Blastobasis repartella ( Dietz 1910 )
|
publication ID |
https://doi.org/10.5281/zenodo.198936 |
|
DOI |
https://doi.org/10.5281/zenodo.6199106 |
|
persistent identifier |
https://treatment.plazi.org/id/9F1BCC50-FFE8-FFA2-FF39-AB366F4CFE93 |
|
treatment provided by |
Plazi |
|
scientific name |
Blastobasis repartella ( Dietz 1910 ) |
| status |
|
Blastobasis repartella ( Dietz 1910)
Adult diagnosis. Blastobasis repartella is similar to B. graminea in wing pattern, but differs from the latter species by having males with a unidentate medial process on the ventroposterior margin of the gnathos, sparse tergal setae, an absence of the dorsal strut of tegumen, an anellus of the phallus that is broadly round apically, and females with a corpus bursae lacking a small lobe on the posterior end, and by having a smaller signum.
Adult redescription. ( Figs. 1–6): Head: Pale grayish orange except, outer surface of labial palpus as above or grayish orange intermixed with few brown scales.
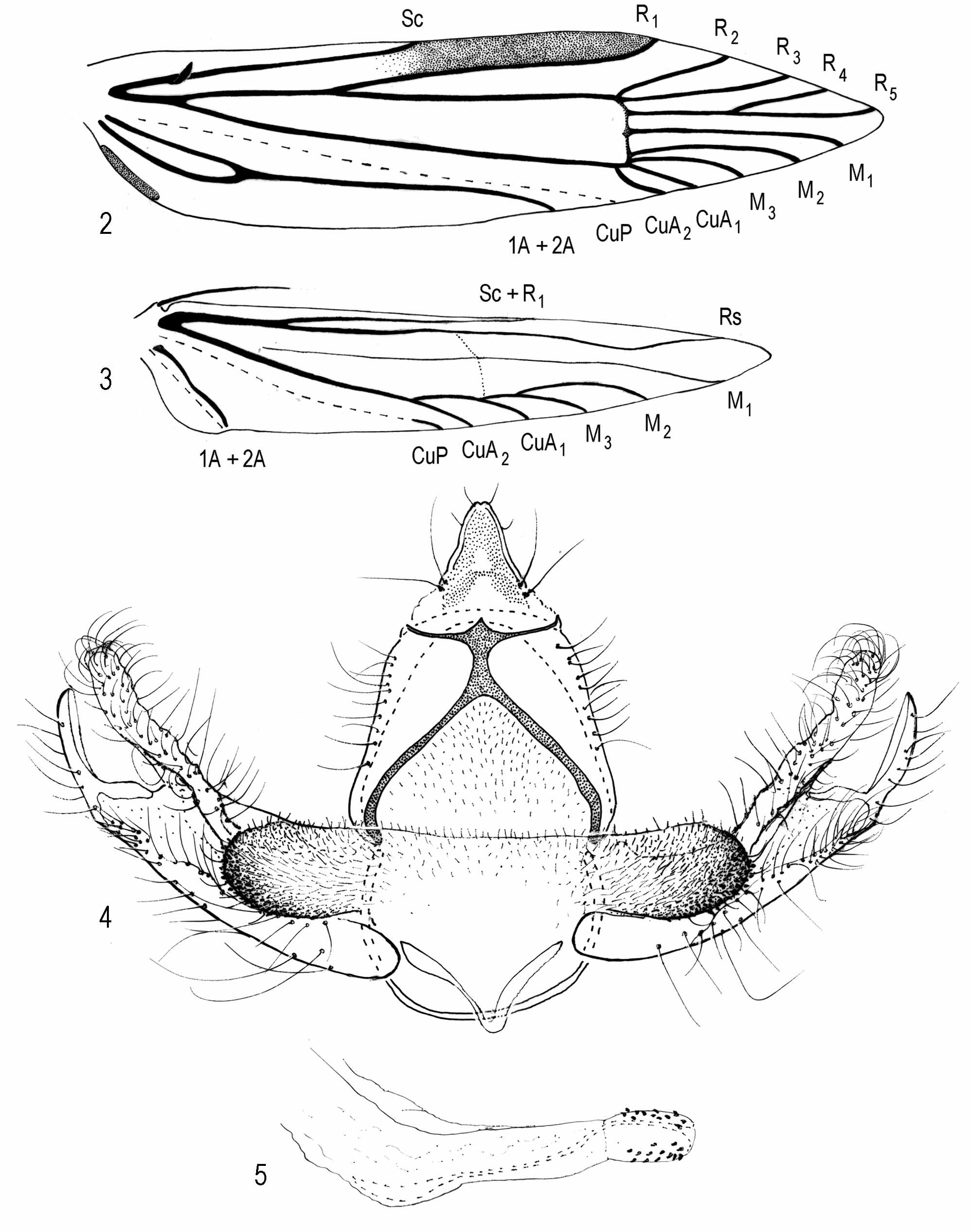
Thorax: Scales of tegula and mesonotum pale grayish orange or pale grayish orange intermixed with a few brown scales. Legs pale grayish orange. Forewing ( Fig. 1), length 3, 5.5−7.2 mm (n = 10); Ƥ, 7.0−9.1 mm (n = 15). Grayish orange to pale grayish orange intermixed with few brown scales, veins overlaid with few brown scales, appearing faintly streaked; discal cell with 2 or 3 dark-brown spots, one present or absent near midcell and two near distal end of cell; undersurface brown. Venation ( Fig. 2 View FIGURES 2 − 5 ), cubitus appearing 4-branched, M3 − CuA1 connate. Hindwing translucent, pale gray. Venation ( Fig. 3 View FIGURES 2 − 5 ); cubitus 4-branched, closer to margin than to chorda, each vein appearing similar in length; frenulum with one acanthus in male, three acanthae in female.
Abdomen ( Figs. 4–6 View FIGURES 2 − 5 ): Anterior 3/4 of terga 1−7 of male and 1−6 of female with 5−6 irregular, transverse rows of spiniform setae, posterior pale yellowish brown; undersurface pale yellowish brown. Male Genitalia ( Figs. 4–5 View FIGURES 2 − 5 ): Uncus sparsely setose, wide basally, gradually narrowing apically to a slightly indented apex; ventroposterior margin of gnathos unidentate medially; dorsal strut of tegumen absent; tergal setae sparse; diaphragma microtrichiate throughout, forming a pleat above phallus, extending laterally from middle to and above ventrolateral margins of proximal flange, forming an elliptical-shaped, densely microtrichiate process; microtrichiate process intermixed with spinules on ventrolateral margin; juxta bandlike; vinculum semicircular; phallus with internal support, acutely curved near apical 1/3, slightly curved near base; anellus broadened apically, bearing several conical setae. Female Genitalia ( Fig. 6): Ovipositor telescopic, with four membranous subdivisions posterior to 8th segment; ostial opening within slightly microtrichiate membrane posterior to seventh segment; ductus bursae narrow, gradually widening anteriorly, about as long as ovipositor, anterior 1/3 lined with two rows of imbricate platelike processes; inception for ductus seminalis near posterior end of seventh segment; corpus bursae elliptical, with a small spinelike signum near midlength.
Larval diagnosis.− Blastobasis repartella is similar to B. graminea in feeding habits, size, and chaetotaxy but differs from the latter species by having darker pinacula, an elliptical diffuse spot on the prothoracic shield within area between SD1, XD2, SD2, and D2, and SD1 hairlike on A8.
Larva: description. ( Figs. 7–21 View FIGURES 7 − 12 View FIGURES 13 − 16 View FIGURES 17 − 21 ): Length 8.3 – 12.0 mm (n = 59 preserved larvae). Body pale gray to white, smooth; head capsule, prothoracic shield, thoracic legs, and anal shield yellowish brown; pinacula small, brown; circular spiracles on T1 and A10 with equal diameters, slightly larger than spiracles on A1–A8.
Head ( Figs. 7 – 12 View FIGURES 7 − 12 , 20 View FIGURES 17 − 21 ): Hypognathous; epicranial suture extending to epicranial notch, beyond apex of frons, dividing head into two hemispheres; adfrontal sclerites delimiting frons; frons widened basally, gradually narrowing to apex ( Fig. 7 View FIGURES 7 − 12 ); AF2s slightly above or at same level of apex of frons; AF2 and AF1 about same lengths, slightly shorter than F1; distance between AF1 and AF2 about 3x distance between AF1 and F1; distance between F1 and AF1 about 3x distance between F1 and C2; C1 slightly longer than C2; P1 and A1 longest cranial setae; P1 about 4x length of P2, both below AF2, P2 slightly dorsal to P1; P 2 in straight diagonal line with MD1 and MD2 ( Fig. 8 View FIGURES 7 − 12 ); L1 about length of and posteroventral to A3; A3 above stemmata 1–2; A1 about 1/3 longer than A2, in near straight line with C2 setae, both perpendicular to median longitudinal axis; of six stemmata, five stemmata (1–4 and 6) in a semicircle, stemmata 3–4 approximate; stemma 5 beneath antenna ( Fig. 9 View FIGURES 7 − 12 ); S2 beneath stemma 1, about 3x longer than S1 and S3 ( Fig. 10 View FIGURES 7 − 12 ); S3 on lower aspect of gena, and posterolateral of SS3; S2 slightly beneath and between stemmata 2–3; SS1 beneath antenna; SS2 between stemmata 5–6; SS3 posterior to and closer to midline than SS2; labrum with six pairs of setae, two subequal pairs medially; two subequal pairs along proximolateral margin, and two pairs of equal lengths along margin lateral to notch; mandible with two large dentitions flanked by two smaller dentitions, and bearing two subequal setae dorsally ( Fig. 20 View FIGURES 17 − 21 ); sensilla of maxillary palpus as in Fig. 11 View FIGURES 7 − 12 sensilla of antenna as in Fig. 12 View FIGURES 7 − 12 ; posterior part of labium with two divergent setae slightly anterior to submental pit; spinneret cylindrical and elongate.
Thorax: T1( Figs. 13 View FIGURES 13 − 16 , 17–18 View FIGURES 17 − 21 ): Prothoracic shield dark brown on posterior 1/3 and along lateral margins, paler to anterior margin, a pale diffuse, elliptical spot on each side of shield within area between SD1, XD2, SD2, and D2 ( Figs. 17–18 View FIGURES 17 − 21 ); shield with six pairs of setae; SD1, XD2, and XD1 along anterior margin in a straight line; XD2 and XD1 about equal in lengths, about shorter than SD1; SD2 shortest seta, slightly closer to SD1 than to XD2; D2 longest seta, slightly posterior to SD2, posterior and between XD2 and XD1, forming a large triangle; D1 slightly longer than SD2, posterior to D2 and closer to median longitudinal axis than XD1; L-group trisetose, in a straight line, with L2 about length of L1 (both pointing anteriorly) and L3 (pointing posteriorly) about 1/3 length of L1; MV2 anterior to SV-group; SV1 about 2x length of SV2, both setae on same or separate pinacula; coxae and V1 s approximate, about closer than on T2–T3 (not shown); pretarsus with two setae above claw and two setae beneath claw ( Fig. 13 View FIGURES 13 − 16 ). T2–T3 ( Figs. 17–18 View FIGURES 17 − 21 ): D2 about 3x length of D1, each on same pinaculum; MD1 slightly above D-group pinaculum; MSD1 and MSD2 on same pinaculum, slightly below D-Group pinaculum; SD2 about 3x length of SD1, both on same pinaculum ventral and slightly anterior to D-group pinaculum; L1 about 3x length of L2, both setae on same pinaculum anteroventral to SD-group pinaculum; L3 equal in length to L2, dorsoposterior to L1–L2 pinaculum, and anterior to or in straight line with SV1; SV1 about same length as L1; MV1 dorsoanterior to SV1.
Abdomen: A1–A2 ( Figs. 14–16 View FIGURES 13 − 16 , 19, 21 View FIGURES 17 − 21 ): D2 posteroventral to and about 2x length of D1; MD1 anteroventral to D2; SD-setae on same pinaculum above spiracle; SD1 about equal in length to D2, SD2 minute ( Figs. 19, 21 View FIGURES 17 − 21 , 14 View FIGURES 13 − 16 (enlarged); L2 about length of SD1, about 2x longer than L1, both setae on same pinaculum beneath spiracle; L3 about same length as L2, in near vertical line with D2; MV1 dorsoanterior to L3; SV-group trisetose, transverse to vertical line; V1 -group as in T2–T3, except on larger pinacula (not shown); A3–A6 ( Figs. 15 View FIGURES 13 − 16 , 21 View FIGURES 17 − 21 ): as above except, SV-group in a triangular pattern, each seta on a separate pinaculum; planta of prolegs bearing uniserial crochets, uniordinal in a circle (as in Fig. 15 View FIGURES 13 − 16 ) or biordinal mesally, gradually shortening laterally ( Fig. 15 View FIGURES 13 − 16 ); A7: as above except, SV-group bisetose, with SV1 about 2x length of SV2; A8 as above except, SD1 hairlike, SD2 absent, SV-group unisetose, and V1 s slightly closer (not shown); A9 with D2 and D1 approximate, with the latter more ventral; D2, SD1, L2, and L 1 in a straight line, anterior to L3 and SV1; MV1 not observed; V1 s slightly farther apart than V1 s on other segments; A10 with shield bearing four pairs of setae ( Figs. 16 View FIGURES 13 − 16 , 21 View FIGURES 17 − 21 ); SD1 and SD2 about equal in lengths, slightly longer than D1 and D2; D2 slightly decumbent; D1s in straight line with SD2s; prolegs with crochets uniordinal (as in Fig. 16 View FIGURES 13 − 16 ) or biordinal.
Pupa. ( Figs. 22–23 View FIGURES 22 − 23 ): Length 5.5–6.2 mm (n = 6): yellowish brown; antennal sclerite widely curved laterally from vertex, extending caudally, converging beyond junction of maxillary sclerites, extending in parallel slightly before wing margins, apically diverging, exposing apices of metathoracic legs; prothoracic leg sclerites extending before apices of maxillae; mesothoracic leg sclerites surrounding caudolateral margins of prothoracic leg sclerites; prothoracic femoral sclerites about length of maxillae; abdominal spiracles protuberant; scars of prolegs on A5–A6.
Types examined. Lectotype ɗ, Valentinia repartella Dietz, 1910 : Denver Colorado, USNM Genitalia slide 80987; Holotype Ψ, Blastobasis graminea Adamski, 1999 : Palmira, Cauca Valley, Colombia. Primary types and larvae of B. graminea from same location of holotype were examined at the United States National Museum of Natural History, Washington, DC.
Distribution. Blastobasis repartella was known only from the type locality, Denver, Colorado for nearly 100 years until it was rediscovered recently from farm locations in Brookings, Hughes, and Marshall Counties, South Dakota ( Fig. 24 View FIGURES 24 − 29 ), and later from Champaign County, Illinois. A regional survey in the central United States ( Prasifka et al. 2009), reported that B. repartella is now known to infest switchgrass in eight northern states including Illinois, Iowa, Michigan, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin but was not detected in Arkansas, Louisiana, Oklahoma, and Texas. Combined with the original collections by Dietz (1910), distribution in the U.S. appears to cover from an area Colorado east to Ohio and as far north as North Dakota.
Parasitoids. Two specimens of Bassus difficilis Muesebeck ( Hymenoptera : Braconidae ) were reared from pupae of B. repartella in South Dakota.
Biology. Larval feeding on switchgrass by B. repartella is visible as partially emerged leaves on a tiller that desiccates and eventually dies ( Fig. 25 View FIGURES 24 − 29 ). These are symptoms similar to those produced by other stem boring insects in grass crops (e.g., Davis & Pedigo 1991). The larva of B. repartella appears to be a monophagous stem-borer in the proaxis, basal nodes, and internodes of Panicum virgatum ( Fig. 26 View FIGURES 24 − 29 ). The proaxis of switchgrass is a region of several compressed nodes and internodes at the proximal end of a tiller. Adventitious roots and axillary meristems that give rise to the subsequent generation of extravaginal and intravaginal tillers are produced in this region ( Brejda et al. 1989). The proaxis is metabolically active and is presumably a rich source of nutrients for herbivores.
Early instars of B. repartella may be found by mid-autumn. Presumably, larvae are inactive during the coldest months but were found to be active in South Dakota when plants were brought into the greenhouse in early spring and forced into early growth. In the field, late-instars are commonly found in late May actively feeding.
Pupae are found primarily during mid to late June within the plant stem ( Fig. 27 View FIGURES 24 − 29 ). However, no viable pupae have been found in the field in early July in Illinois or by the third week of July in South Dakota.
Flight period. Adults of B. repartella are nocturnal with a peak of activity during the early pre-sunrise hours. In eastern South Dakota, adult activity occurs from mid-July to mid-August, however, individuals are occasionally collected at evening lights during late August. Seasonal peak adult activity related to reproductive behavior was measured by the frequency of arriving males (40– 50 males per night and occasionally exceeding 75 males per night) at cages containing calling females ( Figs. 28–29 View FIGURES 24 − 29 ). The flight period at Savoy, Illinois appears to be 2−3 weeks earlier than in South Dakota.
There is no evidence to suggest the occurrence of a second generation or overlapping cohorts in either South Dakota or Illinois populations. This is consistent with the single per year growth of switchgrass and appears to correlate with geographic variations in growing season differences of switchgrass ( Quinn 1969) indicating that these populations lack sufficient phenological opportunity to support multiple generations of B. repartella .
Oviposition. Eggs are deposited in a concealed area between the stem and desiccated sheath found at the base of the tillers ( Prasifka et al. 2009).
Niche competitors. The only known potential niche competitor of B. repartella is Aethes spartinana (Barnes and McDunnough) ( Lepidoptera : Tortricidae ), which was reared from larvae also collected from stems of switchgrass in South Dakota. Previously, prairie cordgrass ( Spartina pectinata Bosc ex Link. ) was the only recorded host of A. spartinana .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Gelechioidea |
|
Family |
|
|
Genus |