L. 2016 |
packardii
| Grote 1865 |
packardii
| Grote 1865 |
Cicinnus packardii
| Grote 1865 |
Cicinnus packardii
| Grote 1865 |