Trinomys yonenagae ( Rocha, 1995 )
|
publication ID |
https://doi.org/ 10.1093/mspecies/sez001 |
|
publication LSID |
lsid:zoobank.org:pub:0E35B506-8135-4235-AF3A-C870790C2F26 |
|
persistent identifier |
https://treatment.plazi.org/id/9F6F87D5-F067-FFD5-FF59-FB63C218137C |
|
treatment provided by |
Felipe |
|
scientific name |
Trinomys yonenagae ( Rocha, 1995 ) |
| status |
|
Trinomys yonenagae ( Rocha, 1995) View in CoL
Yonenaga’s Atlantic Spiny-rat
Proechimys yonenagae Rocha, 1995:541 . Type Locality “Ibiraba, Bahia, Brazil (10°48’S, 42°50’W).”
Trinomys yonenagae: Lara and Patton, 2000:665 View in CoL . First use of current name combination.
T [rinomys]. yonengae Lara and Patton, 2000:668. Incorrect subsequent spelling of Trinomys yonenagae ( Rocha, 1995) View in CoL .
CONTEXT AND CONTENT. Order Rodentia , suborder Hystricomorpha , infraorder Hystricognathi , family Echimyidae , subfamily Eumysopinae , genus Trinomys . The genus Trinomys contains 10 unequivocally valid species according to Pessôa et al. (2015): T. albispinus , T. dimidiatus , T. eliasi , T. gratiosus , T. iheringi , T. mirapitanga , T. moojeni , T. paratus , T. setosus , and T. yonenagae . Trinomys yonenagae was first believed to form a natural clade with T. albispinus due to overlap in geographic distributions, similarities in the counterfolds of their cheek teeth, and the shape of the baculum ( Rocha 1995) but later T. yonenagae was shown to be craniometrically more similar to taxa in the T. iheringi complex ( Pessôa et al. 1998). Lara and Patton (2000), using cytochrome- b gene data, proposed Trinomys genera to contain 3 clades. In clade 2, T. yonenagae is sister to the T. setosus group, which is sister to T. iheringi paratus and T. i. eliasi . IackXimenes (2005) also supported the position of T. yonenagae as the sister species of T. setosus and as a group closely related to T. eliasi based on morphological traits. More recently, a Rodentia phylogeny inferred from a molecular super-matrix supported the T. setosus – T. yonenagae clade ( Fabre et al. 2012, 2016; Upham and Patterson 2012).
NOMENCLATURAL NOTES. Trinomys yonenagae is locally known as “rabo-de-facho” (torch-tail), which refers to its long, penciled tail ( Fig. 1 View Fig ). In English, its common names are Yonenaga’s Atlantic spiny-rat and torch-tail rat. The species name was chosen in honor of Dr. Yatiyo Yonenaga-Yassuda, a researcher at the Cytogenetic Laboratory at Instituto de Biociências of Universidade de São Paulo ( Rocha 1995). When first described, Trinomys was 1 of 2 subgenera included in the Proechimys genus— Proechimys and Trinomys ( Thomas 1921) , distinguished by the number of laminae in the cheek teeth (4 and 3, respectively), and by the wear pattern of the crowns of the cheek teeth ( Moojen 1948). Later, analysis of mitochondrial cytochrome- b sequences elevated Trinomys to generic status ( Lara et al. 1996). Even before designation as monophyletic groups, Proechmys and Trinomys were known to have distinct and disjunct ranges of occurrences, Trinomys being much more restricted and limited to the eastern states of Brazil, mostly within the Atlantic rainforest domain ( Moojen 1948; Pessôa et al. 1998), except for T. yonenagae and T. albispinus , which inhabit the semiarid caatinga of Brazil.
Trinomys yonenagae View in CoL is clearly closer to T. setosus View in CoL , as suggested by the analysis of mitochondrial genes performed by both Galewski et al. (2005) and Tavares et al. (2015) that established the clade T. gratiosus View in CoL + T. dimidiatus View in CoL + T. iheringi View in CoL and T. eliasi View in CoL + T. paratus View in CoL + T. yonenagae View in CoL + T. setosus View in CoL . Tavares et al. (2015) added T. moojeni View in CoL to the first clade. T. setosus View in CoL is sister to T. paratus View in CoL + T. eliasi View in CoL according to Upham and Patterson (2012), and this clade is closely related to T. yonenagae View in CoL based on 1 mitochondrial (12s rRNA) and 3 nuclear genes (vWF, GHR, and RAG1). In the most recent phylogeny published ( Fabre et al. 2016), the T. yonenagae View in CoL + T. setosus View in CoL clade forms a sister group to T. paratus View in CoL , which in turn are a sister group to T. iheringi View in CoL + T. dimidiatus View in CoL . In both phylogenies of ( Fabre et al. 2014, 2016), T. yonenagae View in CoL and T. setosus View in CoL are sister species. At a higher phylogenetic level, molecular analysis of the Echimyidae View in CoL using the Willebrand Factor nuclear gene (vWF) suggests that Trinomys View in CoL forms a monophyletic group with Clyomys View in CoL + Euryzygomatomys View in CoL ( Galewski et al. 2005; Leite and Patton 2002), this is also supported by the molecular super-matrix of Fabre et al. (2012, 2016) for Rodentia View in CoL .
Dental morphology, important in understanding the relationships in the family Echimyidae View in CoL , supports the hypothesis that the 10 species in the genus Trinomys View in CoL form a monophyletic group ( Carvalho and Salles 2004). The shape of the pterygoid fossa also supports the monophyly of Trinomys ( Verzi et al. 2014) View in CoL . Results of a combined parsimony analysis using morphological and molecular data supported T. yonenagae View in CoL diverging from other echimyids in the late Miocene ( Verzi et al. 2015). All Trinomys species are terrestrial and most are forest dwelling, but based on what is currently known, only T. yonenagae View in CoL lives in subterranean burrows ( Rocha et al. 2007).
DIAGNOSIS
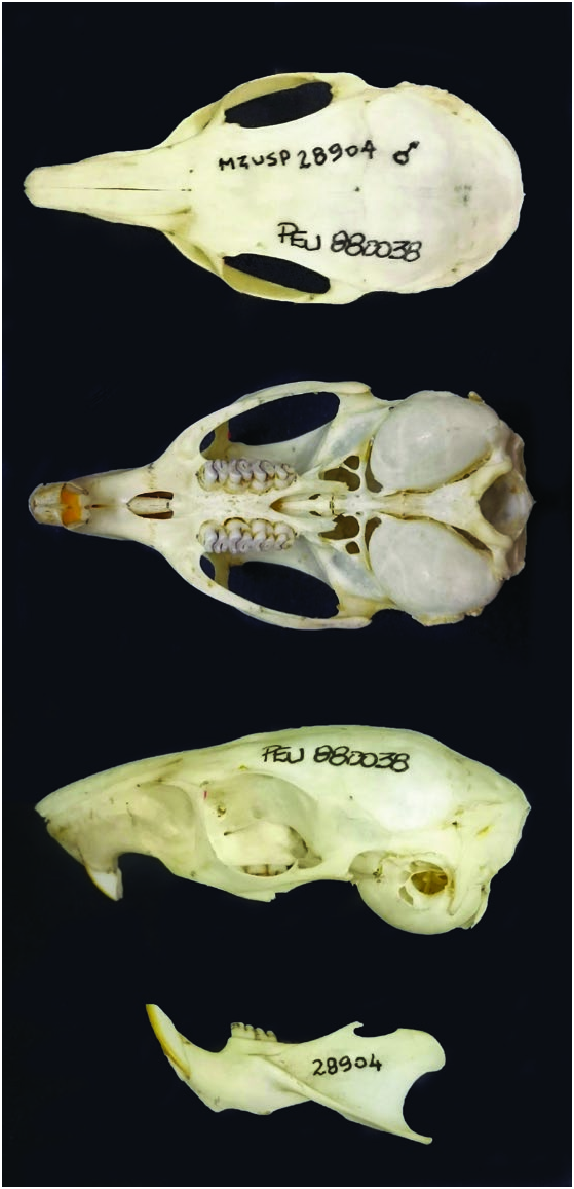
Trinomys yonenagae ( Figs. 1 View Fig and 2 View Fig ) is the smallest species of Trinomys (maximum head and body length 186 mm, mean = 160 mm, SD = 12 mm — Rocha 1995). In comparison, head and body length of other species are: T. albispinus (whitespined spiny rat) 177 mm (n = 1— Moojen 1948); T. dimidiatus (Rio de Janeiro spiny rat), 203 mm SD = 14.5 (n = 132—IackXimenes 2005); T. eliasi (Eliasi’s spiny rat), 197 mm SD = 7.8 (n = 15— Iack-Ximenes 2005); T. gratiosus (gracile Atlantic spiny rat), 200 mm SD = 14.5 (n = 93— Iack-Ximenes 2005); T. iheringi (Sao Paulo spiny rat), 191 mm SD = 16.1 (n = 82—IackXimenes 2005); T. mirapitanga (Pau Brasil spiny rat), 216 mm SD = 15.3 (n = 9— Pessôa et al. 2015); T. moojeni (Moojen’s spiny rat), 187 mm SD = 18.2 (n = 7— Pessôa et al. 2015); T. paratus (rigid-spined Atlantic spiny rat), 212 mm SD = 15.2 (n = 21— Pessôa et al. 2015); T. setosus (elegant-spined Atlantic spiny rat), 204 mm SD = 13.9 (n = 28— Iack-Ximenes 2005). The mean basilar length of the skull is 32.79 mm, considered small to medium sized. T. yonenagae has both a long hind foot (27% of head and body length, discounting the claws) and tail (about 112% of head and body length— Rocha 1995). The tympanic bullae ( Fig. 3 View Fig ) is very inflated (about 173% of the crown length of the upper cheek teeth) and separated by a distance of less than 2.8 mm from the interbullar; the mean bullar length is 11.70 mm. The PM4 width is smaller than the M1 width. The tail ends in a pencil of long hairs covering 1/4 to 1/3 of the tip of tail, body pelage is ventrally white and dorsally dark, and the hair on the dorsal side of the feet barely exceeds onehalf the length of the claws ( Rocha 1995). T. yonenagae has a single counterfold in the cheek teeth, a feature used to distinguish it from all other congeners, which have at least 2 ( Rocha 1995). The inflated bullae, the paler brown dorsal color, and the absence of apical wings in the baculum ( Rocha 1995) are features shared only with the T. albispinus , whose bicolored and brushed tail distinguishes it from T. yonenagae .
GENERAL CHARACTERS
Trinomys yonenagae is on average 160 ± 14.2 mm SD long, from 141 to 195 mm (n = 23— Rocha 1995) has large and prominent ears (the pinna averages 24.04 ± 1.49 mm SD) and soft pelage with aristiform non-spiny hairs with little stiffness ( Fig. 1 View Fig ) that changes in color on the dorsum from gray to brown during development (adults have darker colors with aristiform hairs having distal extensions— Rocha 1995). The head, body, and tail of adults are brown above and white below ( Figs. 1 View Fig and 2 View Fig ). Measurements (mean mm ± SD — Rocha 1995) of adults were: head and body length, 159.83 ± 12.35 mm (maximum 186; n = 24); tail length, 190.26 ± 10.12 mm (n = 23); hind foot length, 43.75 ± 1.54 mm (excluding claws; n = 24); greatest skull length, 47.49 ± 1.88 mm (n = 24); cranial depth, 15.23 ± 0.53 mm (n = 23); incisive foramen length, 33.05 ± 1.32 mm (n = 24); tympanic bulla and mastoid process, 12.18 ± 0.40 mm (n = 23). In captivity, adults weigh 120–138 g (our observation) and the holotype weighed 155 g ( Iack-Ximenes 2005). The small body size with long tail and large hind feet of T. yonenagae are apparently related to the saltatorial locomotion pattern, in convergence with the ricochetal small mammals from Nearctic deserts ( Rocha 1995). There is no apparent sexual dimorphism either in juveniles or in adults.
The skull of T. yonenagae has a unique feature of the dorsal cranium ( Nicola et al. 2003): the incisive foramen is elliptical and its mean ± SD length (33.05 ± 1.32 mm) is twice its width
( Rocha 1995). In addition, the rostrum is short and narrows distally. The inflated bullae seemingly have resulted in the basioccipital and basisphenoid bones to narrow, and the moderately developed postorbital process of the zygoma is formed by the jugal and squamosal bones or only by the jugal ( Rocha 1995).
DISTRIBUTION
Trinomys yonenagae is restricted to sand bank dunes on the western side of the São Francisco River ( Fig. 4 View Fig ). This sand bank dune probably ranges from the municipality of Barra (11°05 ′ 22 ″ S, 43°08 ′ 30 ″ W) to Pilão Arcado (10°02 ′ 12 ″ S, 42°27 ′ 46 ″ W), a linear distance of about 133 km. This species is endemic to Caatinga ( Carmignotto et al. 2012; Gutiérrez and Marinho-Filho 2017) and is assumed to be derived from lineages that diversified in the open areas ( Carmignotto et al. 2012). The estimated western limit of distribution is about 15 km east of the southern portion of the Serra do Estreito mountain range ( Roach and Naylor 2016; Fig. 4 View Fig ). There is no indication of the presence of T. yonenagae on the eastern (right) side of the São Francisco River or in the smaller, northern and smaller dune fields ( Rocha 1995).
FOSSIL RECORD
Based on molecular data, Trinomys yonenagae is estimated to have diverged from T. setosus around 8.5 million years ago, during the late Miocene ( Tavares et al. 2015). Molars of Proechimys (Trinomys) setosus Geoffroy were found in
Pleistocene-Recent cave deposits in Lagoa Santa, Brazil ( Williams and Koopman 1951; Friant 1962) and the molar of unidentified species of Trinomys was recorded from a late Pleistocene-Holocene cave site in southeastern Brazil ( Castro and Langer 2011).
FORM AND FUNCTION
Form. —The dental formula of Trinomys yonenagae , as interpreted from plates 2 and 3 of Rocha (1995), is i 1/1, p 1/1, m 3/3, total 20, consistent with all caviomorph rodents ( Rocha 1995). The cheek teeth have a single transverse counterfold (in 95.1% of 286 teeth analyzed by Rocha 1995), or more rarely none (in M3 or m3 of older animals of age class 9 of Rocha 1995). The upper molariform teeth increase in width from PM4 to M1 and reduce progressively in size to M3 ( Rocha 1995).
It seems that the desert-like environment (caatinga of T. yonenagae and T. albispinus ) has driven the rate of morphological differentiation (in skull shape and size measures). The colonization of the Caatinga produced faster changes in size than in shape, as seen in T. yonenagae and T. albispinus , both of which have smaller body and skull size than all other Trinomys species, that are inhabitants of Atlantic Forest areas ( Tavares et al. 2016b). Also, the mandibular morphology of T. yonenagae has diverged from other Trinomys species, either the result of natural selection and adaptation to the Caatinga semiarid environment ( Monteiro and Dos Reis 2005), or even an evolutionary acceleration of phenotypic change associated with the colonization of Caatinga by T. yonenagae and T. albispinus ( Tavares et al. 2016b) .
A single anal sebaceous gland, which is everted during social encounters, is present and produces a whitish paste with a tutti-frutti-like odor ( Manaf et al. 2003a). Favaretto (2015) described a bilobed gland, possibly a Harderian gland, located on medioposterior portion of the ocular globe. A serous fluid secretion on the infraorbital region, auricular borders, perinasal portion, forelimbs, and lateral body is spread throughout the body during grooming ( Favaretto 2015).
The baculum of T. yonenagae is arched, slender, and long (greatest length of the shaft, 8.15 ± 0.76 mm SD). The proximal portion of the baculum is expanded, to 1.26 ± 0.13 mm SD, but the distal portion narrows to 0.7 ± 0.24 mm SD; the dorsal surface is smooth. The distal margin is straight with a small medial indentation ( Rocha 1995).
Function. —Despite living in xeric environments much different from the forest habitats of its ancestral species, Trinomys yonenagae presents few of the physiological responses to hypercapnic and arid environments seen in other ricochetal small mammals from deserts ( Barros et al. 1998; Rocha et al. 2007). Its resting ventilation is not different from that of the Wistar rat ( Rattus norvegicus domesticus ) or of T. setosus . Under hypercapnic conditions, T. yonenagae increases its ventilation rate in a way that is similar to that of the nonfossorial T. setosus , but its rate does not exceed that of T. setosus . Further, its basal body temperature and O 2 consumption rates are not significantly different from T. setosus , a species that does not live in a xeric environment ( Barros et al. 1998).
On the other hand, behavioral adaptations suggest adjustments to a xeric environment, notably its ability to dig (see “Behavior”). The burrow provides a more mesic microclimate ( Barros et al. 1998) and is a significant adaptation to a desert environment, and the fast locomotion and asymmetrical gait is another ( Rocha et al. 2007). Thus, T. yonenagae has other phenotypic convergences with burrow-dwelling rodents and those that inhabit sand dune habitat, such as small body size, long hind feet and tail, inflated tympanic bullae, and strongly penciled tail ( Rocha 1992; Santos and Lacey 2011). T. yonenagae moves mainly using asymmetrical transverse gallops, half-bounds, and bounds ( Rocha et al. 2007). This kind of gait is considered an autapomorphy by Rocha et al. (2007), one which increases velocity of locomotion and confers high adaptive value in association with the ability to explore open areas in desert-like habitat. Evidence of laterality in motor activity is seen in its preferences for laying down the tail at the right side or choosing between water bottles placed on left or right side of its cage ( Dias 2015). T. yonenagae has a circadian activity-rest rhythm (also seen in its body temperature— Luchesi 2010), being active during the night, and spending 85% of the light phase resting ( Marcomini and Spinelli Oliveira 2003).
Spontaneous limbic idiopathic seizures have been observed in T. yonenagae in captivity ( Cantano 2013) and it was also observed in free-living animals (P. L. B. Rocha, in litt.). Both epileptic mothers and their descendants, when tested, showed a lower anxiety index than non-epileptic animals in an open-field test. A single evolutionary origin of epilepsy is postulated for this species, which does not compromise fitness and may even improve it if associated with antipredatory strategies, at least regarding hereditary and idiopathic seizures ( Cantano 2013).
ONTOGENY AND REPRODUCTION
Ontogeny. — The neonate of Trinomys yonenagae , like the young of other caviomorph rodents ( Kleiman 1974), is precocious ( Manaf and Spinelli Oliveira 2000). It is born with fine gray pelage, opened eyes, and the ability to move around. Mean body mass at birth is 15.8 ± 1.3 g and litters average 2.0 ± 0.6 for infants in captivity ( Luchesi 2010). In 2–3 days they can eat solid food if available. They are sexually mature at about 90 days of age, but copulation was not observed before 120 days of age in both sexes under laboratory conditions and the youngest parturition occurred in a female when 11 months old ( Luchesi 2010). The first opening of the vaginal orifice occurs during the 3rd month of life even though females are housed with their parents (92 ± 17.7 days old— Luchesi 2010). Alloparental care was observed as an important component of social structure in this species, with males protecting and rearing the juveniles ( Manaf and Spinelli Oliveira 2000). Santos (2004) found a high frequency of resident animals and a low index of dispersal for both adults and juveniles. Exploratory movements were observed in both sexes, although usually not associated with their dispersal. Females more than males returned to their original galleries after they had been trapped far from their home burrows (J. W. A. Santos, in litt.).
The skull development has an allometric trajectory (i.e., disproportional changes in relative growth in ontogenetic stages) in relation to shape in the Echimyidae . However, a wide range of size variation was observed in 10 of 13 spiny-rat genera in the Clyomys + Euryzygomatomys + Trinomys clade ( Tavares et al. 2016a). The skull regions of T. yonenagae and T. setosus showed the highest scores of allometry in length of rostrum, palatal, and nasals ( Tavares et al. 2016b). These high resemblance tendencies among closely related species (i.e., phylogenetic signaling or inertia) suggest a common history of these traits in the Echimyidae and the disparity between allometric trajectories compared to other Caviomorph families ( Tavares et al. 2016a). Spiny rats, as well as other recent clades with mild ecological pressures, can maintain strong phylogenetic signals, with similar responses to changes in overall size ( Verzi et al. 2015). Unlike echimyids with the largest skulls, the small skulls of T. yonenagae and T. albispinus show acceleration of phenotypic changes that may be related to the colonization of the Caatinga ( Tavares et al. 2016b).
Reproduction. — Trinomys yonenagae reproduces throughout the year, there being no seasonality either in free-living populations ( Santos 2004) or those in captivity ( Luchesi 2010). It reproduces even during periods of high hydric stress when the rate of mortality of juveniles is high; only offspring born in rainy seasons survive, and even then, survival depends on the fruit of Eugenia stipitata (Myrtaceae) , the araza, locally named araçá-de-boi ( Santos 2004).
Trinomys yonenagae has a spontaneous estrous cycle and postpartum estrus ( Luchesi 2010). There is a vaginal closure membrane (Stockard and Papanicolau 1919) that opens and closes independently from the reproductive phase throughout the estrous cycle ( Spinelli Oliveira et al. 2007; Luchesi 2010). Furthermore, one of us (LCL) has observed that the estrous phase is difficult to identify in histological slides stained by the papanicolau methodology (Stockard and Papanicolau 1919). The presence of lymphocytic cells, that is usually used to identify the diestrus phase, is extended to all phases, as noticed by other authors studying hystricomorph rodents (the nutria Myocastor coypus — Felipe et al. 2001; the degu Octodon degus — Labyak and Lee 1995). Gestation lasts 3 months (in captivity) and, at the late stage of pregnancy (beyond the 46th day), embryos can be detected by palpation ( Santos 2004). Compared to other rodent suborders (Sciuromorpha and Myomorpha), both sexes delay sexual maturity beyond 3 months in captivity and females rarely get pregnant before 4 months old (only one occurrence in 10 years of observation in captivity— Luchesi 2010).
Females gain 25% of their initial body weight during pregnancy, the gain being discrete in the first one-third and pronounced only during the last 4 weeks of pregnancy ( Luchesi 2010). In captivity, females usually get pregnant for the first time around 21.9 ± 18.2 months (youngest 0.30 years and oldest 7 years) with no seasonality pattern, litters occur through the year ( Luchesi 2010).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Trinomys yonenagae ( Rocha, 1995 )
| Luchesi, Lilian Cristina, Cantano, Lais Mendes Ruiz, Takata, Juliana Toshie & Monticelli, Patricia Ferreira 2019 |
Trinomys yonenagae:
| LARA, M. C. & J. L. PATTON 2000: 665 |
Proechimys yonenagae
| ROCHA, P. L. B. 1995: 541 |