Archechiniscus bahamensis, Bartels & Fontoura & Nelson, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4420.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:5509F944-4798-43A1-9179-02F97990FCDA |
|
DOI |
https://doi.org/10.5281/zenodo.5664117 |
|
persistent identifier |
https://treatment.plazi.org/id/A01187D9-FFD2-FFF2-FF44-F903FD07FA3B |
|
treatment provided by |
Plazi |
|
scientific name |
Archechiniscus bahamensis |
| status |
sp. nov. |
Archechiniscus bahamensis sp. nov.
Table 2, Figures 2–6 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6
Material examined: Holotype and 58 paratypes. The holotype (female, slide B: 14-18-9, mounted in PVA) and 17 paratypes were collected from site 9 (8 females, slides B: 3-14-16-4; B: 3-14-17-3; B: 3-14-17-4; B: 3-14-17-7; B: 3- 14-18-1; B: 3-14-18-2; B: 3-14-18-3; B: 3-14-18-8; 4 males, slides B: 3-14-16-1; B: 3-14-16-3; B: 3-14-17-1; B: 3-14- 18-5; 2 specimens of undetermined sex, slides B: 3-14-16-8; B: 3-14-17-5; 2 second-stage larvae, slides B: 3-14-17- 2; B: 3-14-18-7, and 1 first-stage two-clawed larva, slide B: 2-14-18-6). One male (slide B: 1-14-2-1) was collected from site 4. One female (slide B: 2-14-6-1) and 1 male (slide B: 2-14-5-1) were collected from site 5. Three females (slides B: 2-14-7-4; B: 2-14-7-5; B: 2-14-8-3) and 2 males (slides B: 2-14-7-1; B: 2-14-7-3) were collected from site 6. One female (slide B: 3-14-14-1) was collected from site 8. One specimen of undetermined gender (slide B: 3-14- 19-1) was collected from site 10. Four females (slides B: 4-14-23-1; B: 4-14-23-5; B: 4-14-23-7; B: 4-14-23-9), 2 males (B: 4-14-24-6; B: 4-14-24-10), and 5 specimens of undetermined gender (slides B: 4-14-23-2; B: 4-14-23-3; B: 4-14-23-4; B: 4-14-23-6; B: 4-14-23-11) were collected from site 11. Twelve females (slides B: 4-14-25-1; B: 4- 14-25-3; B: 4-14-25-4; B: 4-14-26-2; B: 4-14-26-3; B: 4-14-26-5; B: 4-14-26-6; B: 4-14-27-2; B: 4-14-27-3; B: 4-14- 27-4; B: 4-14-27-7; B: 4-14-27-8), 6 males (slides B: 4-14-26-1; B: 4-14-26-4; B: 4-14-27-5; B: 4-14-27-9; B: 4-14-27- 10; B: 4-14-27-11), 1 second-stage larva (B: 4-14-27-6) and 1 individual of undetermined gender (slide B: 4-14-25- 2) were collected from site 12. The type material (holotype and paratypes) is deposited in the collection of tardigrades of the Department of Biology, Faculty of Sciences, University of Porto, Portugal. We also examined an unnumbered slide in the Pollock collection from Jamaica marked “ Archechiniscus marci ” that we determined to be A. bahamensis sp. nov.
Specific diagnosis: Archechiniscus with large dark brown eyes and cuticular metameric folds that create clearly demarked cephalic and terminal segments. Cuticle with fine punctation. Lenticular secondary clava located between the cephalic cirri, but nearer the external cirrus. Internal and external cephalic cirri with an enlarged crenate proximal portion and a distal portion that is bifurcated or trifurcated, but may appear as a blunt tip. Lateral cirri A with enlarged proximal portion and flagellum and long cirri E present. Median cirrus vestigial. Pedunculated external claws. Short spine on legs I and spherical papilla on legs IV. Legs I–III with an anterior bulbous papilla. Small seminal receptacles with short arced seminal ducts with openings located near, and anterior to, the female gonopore. Female gonopore in a depression surrounded by 3 cuticular platelets.
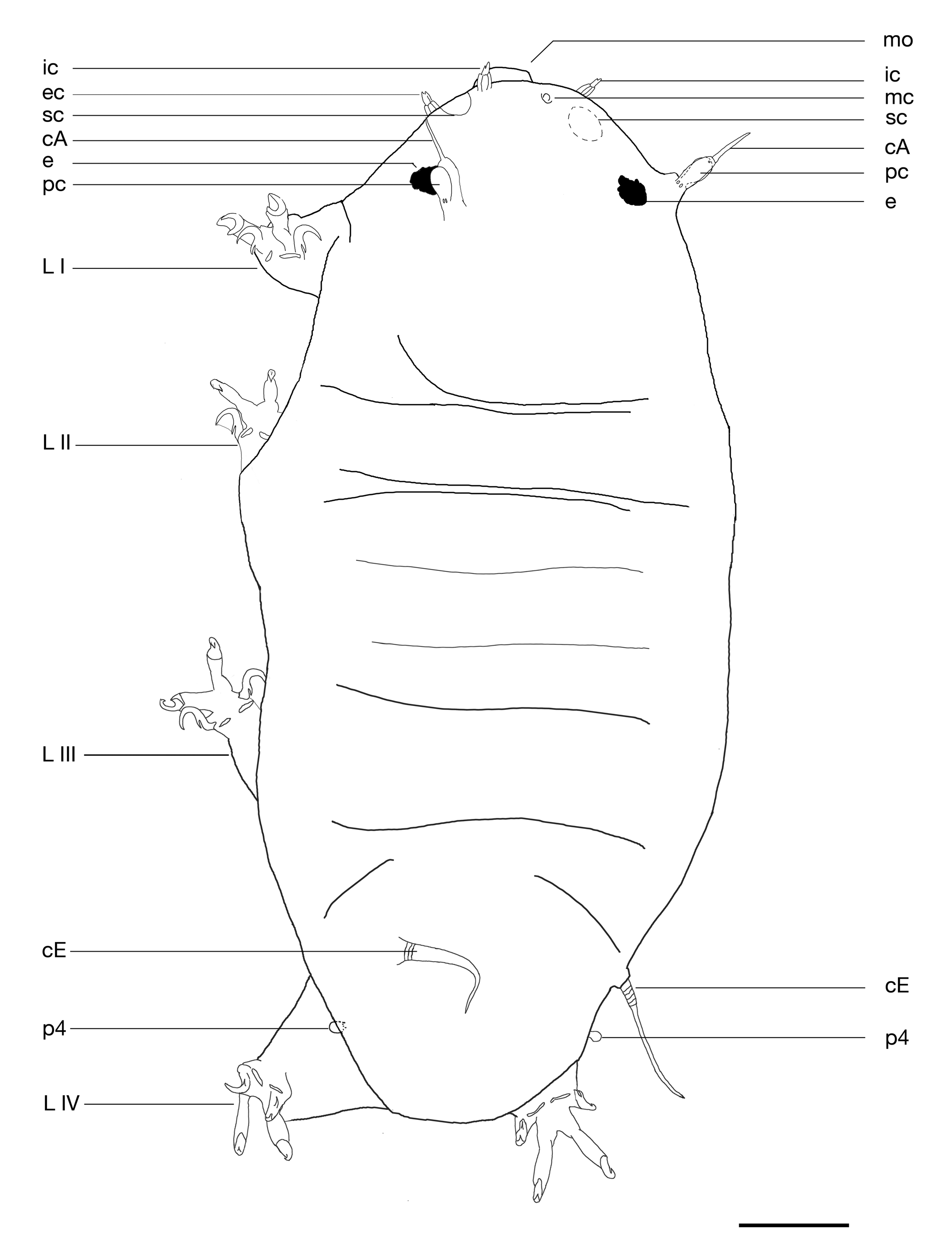
Description of the holotype: Female with a cylindrical body 169 µm long and 69 µm wide. Conical cephalic region with large oblong dark brown eyes (shortest diameter about 6 µm). Rounded caudal region. Cuticle finely punctated (dorsally about 20 pillars/10 µm length, pillars about 0.5 µm high) with cuticular folds creating clearly demarked segments. Cephalic and terminal segments well defined. Other segments (scapular, and at least two other dorsal segments) poorly defined. Habitus shown in Figs. 2 View FIGURE 2 and 3 View FIGURE 3 , cuticular punctations in Fig. 4 View FIGURE 4 .
The cephalic appendages are: short external and internal cephalic cirri (5.3 and 4.5 µm long, respectively) with an enlarged crenate proximal portion and a distal portion that is bifurcated or trifurcated, but may appear as a blunt tip. Lenticular secondary clavae (largest diameter 8.7 µm) located between external and internal cephalic cirri, but nearer the external cirrus. The club-shaped primary clava, 6.2 µm long, with a refracting annular van der Land’s body at its base and a distal pore, and the long lateral cirrus A (15.0 µm long) arising from a short common pedestal. The lateral cirrus A also has an enlarged and transparent proximal portion (about 6.8 µm long) and a thin distal flagellum. A vestigial median cephalic cirrus is present (a short protuberance about 0.7 µm long and 1.5 µm in diameter). Cephalic appendages are shown in Fig. 4 View FIGURE 4 .
Cirrus E (22.3 µm long) consists of a short base and a flagellum with an enlarged proximal portion with accordion-like appearance (about 8.4 µm long) ( Figs. 2 View FIGURE 2 and 3 View FIGURE 3 ).
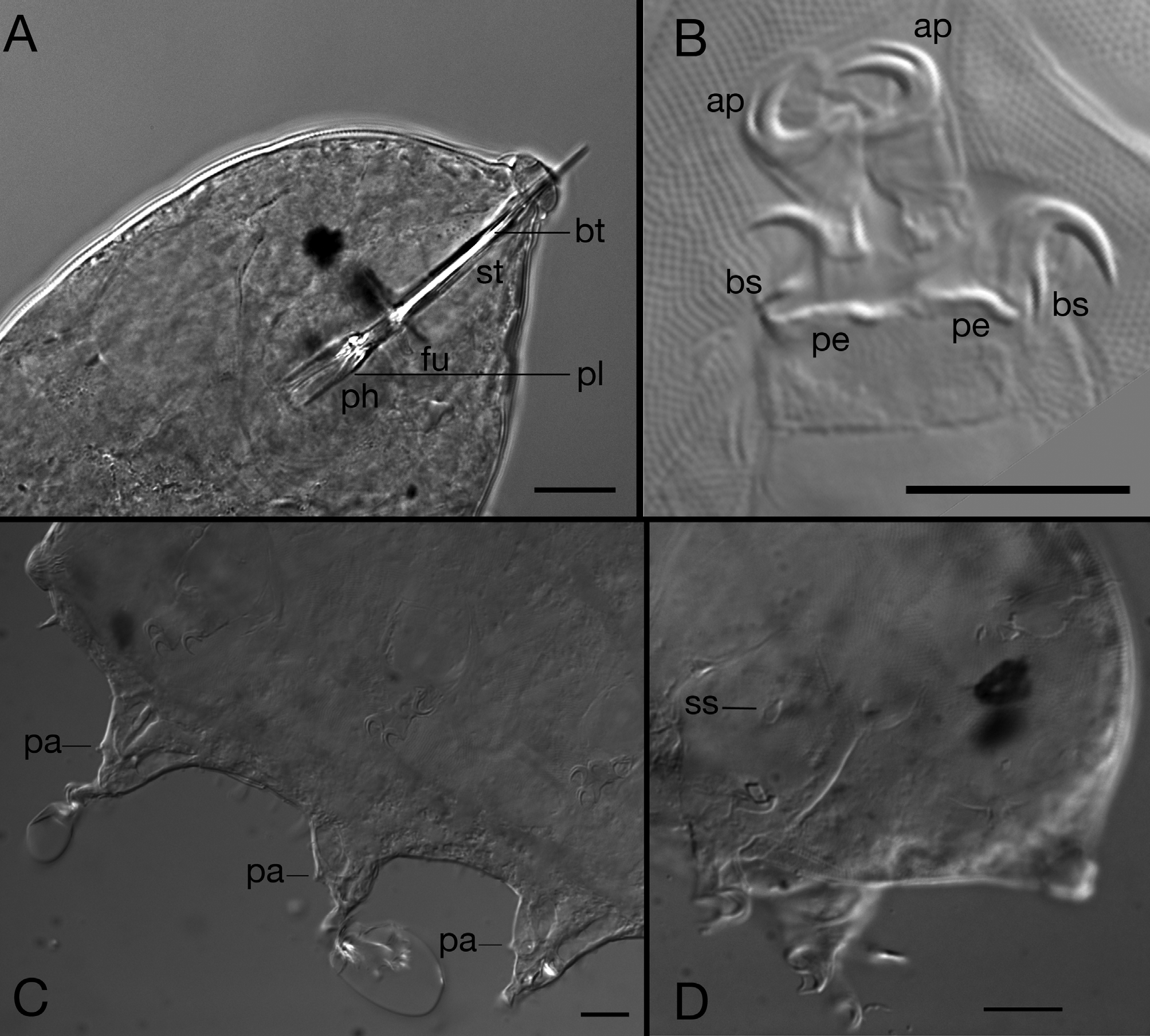
The protruded sub-terminal mouth is followed by a long buccal tube ending in an oval pharyngeal bulb (17.8 x 17.2 µm) containing three placoids (11.6 µm long). Stylets with stylet sheaths (13.2 µm long). Although not visible in the holotype, elongate stylets with large T-shaped furcae and the absence of stylet supports have been confirmed in some paratypes ( Fig. 5A View FIGURE 5 ).
Stubby, non-telescopic legs typical of the genus having two external sessile robust claws and two internal digits (11.4 µm long) ending with crescent shaped claws ( Fig. 5B View FIGURE 5 ). External claws (5.3 µm on legs II/III) with a short spur directed downwards and a conspicuous peduncle (about 3.4 µm long on legs IV) at their base; internal claws (4.7 µm on legs II/III) with accessory points and without peduncles can be integrally retracted inside claw sheaths. Legs I–III with an anterior bulbous papilla (this character is not visible in the holotype but confirmed in several paratypes, Fig. 5C View FIGURE 5 ). Sensory organs on legs I and IV. Small spine (3.1 µm long) with enlarged base and short tip on leg I ( Fig. 5D View FIGURE 5 ), and spherical papilla (3 µm) on leg IV ( Fig. 3 View FIGURE 3 ). This papilla has a refracting annular van der Land’s body at its base.
The female gonopore consists of a six-cell rosette (diameter 4.3 µm), 12.4 µm from the anus ( Fig. 6 View FIGURE 6 and 7 View FIGURE 7 ). Spherical small seminal receptacles are located to each side of the gonopore, with short arced seminal ducts (10.4 µm long and about 1.1 µm in diameter) leading anteriorly from seminal receptacles. The openings of the seminal ducts are anterior to but near the gonopore. The gonopore and the seminal duct openings are located in a triangular depression, protected by three cuticular platelets, two lateral and a smaller one at the vertex. The anal fissure is straight and surrounded by a fan of folds.
Remarks: The males seem to be smaller than females ( Table 2). The male gonopore is circular and, in comparison with females, located much nearer to the anus ( Table 2). Plates or folds surrounding the male gonopore are not visible. All other characteristics are very similar to females. Male and female gonopores are shown in Fig. 6 View FIGURE 6 and 7. View FIGURE 7
A two-clawed larva has been examined (measurements in Table 2). With the exception of missing external claws, all the other characteristics as in the adults. Another examined specimen (slide B: 3-14-17-2) is presumably a second-stage larva, and spurs on the external claws are missing.
......continued on the next page
The specimen from the Pollock collection from Jamaica marked Archechniscus marci matches the new species, including the unique gonopore anatomy. Thus, the known range of Archechniscus bahamensis sp. nov. is the Bahamas and Jamaica. We do not have a date or exact location for this specimen, but according to L.W. Pollock (pers. comm.) it came from subtidal sand, less than 1m depth, Hofstra Marine Station, St. Ann’s Bay, Ocho Rios, St. Ann’s Parish, Jamaica (approximately 18 ° 26.4′N, 77° 12.1′W).
Etymology: The specific epithet refers to the type locality.
Differential diagnosis: Excluding the new species, only four species have been attributed to the genus Archechiniscus Schulz, 1953 : A. marci , A. minutus Grimaldi de Zio & D’Addabbo Gallo, 1987 , A. symbalanus Chang & Rho, 1998 and A. biscaynei Miller, Clark & Miller, 2012 . Archechiniscus bahamensis sp. nov. differs from A. minutus , A. symbalanus and A. biscaynei by the presence of longer cirri A and E, and mainly by the short arced seminal ducts and gonopore peculiarly located in a depression protected by cuticular platelets. In A. minutus the seminal receptacles are also arced, but they are longer, open far away from the gonopore, and there is no cuticular depression associated with the reproductive structures. In A. symbalanus and A. biscaynei the seminal receptacle ducts are S-shaped. The new species also differs from A. minutus by the presence of large conspicuous eyes, a sensory spine on leg I, bulbous papillae on legs I–III, stylet sheaths, and absence of anal platelets. Archechiniscus bahamensis sp. nov. also differs from A. symbalanus by the presence of cuticular folds creating clearly demarked segments, a much shorter median cirrus (a vestigial protuberance about 0.7 µm long in the new species and a conspicuous 2.3 µm long cirrus in A. symbalanus ), longer primary clava, secondary clava located between external and internal cirri (not under external cirri), and retractable claws in sheaths in males and females (only in males in A. symbalanus ). The new species and A. biscaynei can also be distinguished by the presence of eyes, punctate cuticle with cuticular folds forming dorsal segments and sensory spine on leg I.
The comparison of the new species with A. marci is complicated by the convoluted history of A. marci . This species was described by Schulz (1953) based on one specimen from barnacles in El Salvador, the description was brief and the holotype no longer exists. Later, Renaud-Debyser (1963) reported A. pacifici from intertidal sand in Bimini, Bahamas. However, there was no previous species with that name, and no description or drawing was provided in Renaud-Debyser (1963). The name just appeared on a list. Subsequently, multiple specimens of an Archechiniscus species were discovered in subtidal coralline sand in New Caledonia, South Pacific (Renaud- Mornant 1967). Renaud-Mornant provided an excellent description and drawings of these specimens, and she found several distinctions between them and Schulz’s description of A. marci . However, since no type specimen existed for A. marci and the description was brief, she did not feel justified naming a new species based on the New Caledonian specimens, so she called them A. marci , and she claimed that the characters she described completed the description by Schulz. She also synonymized A. pacifici from Bimini with A. marci . After the records from El Salvador, Bahamas, and New Caledonia, A. marci was reported from the Galapagos, Madagascar, the Caribbean, Italy, Australia, Brazil, and California, USA ( Schuster & Grigarick 1966; Renaud-Mornant 1979; Renaud-Mornant & Gourbault 1981, 1984; D’Addabbo Gallo et al. 1987; Grimaldi de Zio et al. 1983a, b; Mackness 1999; Calvacanti da Rocha et al. 2013; Miller et al. 2014), but usually with little to no description. Given this complex history, we have compared our specimens with Schulz’s description of the El Salvador specimen, with Renaud-Mornant’s description of the New Caledonia specimens, and with specimen MNHN# 167Ma from New Caledonia. No specimens or description of Renaud-Debyser’s specimens from the Bahamas exist, so comparisons are not possible.
In the El Salvador specimens of A. marci , the seminal receptacles were not discussed ( Schulz 1953). Our new species differs from the El Salvadorian description by the presence of dark brown eyes, an evident lenticular secondary clava located between the internal and external cirri (in A. marci the eyes are reddish and the secondary clava is a small papilla located under the external cirrus), bifurcated or trifurcated internal and external cirri, sensory spine on leg I, bulbous papillae on legs I–III and by much longer lateral cirri A and E (always longer than 10 µm and 20 µm in the new species compared to 6.4 and 6.5 µm for cirri A and E, respectively, in the description of the holotype of A. marci with a body length of 198 µm). Archechiniscus bahamensis sp. nov. also differs from Schulz’s description of A. marci by some peculiarities of the claws and the buccal apparatus. Unlike A. marci , in the new species the external claws have a conspicuous peduncle and the internal claws have an evident accessory point. In the buccal apparatus of the new species, stylets are smooth and have a T-shaped furca (rounded in A. marci ), and stylet sheaths are present.
The museum specimen marked Archechiniscus marci from New Caldedonia is damaged, but our observations of this specimen together with the original description of the Archechiniscus from New Caledonia (Renaud- Mornant 1967) indicates that this population differs from Schulz’s description of A. marci in a number of ways. Four differences can be noted based on Renaud-Mornant’s description: (1) The cuticle described as smooth by Schultz is dorsally punctated in the New Caledonian specimens. (2) Stylets in the New Caledonian specimens are not smooth but formed by sliding lamellae. (3) The dimension of cirrus E was four times larger in the New Caledonian specimens than in the original A. marci . (4) The female gonopore of the New Caledonian specimens was not rosette-shaped but was a longitudinal slit formed by a cuticular fold. Based on our examination of the MNHN specimen we noted two additional differences: (1) The external claws have a basal peduncle and internal claws have a thin accessory point, and although the peduncles were not mentioned in the original description, they do appear in the drawing ( Fig. 5 View FIGURE 5 , Renaud-Mornant 1967). In Schulz’s description of A. marci these characters were not described and, according to Grimaldi de Zio & D’Addabbo Gallo (1987), the peduncles could not be overlooked by Schulz due to their size and thickness. In addition, in the New Caledonian specimens, claws can be retracted into claw sheaths. (2) The secondary clavae in the New Caledonian specimens, although also located under the external cirri, are not small papillae but they are lenticular (9.3 µm diameter).
There are important differences between A. bahamensis sp. nov. and the New Caledonian specimens. In the new species the stylets are smooth and stylet furcae are T-shaped, and dorsal cuticular folds create clearly demarked segments (less evident in the New Caledonian specimens). The New Caledonian specimen we examined did have bifurcated or trifurcated tips on cephalic cirri like A. bahamensis sp. nov. but they are shorter (about 3.5 µm total length in the New Caledonian specimen which was very large (276 µm body length) versus an average of 4.4 µm in A. bahamensis sp. nov.). The New Caledonian specimen did have basal peduncles on the external claws and accessory points on the internal claws like A. bahamensis sp. nov. but accessory points were considerably thinner. Furthermore, external claws of the New Caledonian specimen have tips that form an angle of about 90o, whereas those in A. bahamensis sp. nov. are only slightly curved. And finally, in the new species the female gonadal apparatus is quite distinct with a rosette-shaped gonopore recessed and surrounded by three obvious cuticular platelets.
Our conclusions are as follows: (1) The New Caledonian and El Salvadorian material are quite distinct, and traits from New Caledonian specimens should not have been used “ pour pouvoir completer ” (p. 114, Renaud- Mornant 1967) the description of A. marci . El Salvador and New Caledonia are on opposite sides of the Pacific Ocean some 12,000 km apart, and the specimens were found in different habitats. (2) A. bahamensis sp. nov. cannot be confused with the El Salvadorian or the New Caledonian descriptions. (3) It is quite possible that the Archechiniscus from Bimini reported by Renaud-Debyser (1963) is A. bahamensis sp. nov. In our collections A. bahamensis sp. nov. was the most common subtidal marine tardigrade in the Bahamas and no other congenerics were found. We have also found it and no other congenerics in the British Virgin Islands (unpublished data), and the Archechiniscus from Jamaica in the Pollock slide collection exactly matches the new species. While these observations support the hypothesis that Renaud-Mornant’s Bimini record was A. bahamensis sp. nov., it is impossible to know this for sure since neither slides nor descriptions exist. Based on these conclusions, we believe A. marci should be used only for the El Salvadorian record. Subsequent records are dubious and need to be confirmed.
Miller et al. (2012) proposed an identification key to the Archechiniscus species. However, that key is based on the presence or absence of the median cirrus that, according to Grimaldi de Zio & D’Addabbo Gallo (1987) and Grimaldi de Zio et al. (1987), is not a useful taxonomic character because, as in A. bahamensis sp. nov., it can be so small that it can be easily overlooked (see also Møbjerg et al. 2016). Miller et al. (2012) also use the location of the secondary clava as an important discriminating character. We agree that the attributes of clavae are important taxonomic characters, however the secondary clavae are often hard to see. Therefore, a new key to the known Archechiniscus species is presented, with the characters for A. marci as described by Schulz (1953).
1 Transverse dorsal cuticular metameric folds present.......................................................... 2
1’ Transverse cuticular dorsal metameric folds absent........................................................... 4
2 Stylets with a rounded furca............................................................. Archechiniscus marci 2’ Stylets with T-shaped furca.............................................................................. 3
3 Gonopore and seminal receptacle openings located in a depression protected by cuticular platelets. Short arced seminal receptacle ducts. Somatic cirri long even in smallest specimens; cirri A> 10 µm long; cirri E> 20 µm long...................................................................................................... A. bahamensis sp. nov.
3’ Gonopore not located in a cuticular depression protected by cuticular platelets. Long arced seminal receptacle ducts. Somatic cirri short even in large specimens; cirri A <10 µm long; cirri E <15 µm.................................. A. minutus
4 Spine on legs I present. Claws not retractable into sheaths in females. Black eyes present.................. A. symbalanus
4’ Spine on legs I absent. Claws retractable into claw sheaths in females. Eyes absent......................... A. biscaynei
Ecological Notes: There may be two different niche specializations in this genus. Archechiniscus symbalanus , A. biscaynei and the holotype for A. marci were collected from barnacles ( Chang & Rho 1998a, Miller et al. 2012, Schulz 1953). In contrast Archechiniscus minutus was collected in coralline sand (Grimaldi de Zio & D’Addabbo Gallo 1987, 2001, D’Addabbo Gallo et al. 1989, Gallo et al. 2007), as was the New Caledonian Archechiniscus ( Renaud-Mornant 1967) and A. bahamensis sp. nov.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |