Elysia zuleicae Ortea & Espinosa, 2002
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4148.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:91353147-FDA8-45CC-A8F1-1DE801C835A6 |
|
DOI |
https://doi.org/10.5281/zenodo.5664213 |
|
persistent identifier |
https://treatment.plazi.org/id/A04A7E6D-9C1A-FFD8-46C9-FADEFA441A09 |
|
treatment provided by |
Plazi |
|
scientific name |
Elysia zuleicae Ortea & Espinosa, 2002 |
| status |
|
Elysia zuleicae Ortea & Espinosa, 2002 View in CoL
( Figs. 6 View FIGURE 6 S, 51–53)
Elysia zuleicae Ortea & Espinosa 2002: 133 View in CoL –139, figs. 3–7; pl. 1, fig. B (Type locality: Marina Hemingway View in CoL , Cuba) — Valdés et al. 2006: 70–71; Händeler et al. 2009: figs. 6, 7; Krug 2009: 361-365, fig. 3B, 4B, 5A,B, 6; Redfern 2013: 286, figs. 792A–E; Ortigosa et al. 2013: 66; Christa et al. 2014: figs. 1, 3; Krug et al. 2015: 990-991, figs. 3B, 4.
Elysia papillosa View in CoL [non Verrill 1901] — Clark 1984: 89–90 [part], figs. 16, 18–20 [non Clark 1984: figs. 15, 17]; Clark 1994: 905
Elysia patina [non Ev. Marcus 1980] — Jensen & Clark 1983; Clark 1994: 905; Jensen 1986: figs. 1,3–4.
Elysia leeanneae Caballer, Ortea & Espinosa in Ortea, Espinosa, Buske & Caballer 2013: 188 View in CoL –190, pl. 11, fig. F–H; pl. 13 (Type locality: Petit Cul-de-Sac Marin, Guadeloupe) n. syn.
Type material. Elysia zuleicae —holotype at IOH, paratypes at MCNT and MZUCR (no registration numbers given); Elysia leeanneae— holotype (MNHN 26978).
Material examined. Great Lameshur Bay , U.S. Virgin Islands , 2014, 1 specimen ( LACM 178662 View Materials ) ; Union Island, Chatham Bay , St. Vincent and the Grenadines, 1987, 2 specimens ( LACM 178660–61 View Materials ) ; Bahamas: Abaco Islands, 2003, 1 specimen ( LACM 178656 View Materials ), Sweetings Cay , July 2007, 1 specimen (LACM 178657), San Salvador, July 2007, 1 specimen ( LACM 178658 View Materials ), Plana Cays , July 2007, 1 specimen ( LACM 178659 View Materials ), Stocking Island, Exumas, 16 February 2009, 1 specimen ( CPIC 00089 ) .
Additional material examined. Geiger Beach , Florida, USA, October 2006, 38 specimens (isolate Ezul__06Gei01-38) ; Bocas del Toro , Panama, December 2004, 51 specimens (isolate Ezul_04Pan01-51); Discovery Bay , Jamaica, 0 7 March 2006, 31 specimens (isolate Ezul_06Jam01-31) ; Bermuda, June 2006, 48 specimens (isolate Ezul_06Ber01-48); Piscadera Bay , Curaçao, 5 January 2009, 12 specimens (isolate Ezul_09Cur01-12), 7 January 2009, 13 specimens (isolate Ezul_09Cur13-25) ; Bahamas: Stirrup Cay, July 2007, 14 specimens (isolate Ezul_07Stir01-14), Sweetings Cay , July 2007, 34 specimens (isolate Ezul_07Swe01-34), July 2010, 25 specimens (isolate Ezul_10Swe01-25), Little San Salvador , July 2007, 22 specimens (isolate Ezul_07LSS01-22), July 2010, 18 specimens (isolate Ezul_10LSS01-18), San Salvador , July 2007, 27 specimens (isolate Ezul_07Ssal01-27), Plana Cays, July 2007, 28 specimens (isolate Ezul_07Plana01-28), Northern Exumas , July 2010, 21 specimens (isolate Ezul_10NEx01-21), New Providence, July 2010, 1 specimen (isolate E_cf_zul10NPr01), Bimini , July 2010, 14 specimens (isolate Ezul_10Bim01-14).
Live animal. Specimens swim readily by undulating their parapodia when disturbed. Slugs mate by hypodermic insemination (sometimes in groups), using a long, highly flexible penis that can extend out for half the body length. Slugs were observed to react violently to touch of a penis, and executed escape maneuvers including back-flips to avoid insemination during bouts of group mating. Juveniles hold parapodia flat against Udotea , and have notably darker coloration than adults.
External anatomy. Coloration variable but generally olive to dark green body, parapodia and head, with variously colored marginal bands along parapodia ( Fig. 51 View FIGURE 51 A–C, E–H). Head light to dark green, sometimes with red, brown or rust-colored patches. Rhinophores long, rolled, colored white to brown-purple along whole length; with scattered, rounded white papillae. White patches of pigmentation concentrated at distal end of rhinophores, which are squared-off and uncurled at tip. Eyes large, usually not surrounded by pigment. White or brown patch sometimes bisecting head along midline between (and sometimes overlying) eyes ( Fig. 51 View FIGURE 51 A, C, E–H). In many specimens, pronounced, narrow tail extending out a few mm beyond end of parapodia, which do not cleanly delimit posterior end of body. Some specimens without a tail at all. Color and pattern of tail usually matching that of parapodial margin. Foot continuous with parapodia, same in coloration, with thin transverse groove separating head ( Fig. 51 View FIGURE 51 D). Light orange streaks on head and parapodia of some specimens.
Parapodia thin, sometimes with slightly undulating edge but not forming siphonal openings. Outer parapodial surface predominantly green with scattered white specks and low, small white papillae. Row of larger white papillae running along parapodial margin, papillae sometimes forming clusters of 3 or 5 with middle papilla being the tallest, creating alternating “crowns” rising and falling along margin ( Fig. 51 View FIGURE 51 C, F–G). Color of margin highly variable, ranging from white to brown. Large adults sometimes with thick, light purple marginal band with submarginal bands of white to brown ( Fig. 51 View FIGURE 51 E); some specimens (especially juveniles) with thin black marginal line surrounded by thicker white submarginal band ( Fig. 51 View FIGURE 51 H). Inside parapodia, dorsum green with scattered brown flecks and iridescent blue to white speckles. Juveniles (<4 mm) tend to lay parapodia completely flat ( Fig. 51 View FIGURE 51 G). Parapodial margin dominated by individual white papillae separated by black marginal line, creating appearance of alternating white peaks and black bars. Juveniles often with proximal pigment patches of black at base of rhinophores, with distal portion white.
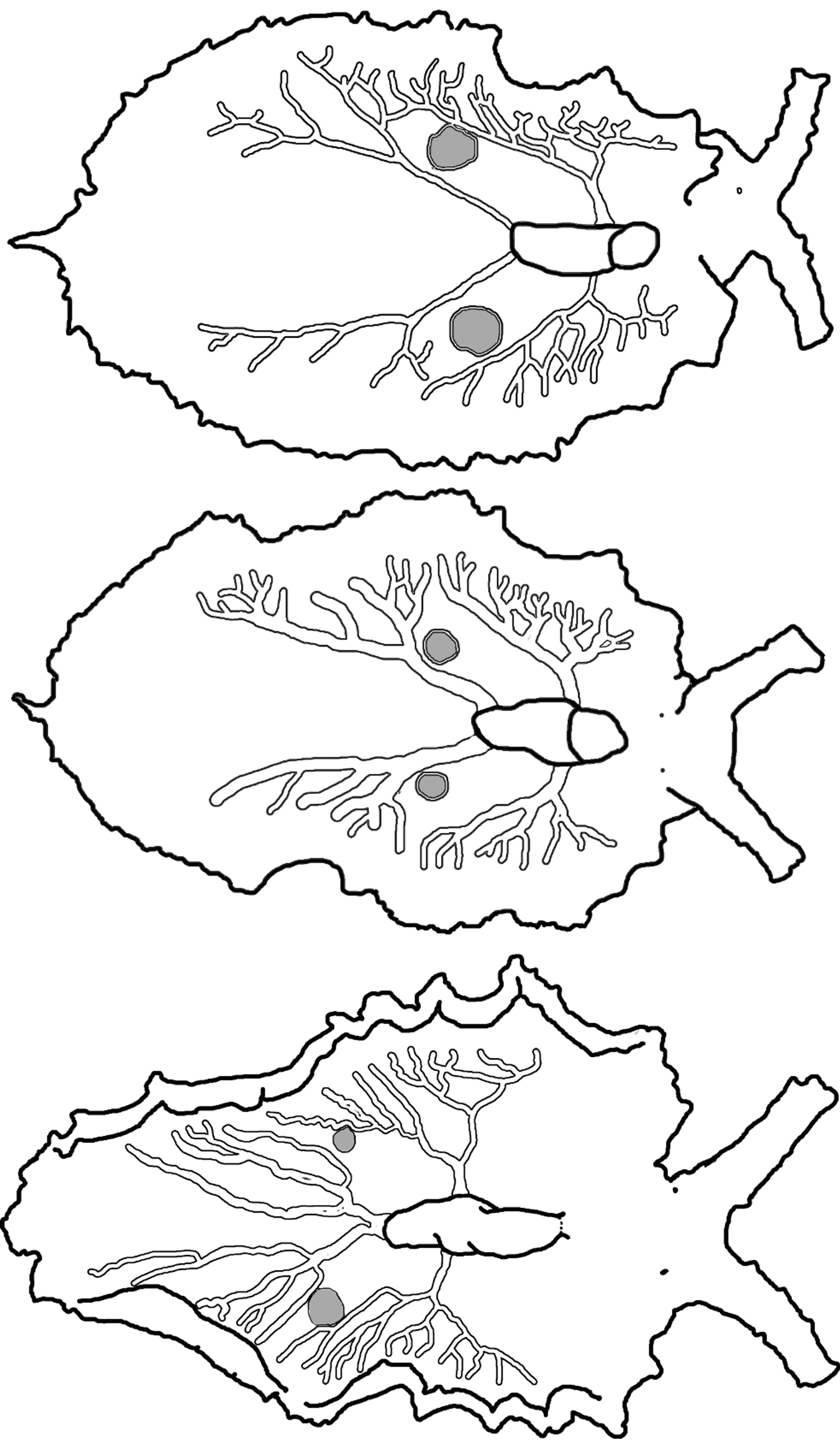
Pericardium small and rounded, with color ranging from white to black but usually white with scattered tiny black or brown speckles. Renopericardial extension wider than pericardium but similar in color, extending about one quarter of body length ( Fig. 51 View FIGURE 51 E–F). Dorsal vessels white, sometimes outlined in iridescent blue. All examined specimens had two paired vessels per side, one pair emerging from renopericardial extension just posterior to pericardial bulge, and a posterior pair extending from corners of squared-off end of renopericardial extension ( Fig. 52 View FIGURE 52 ). Anterior vessels branch immediately, then fork or send out lateral branches at irregular intervals, rarely anastomosing. Posterior vessels extend to about ¾ of body length, with roughly symmetrical pattern of lateral side branches, which sometimes fork 1–2 times while extending towards parapodial margin. Vessels variable in thickness among specimens.
One pair of large sperm-storage vesicles typical present as roughly spherical greyish protrusions ( Fig. 51 View FIGURE 51 E, 52). On all specimens, vesicles positioned between posterior-most branch of 1st pair of vessels, and anterior-most branch of 2nd (posterior) pair of vessels. Vesicles sometimes bordered on one side, but not surrounded, by dorsal vessel branches.
Internal anatomy. Radula with 20–34 teeth (CPIC 0 0 0 89, LACM 178659), 7–12 teeth in ascending limb and 13–22 in descending limb ( Fig. 53 View FIGURE 53 A). Leading tooth elongate and narrow with a curved cusp bearing approximately 45–60 sharp and minute denticles ( Fig. 53 View FIGURE 53 B). Teeth without typical “V”-shaped housing depression of many other elysiids. Instead, teeth overlapping with ½–¾ of tooth cusp resting on lateral edge of tooth base on adjacent tooth ( Fig. 53 View FIGURE 53 A). Base of tooth tall and approximately ½ total tooth length. Ascus either an ordered series of small teeth in sequence ( Fig. 53 View FIGURE 53 A) or a small jumbled heap of discarded teeth.
Penis wide and elongate tapering into a conical apex (CPIC 0 0 0 89, LACM 178660, LACM 178662), bearing a pointed and scoop-shaped stylet ( Fig. 6 View FIGURE 6 S, Fig. 53 View FIGURE 53 C–D). Deferent duct long and convoluted.
Reproduction and development. Mating behavior was observed for specimens from the Bahamas, and conformed to the description given by Jensen (1986) for specimens identified as E. patina . Three specimens performed a prolonged mating ritual that involved jostling for position and probing with the tip of the extended, highly flexible penis to act as a sperm donor, but avoiding contact from the penises of the other two specimens. Contact between a penis and the exterior of the parapodium or foot caused the intended sperm recipient to recoil violently; one specimen did a back-flip to avoid being hypodermically inseminated. Jensen (1986) reported that only contact near the sperm vesicles on the inner parapodial surface would be accepted without triggering a recoil response. The male opening is under the right rhinophore, and the female opening is near the anus in the groove separating the right anterior parapodial edge from the head.
Development is predominantly planktotrophic, but specimens from some Bahamas sites (Sweetings Cay, San Salvador Is., Plana Cays) produced lecithotrophic larvae (Krug 2009). DNA sequencing of the COI gene from lecithotrophic larvae confirmed conspecificity with planktotrophic E. zuleicae (Trathen 2010, and unpublished data). Both developmental morphs produced egg masses containing a ribbon of white ECY. In planktotrophic egg masses ( Fig. 51 View FIGURE 51 I), the ECY ribbon is thin and meanders among the egg capsules, similar to the egg mass of E. patina . In lecithotrophic egg masses, the ECY ribbon is thicker and wider, and tends to cover the upper face of the egg mass, more similar to the ECY ribbon of E. papillosa but white ( Fig. 51 View FIGURE 51 J–K). No other lecithotrophic elysiid from the Caribbean produces a white ECY ribbon.
Mean egg diameter was 67.6 µm (± 2.3 SD, n = 23 ova) for a planktotrophic clutch from Little San Salvador, Bahamas, and 64.5 µm (± 1.9 SD, n = 28 ova) for a clutch from Jamaica; grand mean diameter of planktotrophic eggs was thus 66.1 µm ± 2.3 SD. Planktotrophic larvae developed in 5.7 d (± 0.7 SE, n = 3 clutches), and mean shell length at hatching was 109.5 µm (± 5.1 SD, n = 80) for a clutch from Little San Salvador.
Lecithotrophic clutches contained 104.0 eggs (± 10.4 SE, n = 4 clutches). Lecithotrophic egg diameter was not determined, as clutches were collected after cleavage had begun. Grand mean shell length at hatching for lecithotrophs was 253.9 µm (± 8.9, n = 4 clutches), ranging from 244.6 µm (± 7.4, n = 21) for a clutch from Plana Cays, to 265.3 µm (± 8.9, n = 27) for a clutch from Sweetings Cay. Larval shell sizes previously reported for the lecithotrophic morph of E. zuleicae were plotted on the wrong Y-axis (Krug 2009: fig. 4B). Lecithotrophic larvae developed in 18.5 d (± 0.5, n = 2 clutches) and then hatched over an additional 4–5 d. No intracapsular metamorphosis occurred in four clutches. Significant metamorphosis was induced only by the adult host Udotea flabellum , which triggered about half of larvae to metamorphose; no larvae metamorphosed in the presence of P. capitatus , and fewer than 10% settled in response to Caulerpa verticillata or FSW only (Krug 2009).
Host ecology. Field surveys and laboratory observations indicate that E. zuleicae specializes on the green algal genus Udotea , particularly U. flabellum , the host identified in the species description (Ortea & Espinosa 2002). In field surveys by PJK of 15 sites over a decade,> 400 specimens were collected from Udotea . Juvenile specimens (<5 mm) in particular have only been collected in association with U. flabellum , and both juvenile and adult slugs were observed to feed readily and exclusively on U. flabellum in the laboratory. Elysia zuleicae is generally less abundant than E. papillosa ; a 500 g collection of Udotea (wet weight) yielded only 9 specimens in Jamaica. However, E. zuleicae is far more common and widespread than the related, morphologically similar species E. buonoi n. sp., of which only four specimens were collected from Udotea at a single location (San Salvador Island, Bahamas), two in 2004 and two more in 2007.
Phylogenetic relationships. Elysia zuleicae and its sister species E. buonoi n. sp. were recovered within subclade 1, a lineage of Caribbean elysiids feeding on udotacean algae ( Fig. 4 View FIGURE 4 ). The morphologically similar E. buonoi n. sp. was genetically distinct at the COI locus (minimum distance: 10.2%) and fixed for different alleles at the nuclear H3 locus where the two species co-occurred on San Salvador Island, Bahamas (Trathen 2010; unpublished data); COI divergence, ABGD analysis, and fixed allelic differences at H3 all support treatment of Elysia zuleicae and E. buonoi n. sp. as distinct.
Range. Bahamas (Redfern 2013), Bermuda (present study), Costa Rica (Valdés et al. 2006), Cuba (Ortea & Espinosa 2002), Curaçao (present study), Florida , USA (present study), Jamaica (Valdés et al. 2006), Mexico (Ortigosa et al. 2013), Bocas del Toro , Panama (present study), U.S. Virgin Islands (present study), Union Island , St. Vincent and the Grenadines (present study).
Remarks. Elysia zuleicae was widely confused with E. papillosa and E. patina prior to its description by Ortea & Espinosa (2002). Clark (1984: figs. 16, 18–20) drew E. zuleicae as E. papillosa but noted that his papillosa probably represented a species complex. Contributing to the confusion, radular tooth morphology is highly similar among E. zuleicae , E. buonoi n. sp., and E. patina , and E. zuleicae is common at the type locality of E. patina . Thus, Jensen & Clark (1983) and Clark (1994) identified the host of E. patina as Udotea , which is not correct; E. patina feeds on Halimeda opuntia , but both studies actually focused on E. zuleicae under the name E. patina . Similarly, Jensen (1986) described the mating behavior and reproductive anatomy of “ E. patina ” specimens that were E. zuleicae , having been collected from Udotea and matching E. zuleicae (undescribed at the time) in all respects. The discrepancy in the location of the male opening noted by Jensen (1986) further confirms that her “ E. patina ” was not E. patina Ev. Marcus 1980 , but rather was E. zuleicae .
Ortea & Espinosa (2002) made no mention of penial morphology in the original description of E. zuleicae . Ortea et al. (2005) subsequently stated E. zuleicae lacked a penial stylet, and claimed that the presence of a stylet was a major character difference supporting their new species E. deborahae . However, a penial stylet was present on most specimens of E. zuleicae examined in the present study, as noted by Jensen (1986), and was even visible at low magnification on living specimens. Specimens of E. zuleicae flinch in response to contact by a penis, and execute escape behaviors consistent with traumatic mating by hypodermic insemination (Lange et al. 2013). One specimen of E. zuleicae (confirmed genetically by sequencing two genes) lacked a stylet, suggesting stylets may sometimes be torn out by the violent “escape” movements that occur during mating. Thus, Ortea et al. (2005) may have examined a specimen that lacked its stylet, but a stylet is unambiguously present in E. zuleicae .
The pointed, elongated, black tail of E. zuleicae is a variable character that, when present, distinguishes E. zuleicae from similar Caribbean species. However, the extent to which a tail is present varies within E. zuleicae , especially among juveniles; we misidentified some juvenile E. zuleicae as E. papillosa in three populations due to the lack of an apparent tail, only uncovering the correct identity of these specimens through DNA barcoding. Moreover, half the preserved specimens confirmed genetically as E. zuleicae from U.S. Virgin Islands lacked any tail, whereas half had the typical elongated tail.
Small specimens of E. zuleicae , especially if pale from lack of recent feeding activity, may be confused with small E. papillosa , E. taino n. sp., or E. patina . However, E. zuleicae is distinct from all three species in its pattern of dorsal vessels, and the shape of its penial stylet. Adult specimens of E. zuleicae have only two pairs of dorsal vessels on each side of the renopericardium, in contrast to the greater number seen in equivalently sized specimens of E. patina , E. papillosa or E. taino n. sp. Radular tooth morphology distinguishes E. zuleicae from E. papillosa and E. taino n. sp.
Like the related species in this clade ( E. patina , E. papillosa , E. taino n. sp.), E. zuleicae swims when disturbed, and has gametic vesicles that swell with sperm following insemination. The position of gametic vesicles is distinctive for E. zuleicae : vesicles are located between the primary forks of the posterior dorsal vessel on each side of the body. In contrast, sperm vesicles are located midway along the renopericardial extension in E. papillosa , while those of E. patina are found posterior to the end of the renopericardial extension, surrounded by the terminal branches of the posterior-most vessels. Ecologically, these three species also feed on different host algae.
The recently described E. leeanneae Caballer, Ortea & Espinosa in Ortea, Espinosa, Buske & Caballer, 2013 is highly similar to E. zuleicae , only differentiated by (a) overall white body coloration, (b) a jumbled ascus, and (c) fewer teeth: 10 in the ascending limb and 19 in the descending limb for E. leeanneae , versus a reported 24–25 teeth in the descending limb of E. zuleicae . However, specimens of E. zuleicae can be very white (e.g., Fig. 51 View FIGURE 51 F), and vary considerably in the degree to which parapodial margins are papillose or ruffled. Two of four specimens of E. zuleicae examined in the present study had a disorganized (or jumbled) ascus, while the teeth of ascus were organized in the other two specimens. One specimen had only 22 teeth ( Fig. 53 View FIGURE 53 A). Thus, radular characters do not clearly differentiate E. leeanneae from E. zuleicae . Although the external appearance of the holotype of E. leeanneae looks different from most E. zuleicae , given the intra-specific variability of the latter species, we consider E. leeanneae a junior synonym of E. zuleicae due to their identical dorsal vessel patterns and radular teeth.
| LACM |
Natural History Museum of Los Angeles County |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Elysia zuleicae Ortea & Espinosa, 2002
| Krug, Patrick J., Vendetti, Jann E. & Valdés, Ángel 2016 |
Elysia leeanneae
| Caballer, Ortea & Espinosa in Ortea, Espinosa, Buske & Caballer 2013: 188 |
Elysia zuleicae
| Ortea & Espinosa 2002: 133 |