Elysia patina Ev. Marcus, 1980
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4148.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:91353147-FDA8-45CC-A8F1-1DE801C835A6 |
|
DOI |
https://doi.org/10.5281/zenodo.5664205 |
|
persistent identifier |
https://treatment.plazi.org/id/A04A7E6D-9C06-FFCE-46C9-FD83FEE51AD4 |
|
treatment provided by |
Plazi |
|
scientific name |
Elysia patina Ev. Marcus, 1980 |
| status |
|
Elysia patina Ev. Marcus, 1980 View in CoL
( Figs. 6 View FIGURE 6 G–H, 42–44)
Elysia patina Ev. Marcus 1980: 72 View in CoL –73, figs. 23–24, 36, 41–43, 57 [non Ev. Marcus 1980: figs. 59–60] (Type locality: Florida Keys) — Jensen & Clark 1983: 5; Clark 1994: 905; Krug et al. 2015: 990 -991, figs. 3B, 4.
Elysia papillosa View in CoL [non Verrill 1901: 31, pl. 4, fig. 3] — Jensen & Clark 1983: 5; Clark 1984: 89, figs. 15, 17; Krug 2009: 361-365, figs. 2C, 5D, 6; Händeler et al. 2009: figs. 6, 7; Christa et al. 2014: figs. 1F, 3.
Type material. Elysia patina —10 syntypes (USNM 770515).
Material examined. Bahamas: Sweetings Cay, July 2007, 1 specimen ( LACM 178650 View Materials ) , July 2010, 3 specimens ( LACM 178649 View Materials , LACM 178651–52 View Materials ); Mia Reef Isla Mujeres , Mexico, August 1993, 1 specimen ( LACM 178653 View Materials ) .
Additional material examined. Mote Marine Laboratory and Aquarium, Florida, USA, June 2007, 4 specimens (isolate Epat_07Mote01-04) ; Bahamas: Sweetings Cay , July 2007, 11 specimens (isolate Epat_07Swe01, 0 2, 04-11), July 2010, 8 specimens (isolate Epat_10Swe01-05, 07-09), Stirrup Cay, 1 specimen (isolate Epat_07Stir01), Northern Exumas, 1 specimen (isolate Epat_07NEx01), Bimini, 7 specimens (isolate Epat_07Bim01-07).
Live animal. When resting, slugs hunker down with head tucked between parapodia. Disturbed slugs either cover their head with their parapodia in a similar manner, or swim by flapping (undulating) their parapodia.
External anatomy. Specimens from Sweetings Cay ranged from 1.5– 8 mm in length, while Florida specimens were 6–8 mm in length. Overall color mottled white, grey or yellowish-brown, with scattered brown patches on sides of head and parapodia ( Fig. 42 View FIGURE 42 A–D). Body elongate when crawling. Head predominantly white. Faint orange-brown spots, small, dotting front of face; no moustache of spots on oral lobes. Thin brown line running across side of head under large eyespots, up onto proximal portion of each rhinophore. Rhinophores elongate, rolled, thick, with rounded blunt tips. Outer surface of rhinophores covered with white rounded papillae, both large and small. Thick brown transverse band dividing proximal third of each rhinophore from the distal two-thirds ( Fig. 42 View FIGURE 42 A–D).
Foot not clearly distinct from parapodia. Foot same yellowish-brown color as parapodial base, with scattered faint iridescent blue dots. Transverse groove separates underside of head from foot. End of body narrowing at ends of parapodia; foot then widening to form short, triangular tail with pointed end.
High-arching parapodia covering dorsum, often held over head of live animal. Upper half of parapodia predominantly white, grey or tan, with few scattered brown spots. Parapodial surface with streaks and patches of gold-yellow and white, with scattered dark brown spots. Parapodial surface everywhere dotted with white, rounded papillae varying in size. Parapodial margin even (not scalloped), with row of white papillae sometimes interspersed with light brown dots. Inner parapodial surface yellow or brown with white upper edge along margin; scattered blue iridescent dots speckle inner surface, sometimes highlighting dorsal vessels ( Fig. 42 View FIGURE 42 E–F).
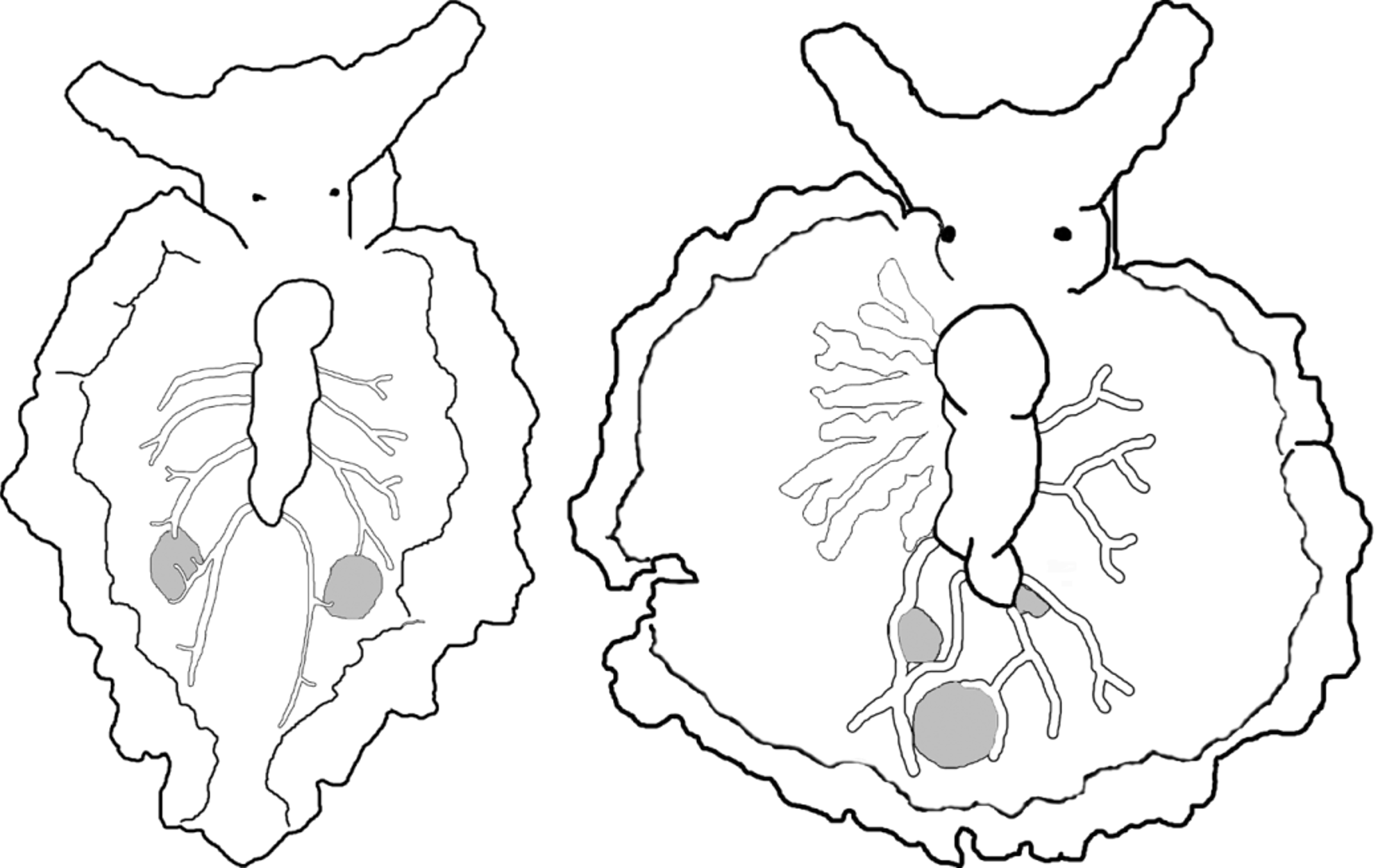
Renopericardium running over half the body length, sometimes bending about halfway along, and may undergo a constriction at posterior end. Coloration of renopericardium white, with sparse brown dots. Dorsal vessels emerging from renopericardial extension, initially wide and straight, bifurcating once about halfway along the distance to parapodial edge, then each branch forking again near parapodial margin ( Fig. 43 View FIGURE 43 ). Vessels transparent, or pigmented by white or iridescent blue speckles ( Fig. 42 View FIGURE 42 E–F). Four to five pairs of vessels on specimens 5–8 mm long, including elongated posterior pair, which sends a short branch over the gametic vesicle on each side of body. Vessels roughly symmetrical, do not anastomose. Prostate gland visible as network of white tubes underlying renopericardium and dorsal vessels.
One pair of large vesicles visible as rounded protrusions inside parapodia, either milky white or cloudy but partly clear ( Figs. 42 View FIGURE 42 E–F, 43). Vesicles irregular in size, each contacted by terminus of one or more branches of dorsal vessels; often located between branches forking from 4th or 5th vessel on each side. Vesicles not apparent on juvenile specimens <3 mm in length; likely function as sperm-storage receptacles.
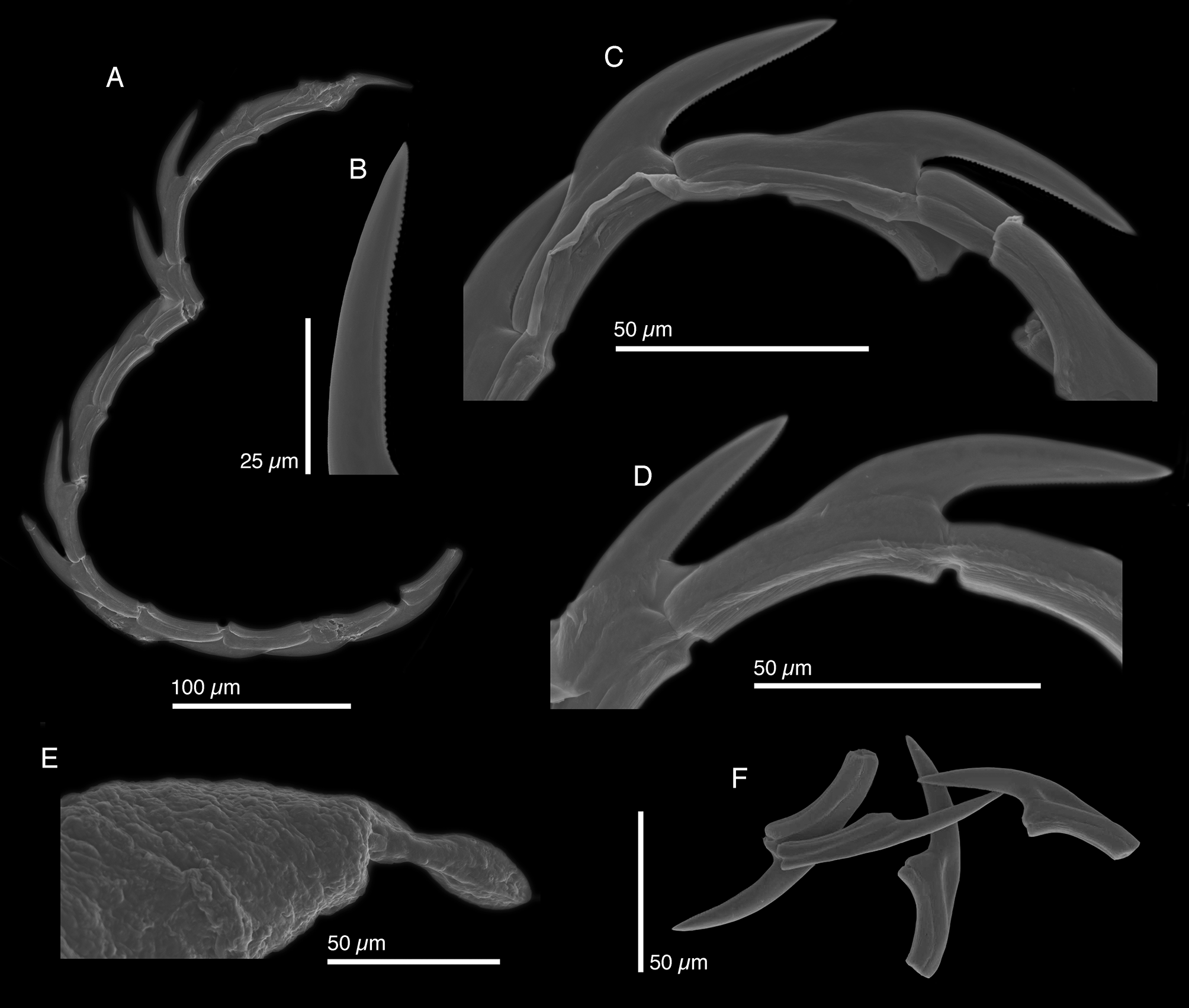
Internal anatomy. Radula with 14 teeth (LACM 178649, LACM 178652–53), 6 teeth in ascending limb and 8 in descending limb ( Fig. 44 View FIGURE 44 A). Leading tooth thin and elongate with approximately 55 very small denticles ( Fig. 44 View FIGURE 44 B–D). Radular teeth smooth according to Marcus (1980). Teeth without typical “V”-shaped depression of many other elysiids. Instead, teeth overlapping slightly with their tips resting in triangular-shaped depressions on side of adjacent teeth ( Fig. 44 View FIGURE 44 F). Base of the tooth approximately ½ total tooth length. Ascus containing jumbled heap of discarded teeth (not figured).
Penis variable in length, with rigid musculature that did not deform after drying (LACM 17 8652–53) ( Fig. 6 View FIGURE 6 G–H), often bent, bearing a folded or scoop-shaped stylet ( Fig. 44 View FIGURE 44 E). Deferent duct long, thin, and loosely convoluted.
Reproduction and development. Marcus noted as unusual placement of the male aperture in the groove separating the head from the right parapodium, which we could not corroborate. Krug (2009) presented data on larval development but reversed the names E. patina and E. papillosa , following Ortea et al. (2005). Development in E. patina is lecithotrophic. Eggs are laid in a typical elysiid spiral, one egg per capsule, with a flat ribbon of bright orange ECY on the upper surface of the egg strand, under the outer covering of the egg mass ( Fig. 42 View FIGURE 42 G). Mean clutch size was 45.0 (± 28.3 SD, n = 2). Mean diameter of uncleaved eggs was 116.3 µm (± 3.4 SD; n = 15 ova). Clutches held at ~25°C hatched after 19.5 d (± 0.7 SD, n = 2), releasing swimming veliger larvae that swam actively for 4 d before undergoing metamorphosis in the absence of any inductive cue. Mean larval shell length was 337.3 µm (± 12.7 SD; n = 65 shells), the largest larval size out of 59 sacoglossan species for which data exist ( Krug et al. 2015). Larvae released white mucus when disturbed. Juveniles fed immediately on H. opuntia when the alga was provided.
Host ecology. No prior report has documented the correct algal host for E. patina . In this study, all specimens of E. patina were collected from clumps of the alga Halimeda opuntia , generally growing in shaded and protected habitats, in water as shallow as a few cm. Slugs maintained in aquaria fed readily on H. opuntia for 6 weeks, but never consumed other udotacean algae ( Udotea , Penicillus , Caulerpa ). In the Florida Keys, E. patina and E. marcusi both feed on H. opuntia but have not been found co-occurring, even in similar habitats a few km apart; the species may be partitioned by competitive exclusion or distinct but as yet unidentified microhabitat preferences. Repeated assertions in the literature that E. patina feeds on Udotea ( Jensen & Clark 1983; Clark & DeFreese 1987; Clark 1994) stemmed from misidentification of E. zuleicae , which does specialize on Udotea . Similarly, assertions that E. papillosa feeds on Halimeda likely reflect misidentifications of E. patina as E. papillosa ( Clark 1984) .
Christa et al. (2014) reported several Halimeda spp. as the food of “ E. papillosa ” but the specimens were actually E. patina based on DNA barcodes of the slugs (and vice-versa: their “ E. patina ” were actually E. papillosa ). For unclear reasons, Christa et al. (2014) did not list H. opuntia as a source of plastids from E. patina even though some algal barcodes matched closely the lone included reference sequence for Caribbean H. opuntia (NCBI accession # AY942174 View Materials , mis-entered as AY942147 on Fig. S1 of Christa et al. 2014). Their data suggest that E. patina may occasionally consume a broader range of Halimeda spp. than is reflected by their tight ecological association with clumps of H. opuntia , the only microhabitat in which we have consistently (and indeed, exclusively) sampled this elysiid.
Phylogenetic relationships. Elysia patina was recovered within subclade 1, but no sister species was identified with meaningful support ( Fig. 4 View FIGURE 4 ). Ortea et al. (2005) casually erected a new genus, Checholysia , with E. patina as the type species, as a “ precaución taxonomica ” or taxonomic precaution, with a penial stylet as the supposed distinguishing synapomorphy of the new genus. However, penial stylets are present in all three sacoglossan superfamilies, and are phylogenetically distributed throughout Elysia ( Fig. 4 View FIGURE 4 ). Therefore, our phylogenetic analysis reveals that Checholysia is polyphyletic and invalid, as stylets have evolved independently several times in Elysia . The proposal was not taxonomically “cautious,” as erecting a new genus rendered Elysia paraphyletic with respect to Checholysia , a problem not discussed by Ortea et al. (2005).
Range. Florida, USA ( Ev. Marcus 1980; present study) , Bahamas: Sweetings Cay, Grand Bahamas Island ; Stirrup Cay; Northern Exumas ; Bimini (present study), Mia Reef Isla Mujeres , Mexico (present study).
Remarks. Marcus (1980) described E. patina from a preserved specimen and had not seen the species alive. Ortea et al. (2005) described E. papillosa as E. patina , inexplicably ignoring the completely different radular tooth morphology shown for E. patina in the original description, and without examining the type material. Ortea et al. (2005) asserted that Marcus (1980) had mixed two species in her type series for E. patina , but we examined the holotype (a mounted series of thin sections) and found no evidence of multiple species; Marcus (1980) clearly states that her diagnosis of E. patina was based on a single specimen from Florida. A specimen from the Bahamas (fig. 59-60 in Marcus 1980) was noted in the text as a different species, distinct from E. patina , but was confusingly labeled as E. patina in the legend to figures 59 and 60 in Marcus (1980). This may have led Ortea et al. (2005) to conclude that two different species were mixed in the description of E. patina , but there is no evidence of that in the type series.
Specimens of E. patina can be superficially similar to whitish specimens of E. papillosa and E. taino n. sp., both in external appearance and in their pattern of dorsal vessel venation, but a number of characters differentiate these species. First, E. patina has a curved finely denticulate radular tooth — the “ Halimeda spur” of DeFreese & Clark (1987) — whereas E. papillosa and E. taino n. sp. both have a coarsely serrulated, blade-shaped tooth. Second, the gametic vesicles of E. patina are located at the posterior end of the renopericardium, often contacted by terminal branches of the vessels; in contrast, the gametic vesicles of E. papillosa and E. taino n. sp. are found midway along the renopericardium, lying between the primary vessels. Third, the coloration of E. patina consistently grades from dark brown to yellow to white moving from the foot up to the parapodial margin, in contrast to the more variable coloration and especially the irridescent speckling inside the parapodia of E. papillosa . E. patina also lacks red spots on, or red lines crossing, the parapodial margins, whereas red spots or bands commonly occur on specimens of E. taino n. sp.
Host use further distinguishes E. patina from all related species: among the udotacean specialists comprising subclade 1, only E. patina feeds on H. opuntia , while similar species feed on Penicillus ( E. papillosa and E. taino n. sp.) or Udotea ( E. zuleicae , E. buonoi n. sp.). Finally, developmental characters also distinguish E. patina from morphologically similar elysiids in subclade 1: only E. patina produces egg masses containing a flat ribbon of orange ECY, from which hatch swimming lecithotrophic larvae of exceptionally large size. Although E. velutinus sometimes feeds on H. opuntia and produces lecithotrophic clutches containing orange ECY, external and radular morphology clearly differentiate E. velutinus from E. patina , and the species are not closely related.
A similar curved radular tooth morphology is shared by E. patina , E. zuleicae and E. buonoi n. sp. However, the dorsal vessel pattern and tail of E. zuleicae differentiate this species from E. patina , as do host use and the production of white ECY, and the shape of the penial stylet. These species were confused in the literature by Jensen & Clark (1983) and Clark (1994), who reported details on E. zuleicae under the name E. patina before E. zuleicae was described.
| LACM |
Natural History Museum of Los Angeles County |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Elysia patina Ev. Marcus, 1980
| Krug, Patrick J., Vendetti, Jann E. & Valdés, Ángel 2016 |
Elysia patina
| Krug 2015: 990 |
| Clark 1994: 905 |
| Jensen 1983: 5 |
| Ev 1980: 72 |