Elysia pawliki, Krug, Patrick J., Vendetti, Jann E. & Valdés, Ángel, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4148.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:91353147-FDA8-45CC-A8F1-1DE801C835A6 |
|
DOI |
https://doi.org/10.5281/zenodo.5664225 |
|
persistent identifier |
https://treatment.plazi.org/id/A04A7E6D-9C2D-FFE4-46C9-FD38FDBF1FFC |
|
treatment provided by |
Plazi |
|
scientific name |
Elysia pawliki |
| status |
sp. nov. |
Elysia pawliki View in CoL new species
( Figs. 56 View FIGURE 56 D, 58–60)
Elysia papillosa [non Verrill, 1901] — Thompson 1977: figs. 26, 27; Espinosa & Ortea 2001: 44; Espinosa et al. 2005: 56; Ortea et al. 2005: 498–502 (part), figs. 1–2, pl. 1, fig. A; Redfern 2001: 162, figs. 672A–F; Collin et al. 2005: 690; Valdés et al. 2006: 65.
Elysia sp. A — Redfern 2013: 286–287, figs. 793A–B.
Elysia cf. tomentosa sp.2 — Krug et al. 2013: 1109-1113, figs. 2C, 4; Krug et al. 2015: 990, fig. 3B.
Type material. Sweetings Cay , Bahamas, 2003, (Holotype LACM 3303 View Materials ), collected by PJK ; Abaco Islands, Bahamas, 2003, (Paratype LACM 3304 View Materials ), collected by Colin Redfern .
Type locality. Sweetings Cay , Bahamas
Material examined. Bahamas: Abaco Islands, 2003, 2 specimens (Paratype LACM 3304 View Materials , LACM 178674 View Materials ) ; Sweetings Cay , 2003, 1 specimen, 35 mm long alive (isolate Epaw_03Swe01, Fig. 58 View FIGURE 58 A); July 2007, 1 specimen, 25 mm long alive (Holotype LACM 3303 View Materials , isolate Epaw_07Swe01, Fig. 58 View FIGURE 58 B–E).
Live animal. Both Sweetings Cay specimens were collected four years apart in the same tidal channel, which opens to a mangrove lagoon. Slugs were collected off a large clump of Caulerpa racemosa . Resting slugs held their parapodia apart, forming a series of siphonal openings ( Fig. 58 View FIGURE 58 A–B). One specimen was observed to associate, and potentially attempt to mate with, a large E. subornata when the two were held together in a container. Slugs often rested with their head tucked inside their expansive parapodial flaps. Both specimens from Sweetings Cay, Bahamas were observed in the laboratory for 3–4 weeks after collection; neither slug was ever observed to swim or flap its parapodia when disturbed. When stressed, slug released a cloud of iridescent blue-white mucus from the parapodial margin, and contorted its parapodia, but did not swim.
External anatomy. Overall coloration yellow-green with patches of brown. Body turning dark green after feeding, due to digestive diverticula ramifying throughout body, head and parapodia, visible through epidermis at low density. Overall body shape dominated by large parapodia with series of three laterally extended side-flaps folding away from body ( Fig. 58 View FIGURE 58 A–B). When parapodia close over dorsum, flaps create three siphonal openings, the middle being the largest. Anterior-most opening small, representing little more than a fold over pericardium. When held open, middle parapodial flaps extending out roughly as wide, tip to tip, as body length from pericardium to tail. Elongated middle flaps forming largest opening when parapodia are closed, giving live animal a cruciform appearance. Third, posterior-most pair of parapodial flaps intermediate in size. Larger specimens with 2–3 additional siphonal openings present along posterior half of body, including a posterior pair of laterally extended parapodial flaps; examples include isolate Epaw_03Swe01 ( Fig. 58 View FIGURE 58 A), and a specimen from Jamaica in the BMHN labeled “ E. papillosa ” by T. E. Thompson, 30 mm long. Exterior surface of parapodia covered by elongate, white papillae rising from tan-brown patches of pigment ( Fig. 58 View FIGURE 58 C). Upper portion of parapodia greywhite inside and out, with occasional blotches of plum color; one large plum-color patch appearing on anteriormost parapodial flap near margin. Brown band running along inner and outer edge of parapodial margin. Row of papillae extending straight up from edge, running to end of parapodia; marginal papillae grey-brown tipped with white. Interior of parapodia featuring white patches, pink at center, scattered about.
Outer surface of body heavily papillose. Irregular, white patches like lichen covering head and upper portion of parapodia; long, conical papillae rising out of these white patches, spotted with irregular small patches of pink. Glands appearing as scattered brown spots cover sides of parapodia, head and rhinophores; black-edged openings slightly elevated above surface of epidermis, with brown spherical inclusion (gland) lying beneath, discharging heavy mucus secretion when animal is alarmed.
Front of head covered by large patches of tan, white and pink, over yellow-green background color. Eyes tiny, located in patch of background coloration posterior to base of rhinophores. Upper lip split into two curved sections; lower lip flattened, broader. Diverticula extend into both top and bottom lips. Upper lip with moustache of brown spots. Rhinophores short relative to body length (3 mm at maximum extension on 25 mm animal); rolled, bluntended, with long white papillae. Surface of rhinophores white-brown, dotted with dark brown spots (glands) and pink spots of equal size, and occasional irregular white patches. Faint, longitudinal white stripes run tip to base. At base, inner surface of rhinophores penetrated by green digestive diverticula, but outer surface white and devoid of diverticula.
Foot with same yellow-green or brown color as rest of body, with rows of minute white papillae but no brown glands or white patches ( Fig. 58 View FIGURE 58 C–D). Transverse groove separating underside of head from foot, opening into wider genital groove on right side of head, with a white genital aperture at the top of the groove. End of foot wide, blunt, not narrowing to a tip; no extended tail.
Pericardium large, rounded, white-pink patch on top, dotted with brown glands. Thick renopericardial extension runs to halfway point of body, between second and third parapodial flaps ( Fig. 58 View FIGURE 58 B, E). Dorsal vessels asymmetric; type specimen with four vessels emerging on left side, five on right side of pericardial complex ( Fig. 59 View FIGURE 59 ). Vessels branch and anastomose forming complex network running up to inner parapodial margin. Main branch of elongated posterior vessel running to tail on each side; posterior vessel otherwise notably asymmetric in placement and branching pattern. Vessels run under, or terminate in, papillae that dot inner parapodial surface.
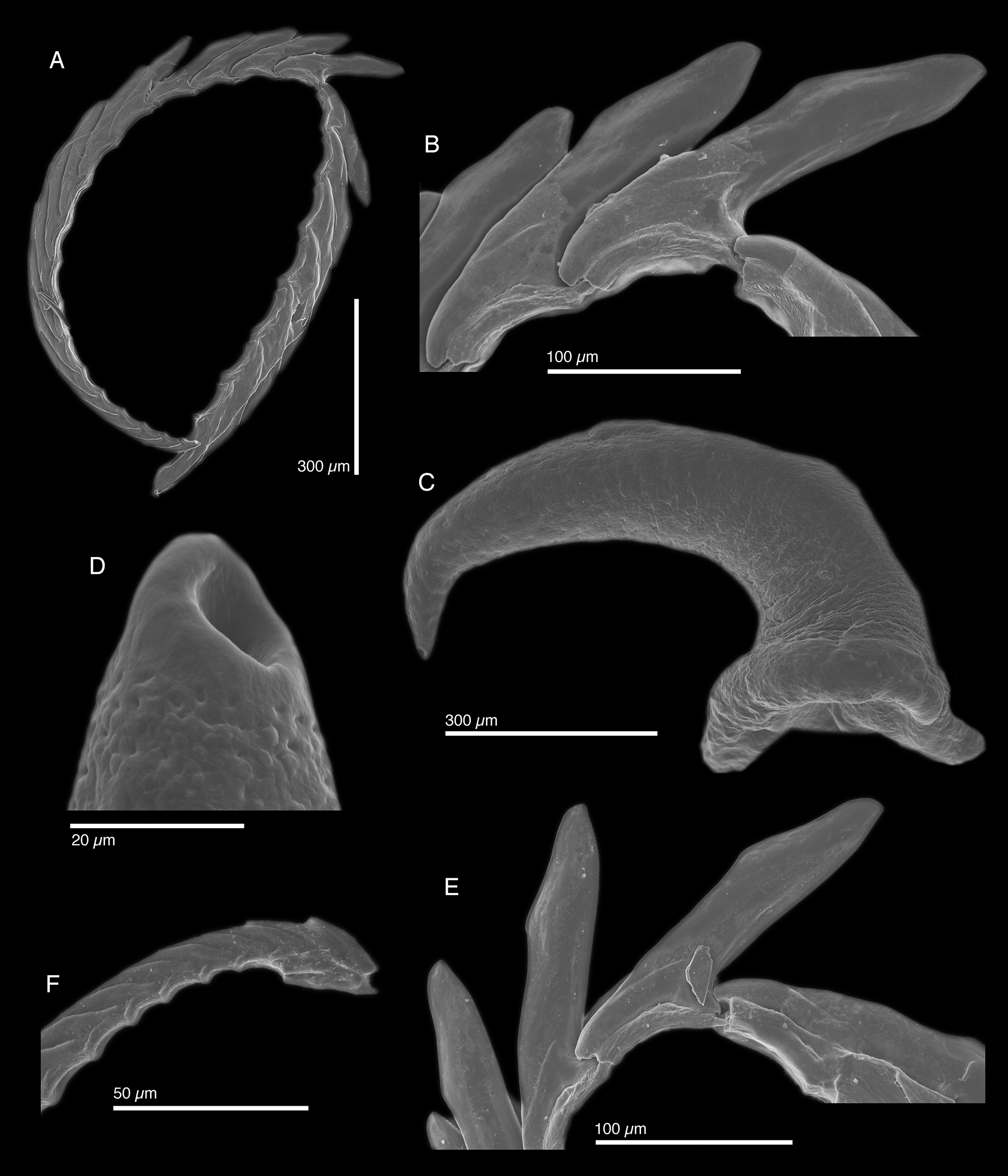
Internal anatomy. Radula with ~24 teeth (LACM 3303, LACM 3304), 6 teeth in ascending limb and ~ 18 in descending limb ( Fig. 60 View FIGURE 60 A). Leading tooth wide and flat, with fine, blunt denticles on cusp, with slightly rounded apex ( Fig. 60 View FIGURE 60 B, E). Housing depression for interlocking teeth “V”-shaped and extending ⅔ of tooth length. Base of tooth approximately ¼ total tooth length. Ascus of small teeth in a single row with some jumbled teeth at the end ( Fig. 60 View FIGURE 60 F).
Penis elongate and curved ( Fig. 60 View FIGURE 60 C) with rigid musculature resistant to desiccation and tapering distally into a conical apex bearing a resistant, hollow tip ( Fig. 60 View FIGURE 60 D). Penial stylet is not a scoop or barb; hardened penial tip visible by SEM, but not light microscopy. Deferent duct long, narrow, and highly convoluted.
Reproduction and development. One egg mass was produced by specimen 03Swe01 ( Fig. 58 View FIGURE 58 F). The egg strand was wound in a typical elysiid spiral on the surface of the bubble-like “grapes” of the host alga C. racemosa .
Egg capsules alternated around a continuous, thick ribbon of bright orange ECY on the upper surface of the egg strand, under the outer covering of the egg mass. The ribbon was molded around each individual capsule. The clutch released swimming, lecithotrophic veliger larvae with eyespots; neither egg nor larval shell size was obtained, however.
Host ecology. Both live specimens of E. pawliki n. sp. were recovered from Caulerpa racemosa , on which both specimens fed readily in the laboratory. Slugs became green upon feeding, but reverted to a brown color after a few days without food. Specimens collected at different times from the Bahamas each had one pair of vivid purple egg masses of a parasitic copepod poking out of the dorsal surface near the pericardium; ( Fig. 58 View FIGURE 58 B, E). Although copepods parasitize a wide range of opisthobranchs, the purple eggs of this unknown copepod species were only observed on the related E. zemi n. sp. and not on any other sacoglossan over the past 12 years, suggesting a specialized relationship.
Phylogenetic relationships. Elysia pawliki n. sp. belongs to the E. tomentosa species complex, together with at least five distinct species from the Indo-Pacific, and three sympatric Caribbean species: E. subornata , E. pratensis and E. zemi n. sp. ( Fig. 4 View FIGURE 4 ). All species in this clade feed on Caulerpa spp., except E. pratensis . We recovered as sister to E. pawliki n. sp. an undescribed, morphologically similar species collected from Caulerpa cupressoides in Australia and an unknown Caulerpa sp. in Thailand ( Elysia cf. tomentosa sp. 5; Krug et al. 2013). Both species are brown, highly papillose, and share a characteristic cruciform body shape due to the wide lateral extension of the parapodia. Molecular data were not available for the morphologically similar species E. manriquei Ortea & Moro, 2009 from the Canary Islands.
Range. Bahamas (Redfern 2001, 2013; present study), Costa Rica (Espinosa & Ortea 2001), Venezuela (Valdés et al. 2006)
Etymology. Named in honor of colleague and “evil twin” Joseph R. Pawlik, in recognition of his landmark achievements studying Caribbean reef ecosystems, and the larval and chemical ecology of sea slugs. Without the opportunity to participate in four research cruises on which Dr. Pawlik was Chief Scientist, the present study would not have been possible, and the holotype specimen would not have been collected.
Remarks. Both Thompson (1977) and Ortea et al. (2005) described material most closely matching E. pawliki n. sp. (but potentially also E. zemi n. sp.) as E. papillosa . However, E. pawliki n. sp. has many features that are incompatible with the details provided by Verrill (1901) in his description of E. papillosa . The only similar feature shared by both species is a highly papillose body surface, but the papillae of E. pawliki n. sp. are notably different from the “small conical papillae” described and figured by Verrill (1901) for E. papillosa . Ortea et al. (2005) claim that Verrill’s illustration shows branching, digitiform papillae, but they misinterpreted Verrill’s drawing (Verrill, 1901: pl. 4, fig 3), which shows the specimen of E. papillosa sitting on a branching stipe of the alga Halimeda incrassatta ; the alga was interpreted as papillae on the slug by Ortea et al. (2005). Further, although Verrill’s type material from the Bermuda expedition was lost, we found among Verrill’s surviving material a preserved specimen of E. pawliki n. sp. marked “ Elysia sp.”; thus, Verrill considered E. pawliki n. sp. to be distinct from his E. papillosa . Moreover, E. pawliki n. sp. has never been reported from the type locality of E. papillosa ( Bermuda) .
A major behavioral difference further confirms that E. pawliki n. sp. is distinct from E. papillosa Verrill 1901 , which was described as swimming freely using its parapodia. Two live specimens of E. pawliki n. sp. were observed over a period of weeks, but never swam no matter the degree to which they were disturbed; thus, E. pawliki n. sp. cannot be E. papillosa Verrill 1901 . At least five Caribbean species swim when disturbed by flattening and rapidly undulating their thin parapodia; all five belong to subclade 1, and are phylogenetically distinct from E. pawliki n. sp.
Morphologically, no named Caribbean species is similar in gross anatomy to E. pawliki n. sp. In E. pawliki n. sp., as in most species belonging to subclade 4, the renopericardial extension runs about halfway down the body, whereas the renopericardial extension of related species E. subornata and E. pratensis runs the whole body length. The most similar Atlantic species is E. manriquei Ortea & Moro 2009 from the Canary Islands off West Africa. In addition to its east Atlantic type locality, E. manriquei differs from E. pawliki n. sp. in its external morphology in having symmetrical and non-anastomosing dorsal vessels, large black spots dotting the entire body and head, shorter rhinophores, and more vertically exaggerated siphonal openings. The radular teeth are also markedly different. The tooth of E. manriquei has a flat, serrated cutting edge, and a curving top edge; in E. pawliki n. sp., both the cutting and non-cutting surfaces taper together towards a rounded tooth tip.
The radular teeth of E. pawliki n. sp. are most similar to those of E. zemi n. sp. in overall morphology, both bearing fine denticles and possessing a sharp tooth tip on a rounded apex, and downward-facing angle at ⅔ the length of the tooth. The teeth of E. pawliki n. sp. are distinct in having straighter tooth cusps and less club-like tooth apices. Like in E. ellenae and E. zemi n. sp. the resistant penial tip in E. pawliki n. sp. is not scoop or barblike, but based on Gascoigne’s (1974) description we refer to it as a stylet.
Developmentally, all members of the E. tomentosa clade studied to date have orange ECY ribbons. However, the lecithotrophic larvae of E. pawliki n. sp. swim upon hatching; thus, development mode (pelagic lecithotrophy) differentiates E. pawliki n. sp. from E. subornata and E. pratensis (non-pelagic lecithotrophy with encapsulated metamorphosis), and from its Pacific sister species E. cf. tomentosa sp. 5, which is planktotrophic (Krug et al. 2013). In terms of host ecology, E. pawliki n. sp. feeds on Caulerpa racemosa , which is also consumed by most related species. The co-occurring E. subornata feeds on several Caulerpa spp. including C. racemosa , but is much more common throughout the Caribbean.
| LACM |
Natural History Museum of Los Angeles County |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.