Andromma Simon, 1893
|
publication ID |
https://doi.org/10.5852/ejt.2022.850.1997 |
|
publication LSID |
lsid:zoobank.org:pub:E8AD897F-2076-4850-9520-BB79B1EAFFEA |
|
DOI |
https://doi.org/10.5281/zenodo.7433845 |
|
persistent identifier |
https://treatment.plazi.org/id/9C479E6B-FFF3-FFD6-FDF1-F7CDFD35FCF9 |
|
treatment provided by |
Felipe (2022-12-12 12:09:14, last updated 2024-11-26 05:13:07) |
|
scientific name |
Andromma Simon, 1893 |
| status |
|
Genus Andromma Simon, 1893 View in CoL
Type species
Andromma aethiopicum Simon, 1893 View in CoL , by monotypy.
Diagnosis
Andromma resembles Hortipes Bosselaers & Ledoux, 1998 by the peculiar retina of the AMEs which, as seen from above, is only visible in the median half of these eyes. It differs from it by the absence of a metatarsal sensory array on the legs I and II ( Bosselaers & Jocqué 2000; Ramirez 2014: 118), by the smaller number of ventral spine pairs on the tibia and metatarsus I and II, by the flat carapace, which is not elevated in the thoracic region, and by the absence of a median apophysis on the male bulbus. Andromma also resembles the equally myrmecophilous Arabelia Bosselaers, 2009 in general somatic aspect, but differs from it by the absence of an anterior epigynal hood, by the smaller copulatory opening, by the absence of spermathecae II, by the complex, bipartite RTA of the male palp, and by the longer embolus. It differs from Cybaeodes by the bipartite RTA, and by the absence of enlarged piriform gland spigots on the ALS of males, and from Paratus Simon, 1898 by the flat carapace, by the shorter and less numerous ventral spine pairs on the tibia and metatarsus I and II, and by the absence of dorsal guanine spots on the abdomen. Andromma differs from Liocraninae in general by the absence of rows of large and erectile bristles with special basal sockets in the ventral scopulae of tibia, metatarsus and tarsus of legs I–III ( Ubick & Platnick 1991: 2).
Description
Translation of original Latin description of Simon (1893: 390)
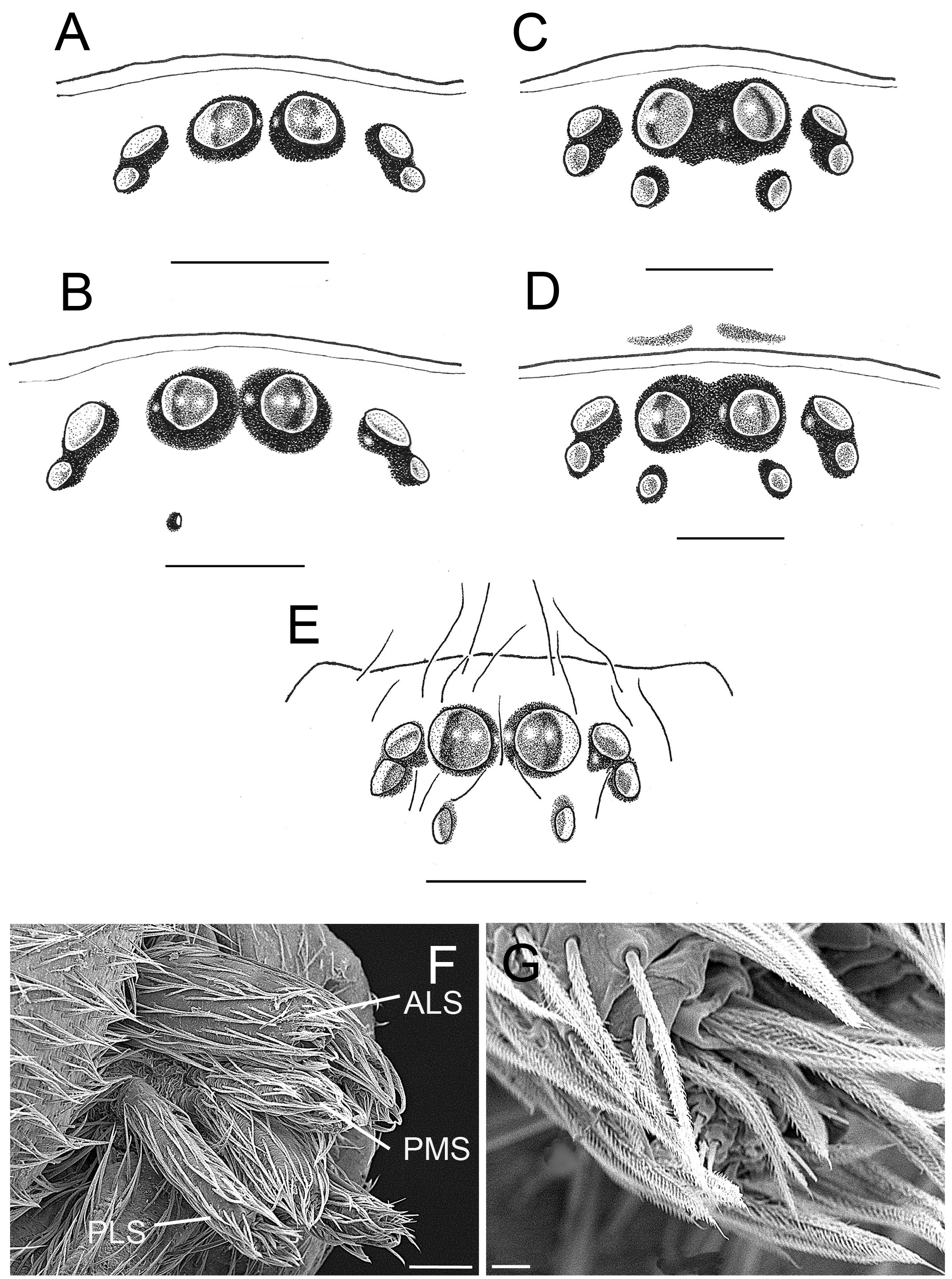
Cephalothorax short and oval, rather convex, without fovea. Four eyes in a straight transverse line, the medians of medium size, the laterals small, minute in males. Clypeus about twice as high as diameter of median eyes. Strong chelicerae with a robust and long fang. Labium much wider than long, transversely semicircular and hardly reaching the middle of the endites. Endites broad and short, not much longer than wide, very blunt, inclined, not curved or transversely grooved. Sternum convex, broadly heartshaped, wider than long. Posterior coxae widely spaced. Leg formula 4123. Legs rather short, tarsi long and hardly shorter than metatarsi, the anterior ones slightly fusiform. Tarsi hardly scopulated, but with dense claw tufts. Tarsal claws shiny and slender, with a series of 5–6 teeth reaching to the apex. Anterior lateral spinnerets closely spaced, longer than posterior medians. Posterior lateral spinnerets also longer than medians, their apical segment at least ⅓ shorter than basal one ( Fig. 2F–G View Fig ).
Additional description data
In the remainder of the text, Simon (1893: 387–389) elaborated on the differences between Andromma and Cybaeodes or Cithaeron O. Pickard-Cambridge, 1872 (erroneously spelled Cythaeron in the text). This allows us to infer the following additional characteristics: legs reddish brown, spineless, tarsal claw tufts consisting of tenent hairs ( Forster 1970: 18; Ubick & Vetter 2005: 69, 71).Anterior lateral spinnerets rather small and cylindrical, separated at the base by their diameter. Posterior median spinnerets of similar shape, as long as anterior laterals or longer. Posterior lateral spinnerets a bit thicker than anterior laterals and slightly more widely separated, resulting in a trapezoidal spinneret area, which is narrower anteriorly, contrary to the situation in gnaphosids, except the Micaria group ( Murphy 2007). Posterior lateral spinnerets consisting of two segments, the apical one conical and at least one third shorter than the basal one. Female palp rather long, tibia much longer than patella, tarsus a bit longer than tibia and slightly fusiform. Male palpal tibia somewhat shorter than patella, with a bifid apophysis: a hook-shaped retrolateral apophysis (RTA) and a thicker ventral apophysis (VTA) with an anterior cavity. Simon also mentions a small tibial prolateral tooth, but we were unable to observe that in any of the species examined. Palpal tarsus longer than patella and tibia together, oval, large, convex, with a tip that largely exceeds the convex, simple bulbus. Abdomen covered in greyish silky hairs.
Remarks on original description
Simon’s original description understandably is solely based on the only species known to him at the time, A. aethiopicum . Unfortunately, this species is one of the least representative for the genus as a whole. The following four corrections are important to note:
1. While some species of Andromma have a sternum that is as wide as long (e.g., female of A. deogratias sp. nov., A. bouvieri and A. ghesquierei sp. nov.), the sternum is longer than wide in most species of Andromma known to date. Andromma aethiopicum , A. anacardium sp. nov. and the female of A. raffrayi are the only species within the genus with a sternum that is clearly wider than long.
2. Most species of Andromma have eight eyes. The only other species with significant eye reduction are A. albinovani sp. nov. and A. deogratias sp. nov. ( Fig. 2A–B View Fig ).
3. Most species of Andromma have at least some leg spines.
4. Andromma does have a thoracic fovea, as our habitus photographs show ( Figs 20A View Fig , 22A View Fig , 30A View Fig ). In very pale specimens, it may be difficult to see ( Fig. 5A View Fig ).
5. In his French description, Simon (1893: 388) refers to the claw tufts as “denses et formés de poils claviformes nombreux”. However, careful observation shows that these hairs are not clavate.
To the above can be added that the AMEs of Andromma are oriented divergently. As a consequence, the retina of the AMEs which, as seen from above, is only visible in the median half of these eyes. ( Fig. 2A–E View Fig ). Such AMEs are also present in Hortipes ( Bosselaers & Jocqué 2000: figs 1–2), Attacobius Mello-Leitão,1925 (Corinnidae) ( Platnick & Baptista 1995: figs 7–8), Piabuna Chamberlin & Ivie, 1933 (Phrurolithidae) ( Bosselaers & Jocqué 2002: fig. 2f; Chamberlin & Ivie 1933: fig. 122), Orthobula Simon, 1897 (Trachelidae) ( Marusik et al. 2013: figs 1–2) and Paratus (Liocranidae) ( Marusik et al. 2008: fig. 4), and others ( Bosselaers & Jocqué 2002: table 1). Furthermore, the male palp has a tibial apophysis split into a ventral (VTA) and retrolateral (RTA) part as well as a globular bulbus without median apophysis ( Figs 4E–F View Fig , 6D–E View Fig ). The epigyne presents conspicuous copulatory openings in most species ( Figs 5C–D View Fig , 9C–D View Fig ) and the vulva generally has more or less globular spermathecae ( Figs 25C– F View Fig , 30E–F View Fig ). The spermathecae have short, tubular fertilisation ducts running in posterior direction. These fertilisation ducts are accompanied by a sickle-shaped sclerite (Sss) oriented laterally or posteriorly ( Fig. 7D View Fig ; Ledoux & Canard 1991: fig. 36). Such sclerites should not be confounded with fertilisation ducts; they are commonly found in dionycha, e.g., in many Gnaphosidae ( Grimm 1985) , Apostenus Westring, 1851 (Liocranidae) ( Grimm 1986), Arabelia Bosselaers, 2009 (Liocranidae) (Bosselaers 2009), Cheiracanthium Wagner, 1887 (Cheiracanthiidae) (Bosselaers 2013), Cteniogaster Bosselaers & Jocqué, 2013 (Liocranidae) ( Bosselaers & Jocqué 2013), Heser Tuneva, 2004 (Gnaphosidae) (Bosselaers 2010), Hortipes Bosselaers & Ledoux, 1998 (Corinnidae) ( Bosselaers & Jocqué 2000), Metatrachelas Bosselaers & Bosmans, 2010 (Trachelidae) ( Bosselaers & Bosmans 2010), Paratrachelas Kovblyuk & Nadolny, 2009 (Trachelidae) ( Bosselaers et al. 2009), Rhaeboctesis Simon, 1897 (Liocranidae) ( Bosselaers & Jocqué 2002), and Trachelas L. Koch, 1872 (Trachelidae) ( Bosselaers et al. 2009). However, these sclerites are never explicitly mentioned in the literature. Engelhardt (1910: 73, fig. 25) and Osterloh (1922: 386, fig. 36) mention sclerotised beams in the vulvae of Platnickina tincta (Walckenaer, 1802) and Allagelena gracilens (C.L. Koch, 1841) respectively, but these structures do not correspond to the sickle-shaped sclerites mentioned here.
Bosselaers J. & Jocque R. 2000. Hortipes, a huge genus of tiny Afrotropical spiders (Araneae, Liocranidae). Bulletin of the American Museum of Natural History 256: 1 - 108. https: // doi. org / bb 8 rs 5
Bosselaers J. & Jocque R. 2002. Studies in Corinnidae: cladistic analysis of 38 corinnid and liocranid genera, and transfer of Phrurolithinae. Zoologica Scripta 31: 241 - 270. https: // doi. org / 10.1046 / j. 1463 - 6409.2002.00080. x
Bosselaers J., Urones C., Barrientos J. A. & Alberdi J. M. 2009. On the Mediterranean species of Trachelinae (Araneae, Corinnidae) with a revision of Trachelas L. Koch 1872 on the Iberian Peninsula. Journal of Arachnology 37: 15 - 38. https: // doi. org / 10.1636 / A 08 - 33.1
Bosselaers J. & Bosmans R. 2010. Studies in Corinnidae (Araneae): a new Paratrachelas Kovblyuk & Nadolny fromAlgeria, as well as the description of a new genus of Old World Trachelinae. Zootaxa 2612 (1): 41 - 56. https: // doi. org / 10.11646 / zootaxa. 2612.1.3
Bosselaers J. & Jocque R. 2013. Studies in Liocranidae (Araneae): a new afrotropical genus featuring a synapomorphy for the Cybaeodinae. European Journal of Taxonomy 40: 1 - 49. https: // doi. org / 10.5852 / ejt. 2013.40
Chamberlin R. V. & Ivie W. 1933. Spiders of the Raft River Mountains of Utah. Bulletin of the University of Utah 23: 1 - 79.
Engelhardt V. von. 1910. Beitrage zur Kenntnis der weiblichen Copulationsorgane einiger Spinnen. Zeitschrift fur Wissenschaftliche Zoologie 96: 32 - 117.
Forster R. 1970. The Spiders of New Zealand. Vol. 3: Desidae, Dictynidae, Hahniidae, Amaurobiodidae, Nicodamidae. Otago Museum Bulletin, Otago Museum, Otago.
Grimm U. 1985. Die Gnaphosidae Mitteleuropas (Arachnida, Araneae). Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg (NF) 26: 1 - 318.
Grimm U. 1986. Die Clubionidae Mitteleuropas: Corinninae und Liocraninae (Arachnida, Araneae). Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg (NF) 27: 1 - 91.
Ledoux J. - C. & Canard A. 1991. Initiation a l'etude systematique des araignees. 2 e edition. J. - C. Ledoux, Aramon, France.
Marusik Y. M., Zheng G. & Li S. Q. 2008. A review of the genus Paratus Simon (Araneae, Dionycha). Zootaxa 1965 (1): 50 - 60. https: // doi. org / 10.11646 / zootaxa. 1965.1.2
Marusik Y. M., Ozkutuk R. S. & Kunt K. B. 2013. On the identity and distribution of the poorly known spider Orthobula charitonovi (Mikhailov, 1986) (Aranei: Corinnidae). Arthropoda Selecta 22: 157 - 162. https: // doi. org / 10.15298 / arthsel. 22.2.05
Murphy J. 2007. Gnaphosid Genera of the World. British Arachnological Society St Neots, Cambridgeshire.
Osterloh A. 1922. Beitrage zur Kenntnis des Kopulationsapparates einiger Spinnen. Zeitschrift fur Wissenschaftliche Zoologie 119: 326 - 421.
Platnick N. I. & Baptista R. L. C. 1995. On the spider genus Attacobius (Araneae, Dionycha). American Museum Novitates 3120: 1 - 9.
Ramirez M. J. 2014. The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae). Bulletin of the American Museum of Natural History 390: 1 - 374. https: // doi. org / 10.1206 / 821.1
Simon E. 1893. Histoire naturelle des araignees. Deuxieme edition, Tome premier: 257 - 488. Roret, Paris. https: // doi. org / 10.5962 / bhl. title. 51973
Ubick D. & Platnick N. I. 1991. On Hesperocranum, a new spider genus from western North America (Araneae, Liocranidae). American Museum Novitates 3019: 1 - 12.
Fig. 2. Andromma Simon, 1893. A–E. Eye patterns, dorsal view. F. Spinnerets, ventral view. G. ALS, ventral view. A–B. Andromma deogratias sp. nov.A. ♂, paratype (BE_RMCA_ARA.Ara 246048). B. ♀, paratype (BE_RMCA_ARA.Ara 246054). C–D. Andromma divinagraciae sp. nov. C. ♂, holotype (BE_ RMCA_ARA.Ara 246051). D. ♀, paratype (BE_RMCA_ARA.Ara 246055). E. Andromma ghesquierei sp. nov., ♀, paratype (BE_RMCA_ARA.Ara 84109). F–G. Andromma divinagraciae sp. nov., ♂, paratype (BE_RMCA_ARA.Ara 246056). Scale bars: A–E= 0.2 mm; F =0.1 mm; G = 0.01 mm.
Fig. 20. Andromma didrepanum sp. nov., ♂, holotype (BE_RMCA_ARA.Ara 216034). A. Habitus, dorsal view. B. Same, ventral view. C–D. Left palp, retrolateral view. E–F. Same, ventral view. Scale bars: A–B= 1 mm; C–F =0.2 mm.
Fig. 22. Andromma divinagraciae sp. nov., ♀, paratype (BE_RMCA_ARA.Ara 246055). A. Habitus, dorsal view. B. Same, ventral view. C–D. Epigyne, ventral view. E–F. Same cleared, dorsal view. Scale bars: A–B =2 mm; C–D=0.2 mm; E–F =0.1 mm.
Fig. 30. Andromma ophiophagum sp. nov., ♀, holotype (RMCA_ARA_Ara 144686). A. Habitus, dorsal view. B. Cephalothorax, ventral view. C–D. Epigyne, ventral view. E–F. Same cleared, dorsal view. Scale bars: A=2 mm; B= 1 mm; C–F = 0.2 mm.
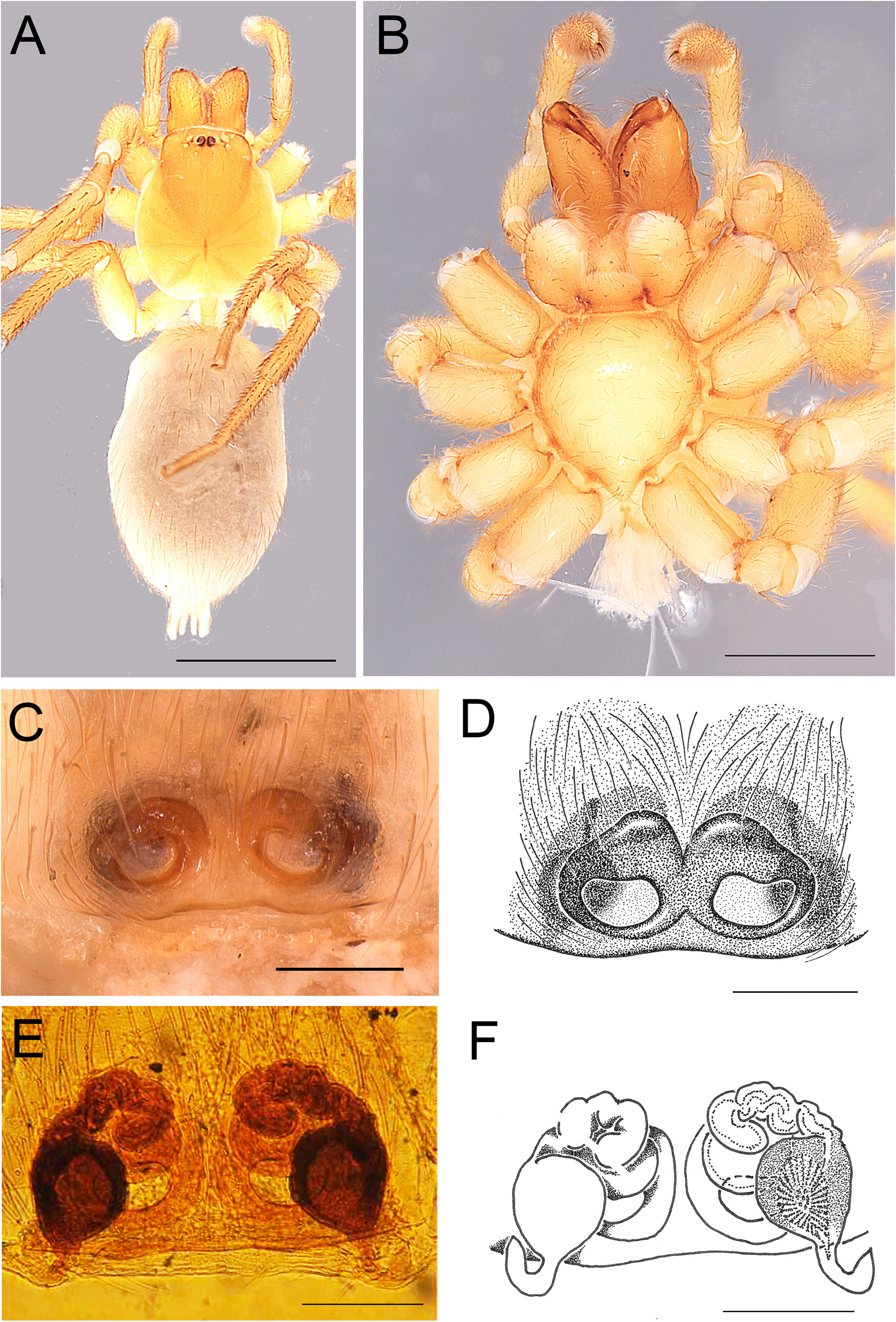
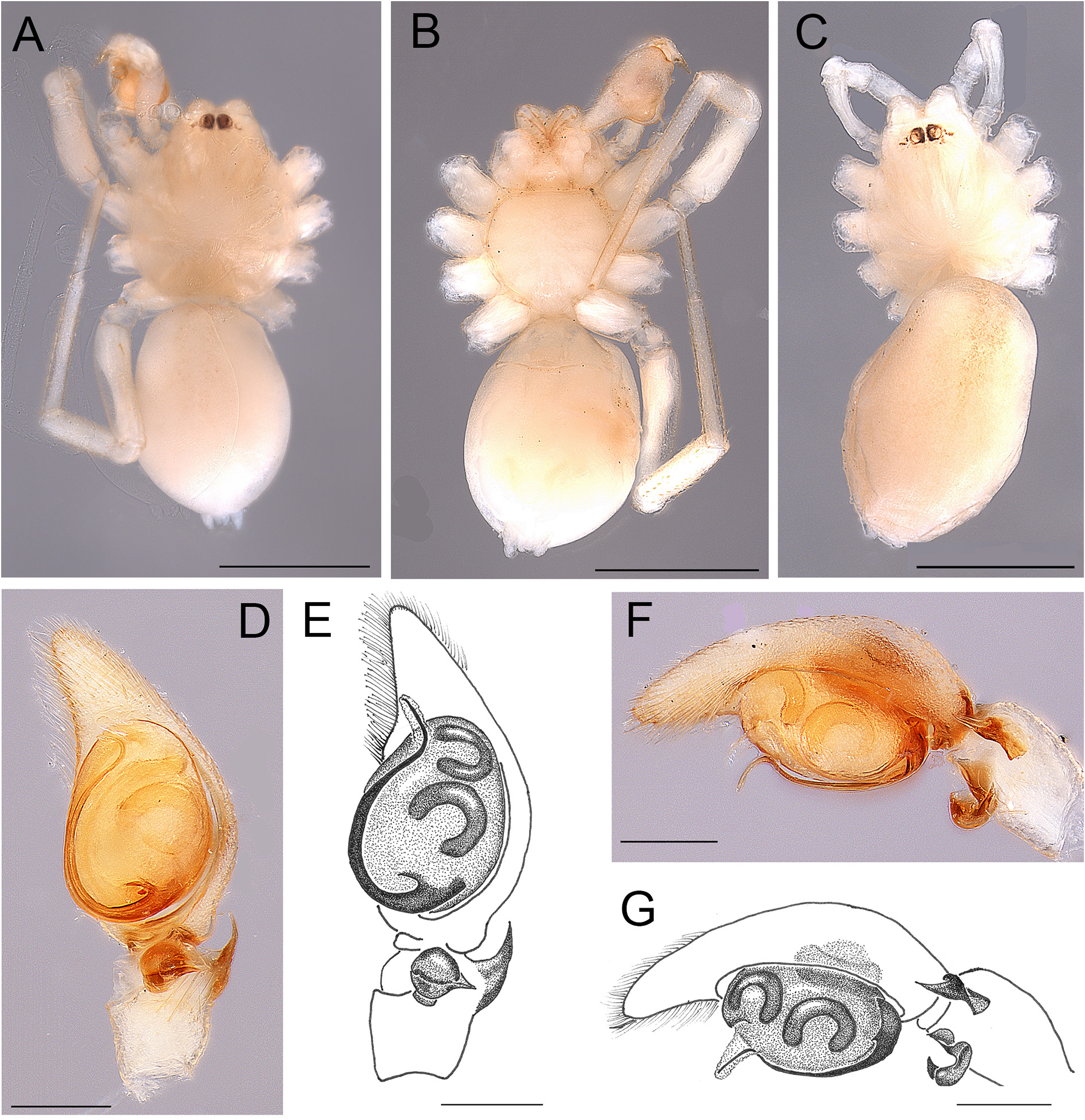
Fig. 5. Andromma aethiopicum Simon, 1893, ♀, paralectotype (MNHN AR1678). A. Habitus, dorsal view. B. Same, ventral view. C–D. Epigyne, ventral view. Abbreviation: CO=copulatory opening. Scale bars: A–B =1 mm; C–D=0.2 mm.
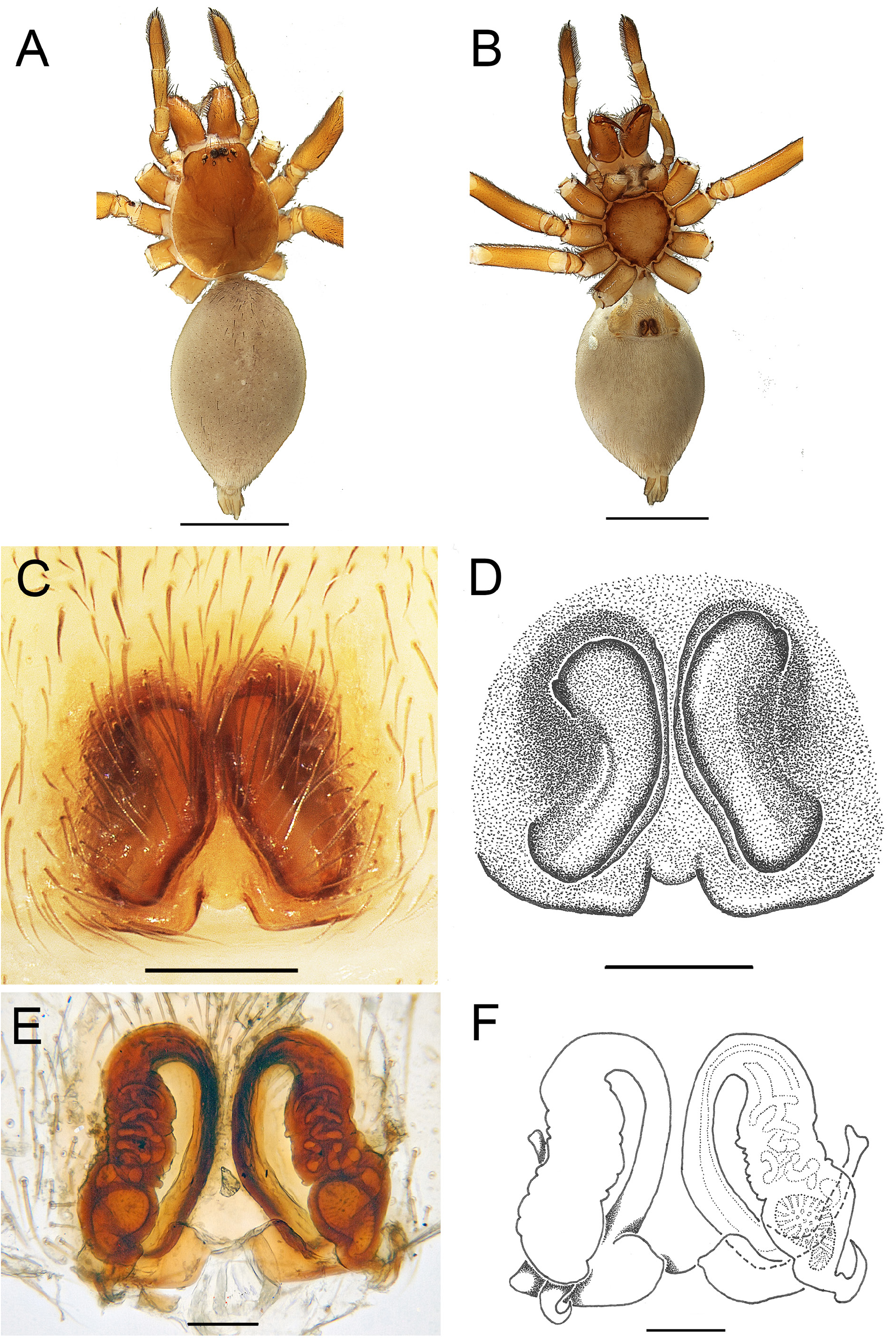
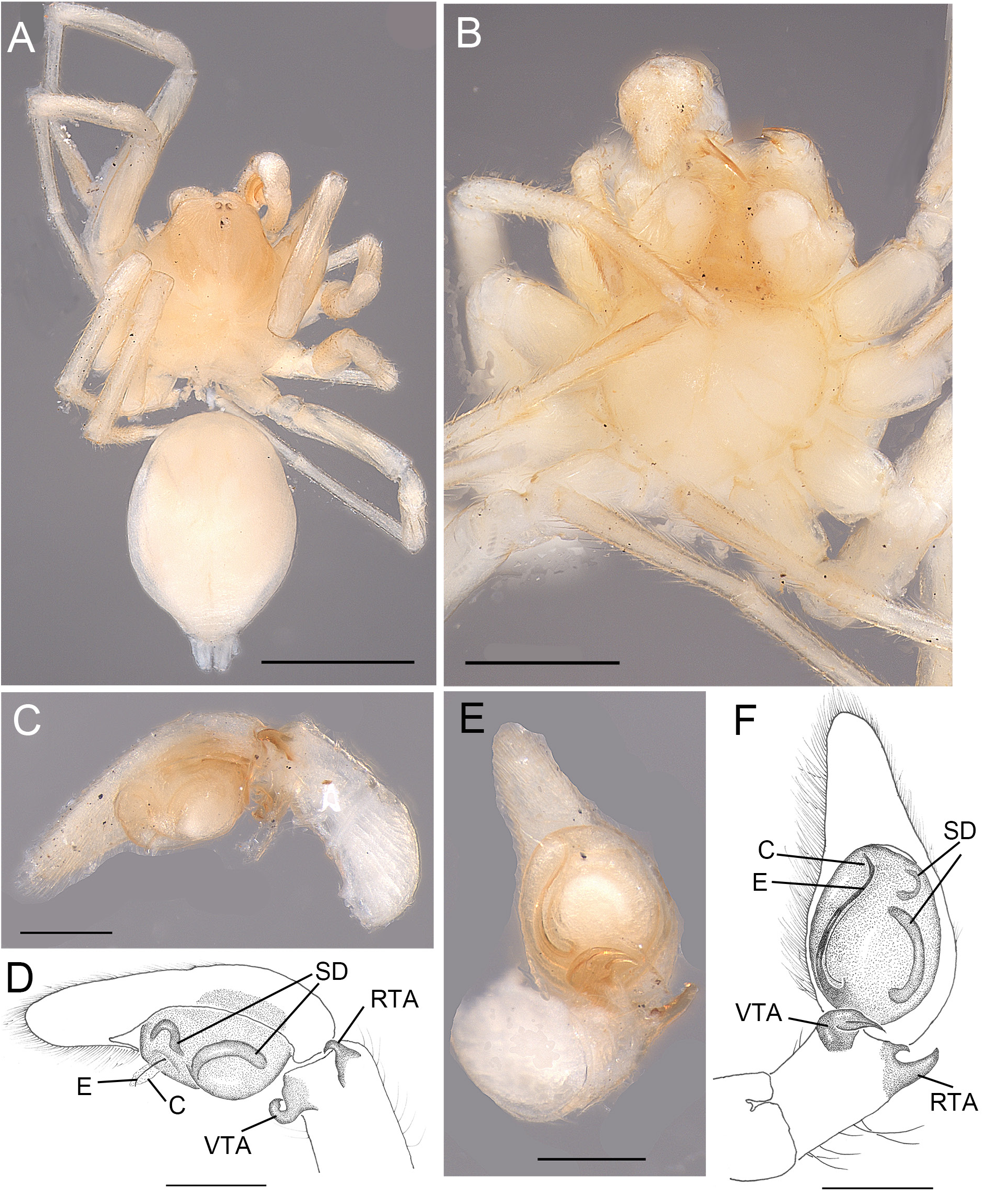
Fig. 4. Andromma aethiopicum Simon, 1893, ♂, lectotype (MNHN AR1678). A. Habitus, dorsal view. B. Cephalothorax, ventral view. C–D. ♂, left palp, retrolateral view. E–F. Same, ventral view. Abbreviations: C =conductor; E =embolus; RTA=retrolateral tibial apophysis; SD =sperm duct; VTA=ventral tibial apophysis. Scale bars: A= 1 mm; B=0.5 mm; C–F = 0.2 mm.
Fig. 6. Andromma albinovani sp. nov. A–B, D–G. ♂, holotype (BE_RMCA_ARA.Ara 177497). C. ♀, paratype (BE_RMCA_ARA.Ara 177497). A, C. Habitus, dorsal view. B. Same, ventral view. D–E. ♂, left palp, ventral view. F–G. Same, retrolateral view. Scale bars: A–C= 1 mm; D–G =0.2 mm.
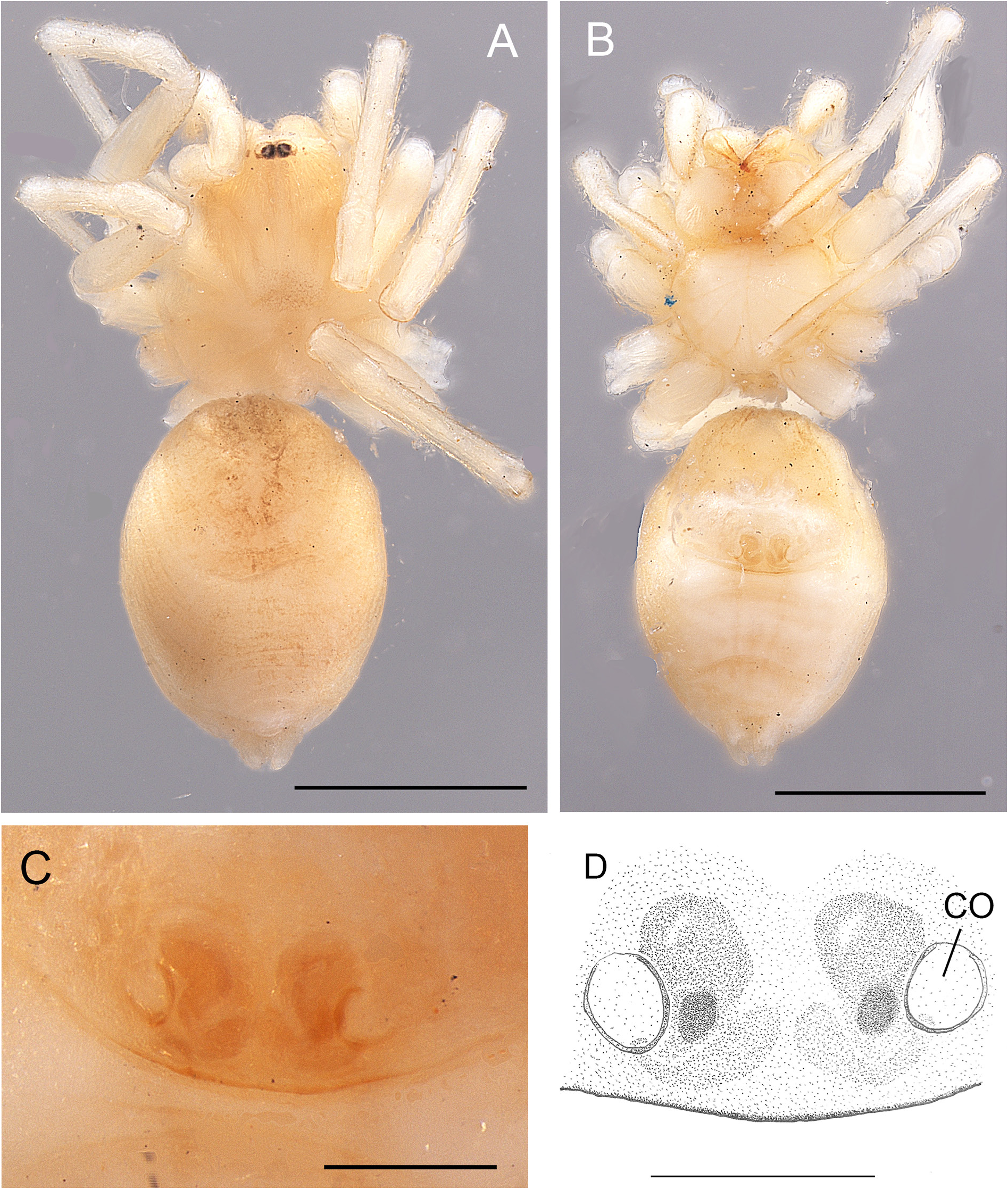
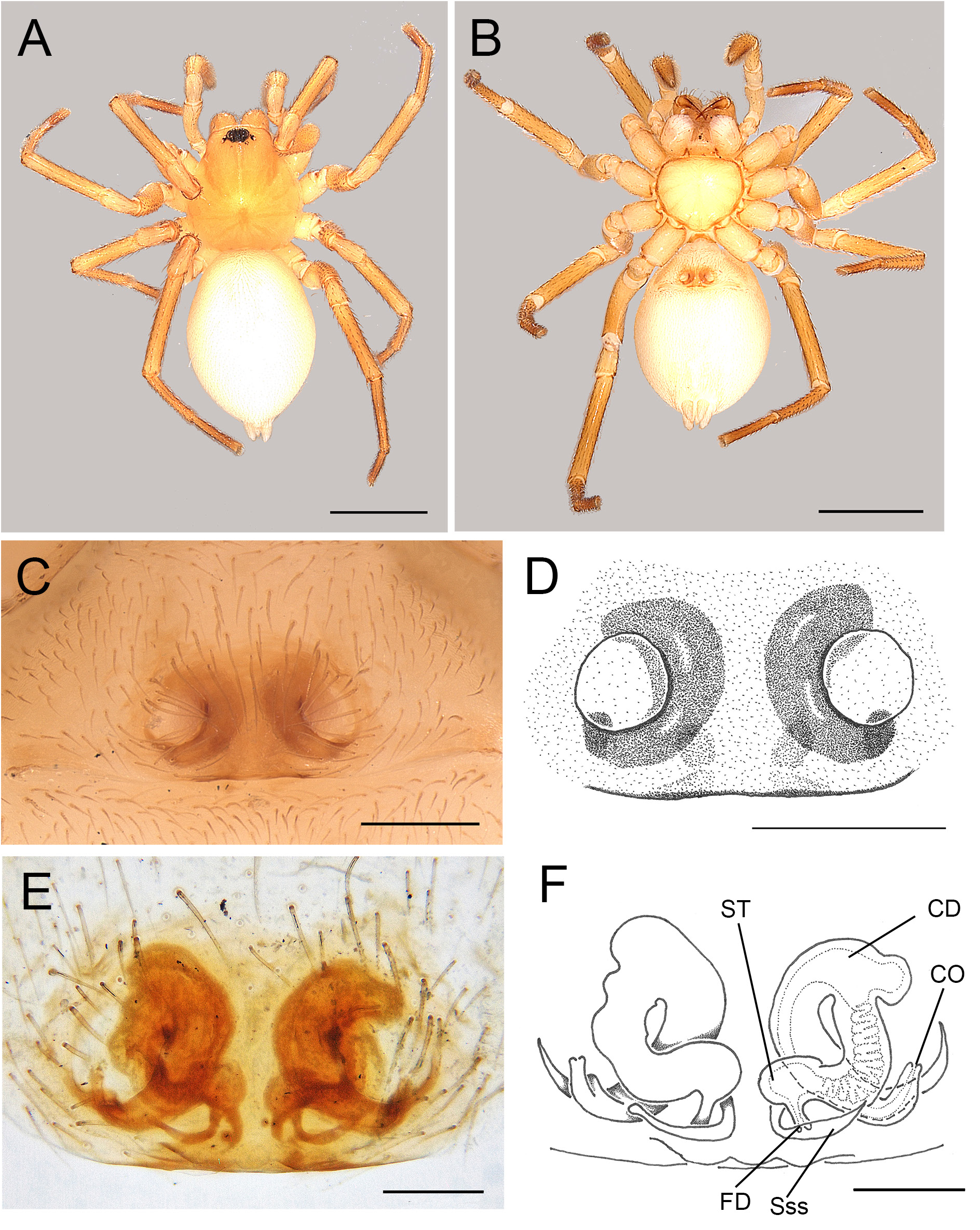
Fig. 9. Andromma anacardium sp. nov., ♀, holotype (BE_RMCA_ARA.Ara 90890). A. Habitus, dorsal view. B. Same, ventral view. C–D. Epigyne, ventral view. E–F. Epigyne, cleared, dorsal view. Abbreviations: CD=copulatory duct; CO= copulatory opening; FD = fertilisation duct; Sss =sickle shaped sclerites; ST =spermatheca. Scale bars: A–B =1 mm; C–D=0.2 mm; E–F =0.1 mm.
Fig. 25. Andromma ghesquierei sp. nov., ♀, paratypes. A–B. BE_RMCA_ARA.Ara 84109. C, E. BE_RMCA_ARA.Ara 177091. D, F. BE_RMCA_ARA.Ara 22878. A. Habitus, dorsal view. B. Cephalothorax, ventral view. C–D. Epigyne, ventral view. E–F. Same cleared, dorsal view. Abbreviations:CD =copulatory duct; CO=copulatory opening; FD =fertilisation duct; ST= spermatheca. Scale bars: A–B =1 mm; C–E =0.2 mm.
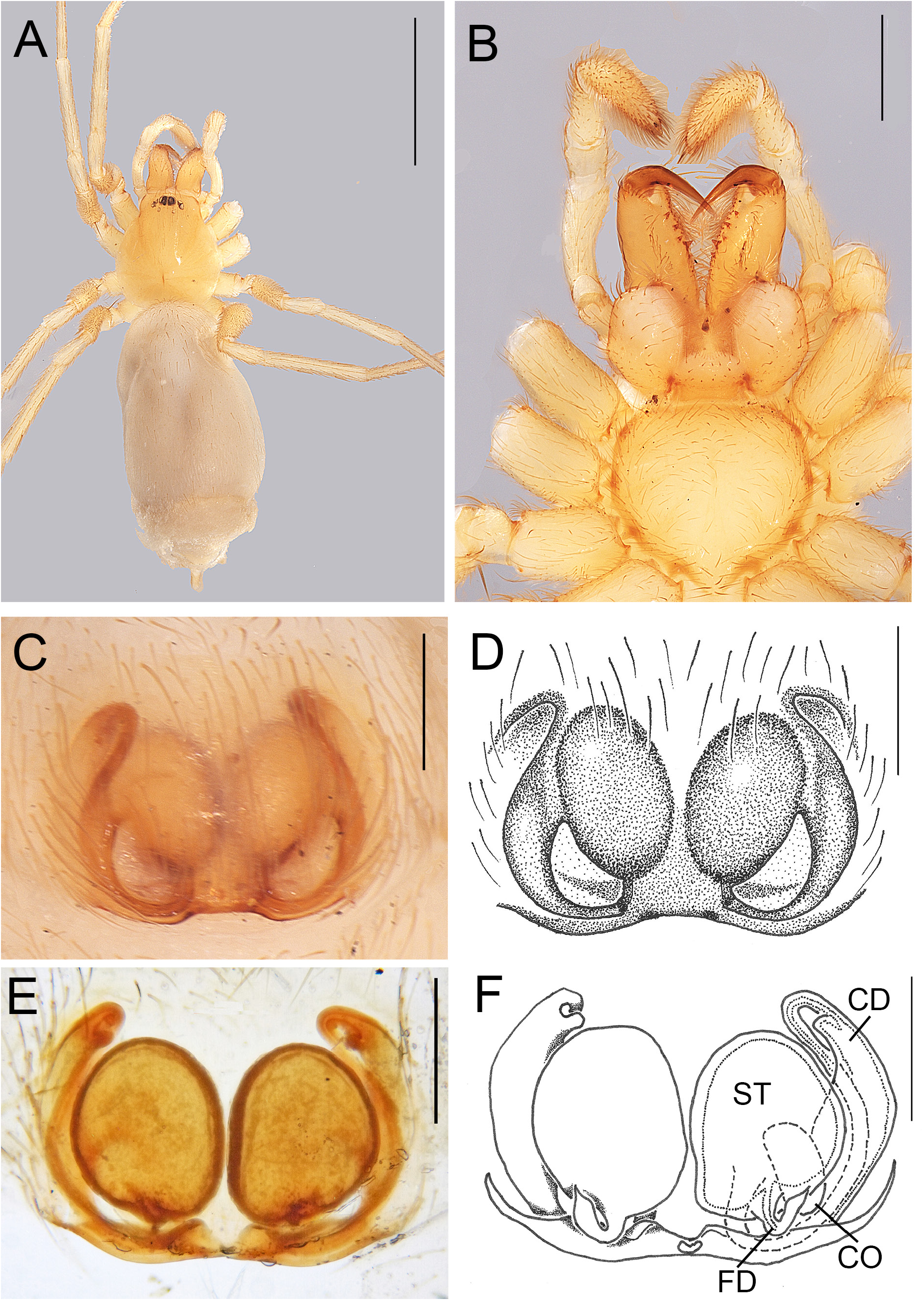
Fig. 7. Andromma albinovani sp. nov., ♀, paratype (BE_RMCA_ARA.Ara 177497). A–B. Epigyne, ventral view. C–D. Same, cleared, dorsal view. Abbreviations: CD = copulation duct; CO = copulatory openings; FD = fertilisation duct; Sss = sickle-shaped sclerite; ST = spermathecae. Scale bars: A–B =0.2 mm; C–D= 0.1 mm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Andromma Simon, 1893
| Bosselaers, Jan & Jocqué, Rudy 2022 |
Andromma aethiopicum
| Simon 1893 |