Elysia crispata Mörch, 1863
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4148.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:91353147-FDA8-45CC-A8F1-1DE801C835A6 |
|
DOI |
https://doi.org/10.5281/zenodo.5664176 |
|
persistent identifier |
https://treatment.plazi.org/id/A04A7E6D-9C59-FFA0-46C9-FC88FEE91C6C |
|
treatment provided by |
Plazi |
|
scientific name |
Elysia crispata Mörch, 1863 |
| status |
|
Elysia crispata Mörch, 1863 View in CoL
( Figs. 4 View FIGURE 4 , 6 View FIGURE 6 B–E, 9–14)
Tridachia ornata [non Elysia ornata ( Swainson, 1840) View in CoL ]— White 1952: 118 –120, text figs. 19–20, pl. 6, fig. 6.
Elysia (Tridachia) crispata Mörch View in CoL aff. Ørsted 1863: 40 (Type Locality: St. Croix).
Elysia crispata Mörch View in CoL aff. Ørsted 1863— Clark 1994: 904; Redfern 2001: 162, figs. 670; Espinosa & Ortea 2001: 44; Collin et al. 2005: 690; Espinosa et al. 2005: 56, fig. 339; Valdés et al. 2006: 62 –63; Redfern 2013: 282 –283, figs. 784A–C; Zamora-Silva & Ortigosa 2012: 366, fig. 2H.; Händeler et al. 2009: figs. 6, 7; Krug 2009: 362–365, figs. 4D, 6; Christa et al. 2014: fig. 3; Krug et al. 2015: 990 –991, figs. 3B, 4.
Tridachia crispata View in CoL (Mörch aff. Ørsted, 1863)— Engel 1927: 115, fig. 32; Ev. Marcus & Er. Marcus 1960: 153 –159, figs. 36–43; Ev. Marcus & Er. Marcus 1962: 461 –463, figs. 7–8; Ev. Marcus & Er. Marcus 1967: 33 –34, text fig. 38, pl. 1, figs. 7–8; Ev. Marcus & Er. Marcus 1963: 23 –24; Er. Marcus & Ev. Marcus 1970: 50; Bandel 1976: 96 –97, fig. 9 (egg mass); Ev. Marcus 1976b: 128 –129; Ev. Marcus & Hughes 1974: 509, figs. 21–22; Thompson 1977: 131 –132, figs. 22f, 28a–b; Ev. Marcus 1980: 75; Jensen & Clark 1983: 6, fig. 2A; Hess et al. 1994: 161 –162, figs. 9.5–9.6.
Elysia (Tridachia) crispata var. schiadura Mörch 1863: 40 View in CoL –41 (Type locality: St. Croix).
Elysia schrammi Ørsted & Mörch in Mörch 1863: 41 View in CoL (Type locality: Guadeloupe)—Er. Marcus 1957: 415–416.
Tridachia whiteae Er. Marcus 1957: 416 (Type locality: Dry Tortugas)—introduced for Tridachia ornata sensu White (1952) [non Swainson (1840)].
Elysia clarki Pierce et al. 2006: 26 View in CoL –36, figs. 1B, 1D, 2, 4A, 5A, 5C, 5E, 5G, 6A–B, 7 (Type locality: Eastern end of Vaca Key, Florida Keys, USA) n. syn.; Curtis et al. 2006: 340–343, figs. 3–6; Curtis et al. 2010: 299–302, figs. 1A, 1B, 2A, 3; Middlebrooks et al. 2011, 2014; Christa et al. 2014: fig. 1E; Curtis et al. 2015: 27, fig. 1
Type material. Elysia crispata— 3 syntypes from St. Croix ( ZMUC GAS- 1584 ) ; Elysia crispata var. schiadura— 1 syntype from St. Croix ( ZMUC GAS- 1572 ) ; Tridachia schrammi— 4 syntypes from Guadeloupe ( MHNH).
Material examined. A total of 189 specimens examined morphologically by PJK, 155 of which were also sequenced for the mitochondrial COI and nuclear H3 loci. Of these specimens, those with LACM specimen numbers range from LACM 178584–96.
Bocas del Toro, Panama, 19 February 2004, 1 specimen ( LACM 2004-5.1 ) ; New Providence , Bahamas, July 2010, 3 specimens ( LACM 178588–89 View Materials , LACM 178641 View Materials ) ; Discovery Bay, Jamaica, 7 March 2006, 5 specimens ( LACM 178591–92 View Materials , LACM 178636–37 View Materials , LACM 178640 View Materials ) ; Florida, USA: Geiger Beach , August 2007, 2 specimens ( LACM 178590 View Materials , LACM 178596 View Materials ), Dry Tortugas National Park, 2010, 2 specimens ( LACM 178593–94 View Materials ), Mote Marine Laboratory and Aquarium , June 2007, 2 specimens ( LACM 178587 View Materials , LACM 178635 View Materials ), Lake Surprise Inlet , Key Largo, 26 October 2009, 2 specimens ( LACM 178595 View Materials , LACM 178638 View Materials ) , November 2010, 4 specimens (LACM 178584–86, LACM 178639).
Additional material examined. Bocas del Toro , Panama, December 2004, 17 specimens (Ecri_04Pan01-17) ; Bahamas: New Providence , July 2010, 17 specimens (isolate Ecri_10NPr01-07, isolate Ecri_10NPr10-20), Sweetings Cay, July 2007, 20 specimens (isolate Ecri_07Swe01-20) , Little San Salvador, July 2007, 45 specimens (isolate Ecri_07LSS01-45) , San Salvador, July 2010, 6 specimens (isolate Ecri_10SSal01-06), Compass Cay, July 2010, 1 specimen (isolate Ecri_10Comp01) , Northern Exumas, July 2010, 3 specimens (isolate Ecri_10NEx01-03) , Bimini, July 2010, 14 specimens (isolate Ecri_10Bim01-14); Discovery Bay , Jamaica, 7 March 2006, 16 specimens (isolate Ecri_06Jam04, isolate Ecri_06Jam08-23) ; Florida, USA: Geiger Beach, August 2007, 9 specimens (isolate Ecri_07Gei03-11), Dry Tortugas National Park, 2010, 16 specimens (isolate Ecri_10Dry03-18), Mote Marine Laboratory and Aquarium, June 2007, 1 specimen (isolate Ecri_07Mote02), Lake Surprise Inlet , Key Largo, 26 October 2009, 8 specimens (isolate Ecri_09LKS04-11), November 2010, 1 specimen (isolate Ecri_10LKS03); English Harbor , Antigua, Antigua and Barbuda, 25 April 2008, 16 specimens (isolate Ecri_08Ant01-16) ; Dominica, 2007, 26 specimens (isolate Ecri_07Dom01-26); Son Friere Bay , St. Lucia, 27 March 2008, 12 specimens (isolate Ecri_08 STL 01-12 View Materials ); Spanish Waters inlet , Curaçao, 8 January 2009, 7 specimens (isolate Ecri_09Cur01-07) ; Bonaire, May 2012, 1 specimen (Ecri_012Bon01).
Live animal. Elysia crispata is both the largest Caribbean elysiid, and the only species not commonly associated with a particular host alga. Most specimens in the present study were found resting or crawling on hard substrata, although some specimens were collected on Halimeda incrasatta (Key West, FL), Penicillus capitatus ( Curaçao) , and Bryopsis plumosa ( Jamaica, Dry Tortugas). Lighter morphs with white foot are associated with areas of fast water flow and high light (e.g., tidal surge channels, patch reefs), whereas darker morphs are found in shaded areas of low light and reduced flow (e.g., rocky banks in mangrove lagoons, coastal borrow pits). The frilled, undulating parapodia usually cover the dorsum. A few specimens were found ovipositing in the field on upright algal thalli ( Avrainvillea , Key Largo, FL; Penicillus , Curaçao; Udotea , Sweeting’s Cay, Bahamas).
External anatomy. Large-bodied Elysia with highly variable external coloration, ranging from predominantly creamy white with green patches between large white spots ( Fig. 9 View FIGURE 9 A–C), to dark green ( Fig. 9 View FIGURE 9 D) or in the ‘ clarki’ morph purple with white spotting to entirely blue ( Fig. 9 View FIGURE 9 E). Dorsal surface between parapodia also highly variable in color, generally green with pale cream ( Fig. 9 View FIGURE 9 A–B) to white spots ( Fig. 9 View FIGURE 9 C) varying in size and number, to uniformly green ( Fig. 9 View FIGURE 9 D). Foot also highly variable in color, ranging from green with small pale spots ( Fig. 9 View FIGURE 9 I, 9L) or large white spots ( Fig. 9 View FIGURE 9 J) on specimens from lower light environments, to uniformly pale cream with no spots on specimens from high-light, high-flow habitats ( Fig. 9 View FIGURE 9 K). Head relatively small for body size; ground color green, with scattered large or small white spots, and/or iridescent blue pigment specks. Rhinophores short and wide, having same color pattern as rest of dorsum or lighter and lacking spots.
Parapodia undulating to varying degrees, often correlated with overall coloration and microhabitat. In typical specimens with lighter coloration from brightly lit, high-flow environments, undulations numerous and highly convoluted, resulting in parapodia with large surface area that cover entire dorsum ( Fig. 9 View FIGURE 9 E). Undulations shallow and less numerous (‘ clarki’ morph) on darker specimens from low-light, low-flow habitats, leaving most of dorsum uncovered ( Figs. 9 View FIGURE 9 A–D). Anterior ends of parapodia either fused together ( Fig. 9 View FIGURE 9 F) or separate ( Fig. 9 View FIGURE 9 G) varying among specimens, but usually fused on adults with highly undulating parapodia, and unfused on clarki morphotypes and all juveniles. Parapodial margin thin, typically edged with thin white line followed by submarginal band of darker green or grey, and pale yellow to orange line. Submarginal band either continuous or interrupted; some specimens with neon blue pigment surrounding yellow-orange line. Specimens from Lake Surprise, Key Largo, Florida with distinctive orange marginal line.
Pericardium typically differing in color from rest of dorsal surface, being either darker or lighter. Renopericardial extension short. Two to three anterior dorsal vessels emerging from renopericardium on either side, asymmetrically placed, running perpendicular to body axis and branching near upper edge of parapodium ( Fig. 10 View FIGURE 10 ). Posterior paired vessels emerging before terminus of short renopericardial extension, running to posterior end of body and sending off numerous lateral vessels that fork once or not at all.
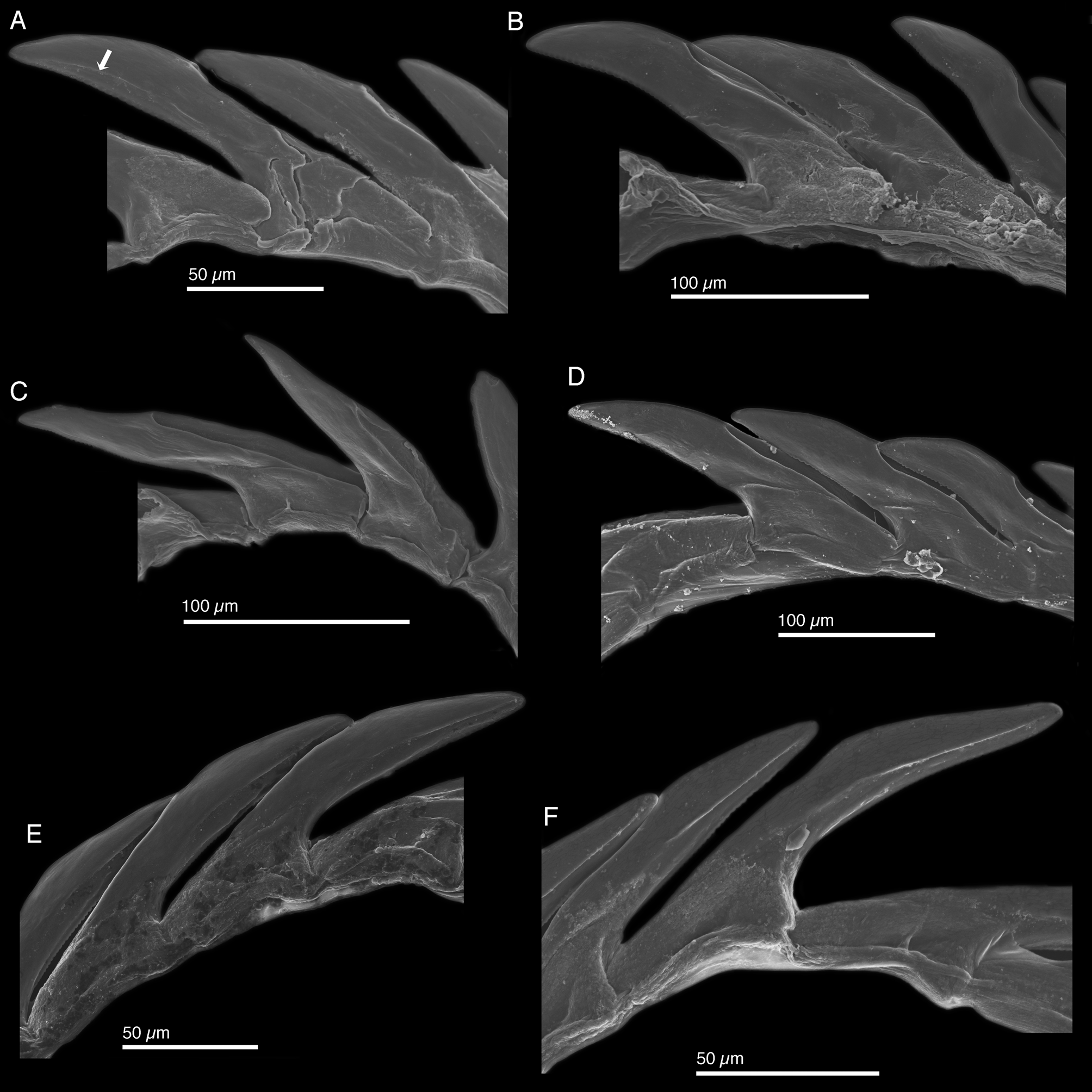
Internal anatomy. Radula with 12–18 teeth (LACM 178584, LACM 178586–89, LACM 178590–94, LACM 178636–38), 6–9 in ascending limb and 6–9 in descending limb ( Fig. 11 View FIGURE 11 A). Leading tooth elongate and variable in shape from slender ( Figs. 11 View FIGURE 11 E–F, 8C) to robust ( Figs. 12 View FIGURE 12 A–B, D). Cusp bearing numerous very small denticles (e.g. Figs. 11 View FIGURE 11 C–D) not evident in all figured specimens (e.g. Fig. 12 View FIGURE 12 B). Housing depression for interlocking teeth “V”- shaped and extending ⅔ of tooth length ( Figs. 11 View FIGURE 11 D, F, 12A–D). Some specimens with a sharp transition between denticulate edge and rest of tooth visible as a longitudinal line (labeled with white arrow in Fig. 12 View FIGURE 12 A; visible but unlabeled in Figs. 11 View FIGURE 11 B–F, 12E–F), but absent in other specimens ( Fig. 12 View FIGURE 12 B–D). Base of tooth approximately ¼ total tooth length. Ascus containing jumbled heap of discarded teeth (not figured).
Penis highly variable ( Figs. 6 View FIGURE 6 B–E), with no correlation between penial shape and position of an individual slug on a COI gene tree of all specimens. Penis typically curved slightly and devoid of armature. Deferent duct long, thin, and convoluted (e.g. Fig. 6 View FIGURE 6 E).
Reproduction and development. Development is lecithotrophic ( Fig. 9 View FIGURE 9 M–N). Like all members of subclade 2, E. crispata does not produce ECY. From field observations, slugs preferentially oviposit on upright, flat surfaces such as the vertical thalli of Udotea or Avrainvillea , or the tops of Penicillus capitatus . Laboratory-held slugs that had not oviposited for several weeks rapidly laid eggs on plastic aquarium plants once introduced to the tank; the response to these structural mimics suggests that egg-laying behavior may be inhibited in the absence of preferred algal substrates.
Larvae lack any host-associated settlement cue and undergo spontaneous metamorphosis before hatching, or within a few days of hatching (Krug 2009). Earlier work described E. crispata as poecilogonous, with mangrove populations producing ‘ type 2’ or swimming lecithotrophic larvae, and reef slugs producing ‘ type 3’ larvae that underwent intracapsular metamorphosis and hatched as crawl-away juveniles ( Clark & Jensen 1981; Defreese & Clark 1983; Clark 1994). These are not distinct types of larvae, however, but rather reflect inter-clutch variation in the timing of hatching or attainment of competence, common in many lecithotrophic species (Krug 2009).
Development of mangrove specimens from the Bahamas, and reef specimens from Curaçao, was described (Krug 2009). For two clutches, mean egg diameter was 113.8 ± 3.9 µm ( Bahamas; n = 7) and 106.1 ± 2.2 µm ( Curaçao; n = 67). Much larger mean egg sizes were previously reported: 205 mm by Clark & Jensen (1981), and 209 mm by Defreese & Clark (1983). No measures of variance were reported by Clark and coworkers, making it difficult to evaluate the reliability of these values. No sacoglossan has been reported to have eggs larger than ~130 µm in any recent study, and only three older studies reported egg diameters on the order of 200 µm ( Limapontia senestra, Chia 1971 ; Berthelinia [= Tamanovalva ] limax, Kawaguti & Yamasu 1960 ; Thuridilla hopei, Thompson & Salghetti-Drioli 1984 ). The older values for E. crispata and other taxa may indicate a recent decline in mean egg sizes in Sacoglossa .
Mean time to hatching was 14.9 ± 0.4 d at ~22°C (n = 9 clutches). In nine of 11 clutches laid by Bahamas slugs, all larvae metamorphosed prior to hatching, while in two clutches all larvae hatched but metamorphosed in filtered seawater within 2 d (Krug 2009). In egg masses laid by specimens of the clarki morph from Lake Surprise, Florida, larvae emerged in the early stages of metamorphosis, primarily crawling and in the process of velar resorption. Longer post-hatching larval periods of 4–5 d were reported by Pierce et al. (2003), and a longer encapsulated period for the clarki morph (28–35 d), for clutches incubated at 20°C. Differences in conditions during incubation of egg masses among studies may explain such variation in time to hatching and the proportion of encapsulated metamorphosis.
Significant among-clutch variation in larval shell size at hatching was reported, ranging from a mean shell width of 238.7 ± 5.93 µm (n = 40) for a clutch from Curaçao to a mean width of 299.2 ± 13.9 µm (n = 33) for a clutch from Sweetings Cay, Bahamas. Overall mean shell size per clutch was 283.0 ± 25.1 µm (n = 5 clutches).
Mean body length of post-metamorphic juvenile slugs was 517.1 ± 64.6 µm (n = 2 clutches) prior to feeding. Juveniles from egg masses laid by clarki morphs fed on Bryopsis plumosa or Derbesia tenuissima , but not Caulerpa verticillata ; juveniles of typical crispata morphs did not feed on other Bryopsis spp., but B. plumosa was evidently not tested ( Pierce et al. 2003).
Host ecology. Elysia crispata is one of the most polyphagous sacoglossans, but studies on its feeding ecology have a long and complicated history. Uncertainty has surrounded whether animals consume algae that they may be spatially associated with in the field. Confusion stems in part from the wide range of algae included in the diet of E. crispata ; further, due to the highly photosynthetic nature of this species, specimens of E. crispata are not often observed feeding or physically resting upon a particular host. Feeding has been inferred from various lines of evidence including preferential association in field surveys or laboratory assays, visual observation, chlorophyll content of slug tissue, electron microscopy of retained chloroplasts, and DNA barcoding of chloroplasts retained in digestive gland cells of field-collected slugs. The latter two methods may best reflect algal consumption under ecologically relevant conditions; however, these methods may not fully capture all species eaten in the field if there is biased retention of (or PCR amplification from) plastids from a subset of consumed species.
It was long asserted that juveniles fed preferentially on Caulerpa verticillata ( Jensen 1980; Jensen & Clark 1983; Clark 1994). The study cited to support this assertion contains no relevant data, however; Clark & Busacca (1978) tested adult feeding, and C. verticillata was not among the five Caulerpa spp. used in assays. Adding further confusion, Clark & Busacca (1978) state “… Tridachia used no species of Caulerpa ” as a food source, yet listed “40% of Caulerpa spp.” as “accepted” by Tridachia in their Table 2, and also reported that Penicillus spp. were consumed but P. capitatus was not.
The adult diet of E. crispata was reported to include Bryopsis plumosa and at least one species each of Penicillus , Halimeda , Cymopolia , and Batophora ( Clark & Busacca 1978; Jensen 1980; Jensen & Clark 1983). In separate laboratory feeding assays, slugs were reported to feed on Halimeda discoidea , Chaetomorpha sp., and three Caulerpa spp. ( C. verticillata , C. racemosa and C. sertularioides ); however, slugs performed poorly on C. verticillata and died after a week on C. sertularioides ( Thompson & Jarman 1989) . The ecological relevance of captive feeding on toxic algae remains unclear, however.
Pierce and colleagues used a combination of microscopy, DNA barcoding, field surveys and lab feeding trials to establish the diet of Florida populations of the ‘ clarki ’ morph of E. crispata . In the field and lab, slugs consumed at least six genera, including Derbesia tenuissima , seven Bryopsis spp. ( B. plumosa , B. pennata , B. pennatula , four unidentified species), three Penicillus spp. ( P. capitatus , P. lamourouxii , P. pyroformus ), three Halimeda spp. ( H. incrassata , H. monile , H. opuntia ), Acetabularia , and an alga with genetic affinity to Pseudochlorodesmis ( Pierce et al. 2003; Curtis et al. 2004, 2006; Middlebrooks et al. 2014). Plastids from Caulerpa were never detected in slugs preserved immediately after field collection ( Middlebrooks et al. 2014); thus, Caulerpa is not consumed by E. crispata under field conditions. Consumed algae were not consistent among sites and did not always reflect algal abundance, indicating dietary preferences despite the breadth of suitable hosts. Using plastid barcoding, Christa et al. (2014) also documented the presence of retained chloroplasts from some of the above listed algal taxa, plus unidentified species in Pseudocodiaceae , Rhipiliaceae and Ulvophyceae. We have observed feeding in situ only on Bryopsis ; however, we have occasionally observed a close association between slugs and either H. incrassata or P. capitatus , consistent with barcoding data showing preferential feeding on these algae.
Phylogenetic relationships. Intra-specific relationships. Within E. crispata , populations are highly genetically structured due to the limited dispersal ability of larvae. We compared the evolutionary relationships of mitochondrial lineages as inferred from ML analysis of COI haplotypes ( Fig. 13 View FIGURE 13 ) to the external morphology of 15 specimens ( Fig. 14 View FIGURE 14 ). Bolded isolate labels ( Fig. 13 View FIGURE 13 ) link a haplotype to the corresponding photographic vouchers ( Fig. 14 View FIGURE 14 ); plain text labels indicate other specimens that shared a given haplotype but that were not included in Fig. 14 View FIGURE 14 . The maximum distance between COI haplotypes, 7.8%, was greater than that noted between ‘ clarki ’ from the FL Keys and E. crispata from the U.S. Virgin Islands (Pierce et al. 2006), yet was below our 8% cutoff for specieslevel distances in Elysia . Clades were not separated into different species by ABGD. On the ML gene tree, COI haplotypes fell into five divergent groups among which mean COI distances (TrN) ranged from 4.4 to 6.7%. Within each clade, mean distance between haplotypes was <1.5%. These results are consistent with a comprehensive phylogeographic analysis of E. crispata (216 specimens from 17 populations), which recovered eight COI clades ranging from 5–8% distant ( Vo 2013). However, all populations shared at least one of three common H3 alleles, indicating populations likely represent one biological species.
In our analysis, Group 1 comprised a grade of closely related COI haplotypes (maximum distance, 0.8%) from four Florida populations. Specimens from three Florida Keys sites (Lake Surprise, Mote Tropical Research Laboratory canal, Geiger Beach) had ‘ clarki ’ features (unfused and unruffled parapodia, green foot; Fig. 14 View FIGURE 14 A-C), but grouped with two specimens sampled 120 km away in the Dry Tortugas that had typical crispata features (fused and ruffled parapodia, white foot; Fig. 14 View FIGURE 14 D). Notably, one E. crispata morph (10Dry08) shared a COI haplotype with a clarki morph from Lake Surprise, Florida ( Fig. 14 View FIGURE 14 A, 09LKS05). The lack of genetic divergence at COI between morphologically distinctive specimens sampled from the area surrounding the Florida Keys supports the synonymy of E. clarki with E. crispata .
The suite of morphological characters that supposedly distinguish E. clarki from E. crispata also failed to covary among specimens sampled from shaded microhabitats across the Bahamas. For instance, Group 2 comprised one haplotype shared by three specimens from Sweeting’s Cay, Bahamas, of intermediate morphology; specimen 07Swe07 ( Fig. 14 View FIGURE 14 G) had features of both clarki (unruffled parapodia, green foot continuous with outer parapodia) and crispata (fused anterior parapodia). Group 3 comprised a specimen from Sweeting’s Cay with a dark clarki morphology but fused parapodia ( Fig. 14 View FIGURE 14 F), plus two specimens from the Northern Exumas.
Both Exumas specimens had symmetrically ruffled parapodia ( crispata -type) and a green foot ( clarki -type); one had a parapodial notch ( Fig. 14 View FIGURE 14 H), but the other did not ( Fig. 14 View FIGURE 14 I). Group 4 included specimens from Sal Salvador and Little San Salvador with intermediate features such as a mostly white foot but pronounced parapodial notch ( Fig. 14 View FIGURE 14 J). Thus, morphology could not be used to classify most specimens as either nominal species, based on the features used in the description of E. clarki .
Group 5 comprised typical E. crispata specimens collected from high-flow sites in Curaçao ( Fig. 14 View FIGURE 14 K) and New Providence, Bahamas ( Fig. 14 View FIGURE 14 L), together with 16 out of 18 Dry Tortugas specimens. Most Dry Tortugas slugs had a typical crispata morphology, but one specimen ( Fig. 14 View FIGURE 14 E, 10Dry07) had an anterior parapodial notch (‘ clarki ’) together with ruffled parapodia and a white foot ( crispata ); this intermediate morph shared a haplotype with nine typical crispata morphs from Dry Tortugas, nine from Dominica, and two from St. Lucia. Moreover, most specimens from the sheltered lagoon at Little San Salvador also belonged to Group 5, yet presented a range of intermediate morphologies; all had reduced parapodial ruffling and dark coloration, but some had clarki features (green foot, parapodial notch; Fig. 14 View FIGURE 14 M, O) while genetically indistinguishable specimens had typically crispata features (white foot, no notch; Fig. 14 View FIGURE 14 N). Overall, the clarki morphotype was not monophyletic at the fast-sorting COI locus ( Fig. 13 View FIGURE 13 ) or the slow-sorting H3 locus ( Vo 2013), and no suite of characters consistently covary and distinguish ‘ clarki ’ morphs from co-occurring E. crispata . Thus, E. clarki cannot be considered a distinct species.
Inter-specific relationships. Elysia crispata is a member of subclade 2 ( Fig. 4 View FIGURE 4 ), which is largely restricted to the north and western Atlantic. The recently described E. ellenae (see Ortea et al. 2013) was recovered as sister to E. crispata , together forming a clade sister to the east Pacific E. diomedea ( Fig. 4 View FIGURE 4 ). This clade of three species occupies a derived position within subclade 2, recovered as sister to a clade comprising E. viridis (cold-temperate) and E. evelinae (tropical) with weak support. Both E. crispata and E. ellenae share a laterally undulating parapodial edge, but the parapodia of E. ellenae are greatly thickened. Speciation may have occurred within the Caribbean after formation of the Panamanian Isthmus isolated the ancestor of E. diomedea from Caribbean populations, but ecological and life-history data on E. ellenae are needed to formulate hypotheses regarding its divergence from E. crispata .
Range. Antigua (present study), Aruba ( Ev. Marcus & Er. Marcus 1963; Valdés et al. 2006), Bahamas ( Redfern 2001, 2013; Valdés et al. 2006), Barbados ( Ev. Marcus & Hughes 1974), Belize ( Valdés et al. 2006), Bonaire ( Ev. Marcus & Er. Marcus 1963; Er. Marcus & Ev. Marcus 1970), Cayman Islands ( Hess et al. 1994), Colombia ( Ev. Marcus 1976b), Costa Rica ( Espinosa & Ortea 2001), Cuba (Espinosa et al. 2005), Curaçao ( Ev. Marcus & Er. Marcus 1963; Er. Marcus & Ev. Marcus 1970), Dominica (present study), Dry Tortugas ( White 1952), Florida , USA ( Ev. Marcus & Er. Marcus 1962; Jensen & Clark 1983; Clark 1994; Valdés et al. 2006), Guadeloupe ( Valdés et al. 2006), Haiti ( Valdés et al. 2006), Honduras ( Ev. Marcus & Er. Marcus 1962; Valdés et al. 2006), Jamaica ( Thompson 1977; Valdés et al. 2006), Mexico ( Zamora-Silva & Ortigosa 2012), Panama ( Collin et al. 2005), St. Lucia (present study), St. Martin / St. Maarten ( Ev. Marcus & Er. Marcus 1963) , Trinidad & Tobago ( Valdés et al. 2006), Venezuela ( Valdés et al. 2006), Virgin Islands ( Mörch 1863; Ev. Marcus & Er. Marcus 1962; Valdés et al. 2006).
Remarks. Ørsted & Mörch in Mörch (1863) described Elysia (Tridachia) crispata in a brief Latin description based on unpublished drawings by Anders Sandøe Ørsted, later published by Bergh (1871: pl. 9, figs. 4–5). The main diagnostic characteristics included in the original description are the slug’s curled parapodial edges (each side having 6–7 strong folds) that are fused together, as well as green body color with large, white, regularly arranged spots on the sides of the body. These external characteristics are only found in the species known as Elysia crispata in the Caribbean literature. Some years earlier, Deshayes (1857) described the genus Tridachia based on a species to be named after Schramm, but did not name the species. Mörch (1863) introduced for the first time the binominal name Tridachia schrammi in reference to Deshayes’ (1857) description, which he considered to be a different species but the same genus as Elysia crispata . Since then, most authors have included E. crispata as the only member of Tridachia . Gosliner (1995) used a morphological phylogenetic analysis to show that E. crispata nests with other members of Elysia , thus rejecting the validity of Tridachia . Our molecular phylogenetic analysis confirms that Tridachia is a synonym of Elysia , as have all prior molecular analyses of Plakobranchidae or Sacoglossa (Bass & Karl 2006; Händeler et al. 2009; Wägele et al. 2010, 2011; Christa et al. 2014; Krug et al. 2015).
Despite the abundance of Elysia crispata in the field and its extreme variation in external morphology, few synonyms exist. Morphological examination of the type specimens of Elysia crispata (ZMUC GAS-1584), Elysia crispata var. schiadura (ZMUC GAS-1572) and Tridachia schrammi (MNHN) confirmed that they all conform to the current use of the species name E. crispata and are therefore synonyms. White (1952) described a specimen from Dry Tortugas under the name Tridachia ornata ( Swainson, 1840) . However, the illustration of the external morphology and the radula of this animal match the description of Elysia crispata . Er. Marcus (1957) recognized that White’s (1952) specimen was different from the original description of Elysia ornata but also from that of Tridachia schrammi (= Elysia crispata ) and therefore he named it Tridachia whiteae Marcus, 1957 . We here regard Tridachia whiteae as a synonym of E. crispata , as all 18 specimens examined from the Dry Tortugas were morphologically and genetically confirmed to be E. crispata .
Pierce et al. (2006) described Elysia clarki from mangrove swamps and canals in the Florida Keys. These authors compared specimens from low-flow Florida habitats with specimens identified as E. crispata from the Virgin Islands, and reported morphological differences including a nearly uniform green color with small white spots across parapodia and an almost transparent foot, non-fused anterior parapodial edges, and asymmetrical nonfixed folds in the parapodia of E. clarki . Pierce et al. (2006) also reported that radular teeth of E. clarki were ~ 10% longer (129 µm ± 4.1) than in E. crispata (114 µm ± 4.2), and also had a deeper and broader groove, and more prominent and widely spaced basal articulations. Finally, Pierce et al. (2006) reported a COI distance of ~7% between Florida specimens and specimens from the U.S. Virgin Islands, which was interpreted as a species-level genetic distance.
In this study, we examined over 200 specimens of E. crispata from across its range and found substantial variation in coloration and internal and external morphology, including all of the distinctive traits used to separate E. clarki . However, all specimens were less than 8% divergent at COI, consistently supported here and in prior work (Krug et al. 2013) as a threshold inter-specific COI distance for Elysia . The phylogenetic position of COI haplotypes (e.g., Fig. 13 View FIGURE 13 ) was not related to morphology, but rather to location, with a high degree of differentiation among most populations. The 7% distance originally noted between Florida ‘ clarki ’ and U.S. Virgin Islands ‘ crispata ’ haplotypes is typical for among-population differentiation in this low dispersal species: Vo (2013) recovered seven COI clades that were 5–8% divergent in E. crispata , five of which are presented in Fig. 13 View FIGURE 13 . The high apparent COI distance between Florida ‘ clarki ’ and U.S. Virgin Islands E. crispata thus reflected population differentiation, not genetic differences among morphotypes; U. S. Virgin Island specimens cluster with our group 5 samples ( Vo 2013). The Florida group (including both ‘ clarki ’ and E. crispata morphs) was no more divergent than any other COI clade of E. crispata from around the Caribbean.
More importantly, the broader sample size examined in the present study and in Vo (2013) revealed that specimens of E. clarki did not form a clade excluding E. crispata , based on molecular phylogenetic analysis of COI haplotypes. Most specimens from the Florida Keys were collected in low-flow, low-light habitats and had ‘ clarki ’ morphology, but grouped genetically with some typical E. crispata from the nearby Dry Tortugas, and not with ‘ clarki ’ morphs from low-flow habitats in the Bahamas, which grouped with typical E. crispata from the same location. Moreover, the three H3 alleles sampled in specimens from the Florida Keys and Dry Tortugas were common throughout the range of E. crispata ( Vo 2013) ; thus, both mtDNA and the nuclear H3 gene fail to distinguish clarki morphs from E. crispata . Combined, all available morphological and molecular data indicate E. clarki cannot be considered a distinct species, as it is a polyphyletic ecotype.
Finally, the features proposed to distinguish E. clarki do not consistently co-occur in specimens from low-flow environments, but are frequently intermingled with crispata - type characters. For example, paired specimens of E. crispata of equivalent size collected side by side often had fused and unfused parapodia, respectively, including pairs from Yucatan, Mexico ( Fig. 9 View FIGURE 9 F–G), Dry Tortugas ( Fig. 14 View FIGURE 14 D–E), Northern Exumas ( Fig. 14 View FIGURE 14 H–I), and Little San Salvador ( Fig. 14 View FIGURE 14 N–O). The degree of undulation of the parapodia is extremely variable and the folds are not consistently fixed in all specimens assigned to E. crispata . The radular morphology of this species is also extremely variable, and we found no consistent differences between specimens of E. crispata from mangrove areas in the Florida Keys and the rest of the range, nor did size delineate clarki morphs from typical E. crispata ( Figs. 11–12 View FIGURE 11 View FIGURE 12 ). Overall, neither morphological nor molecular analyses found consistent differences distinguishing E. clarki from E. crispata , and for all these reasons, E. clarki is here considered a synonym of E. crispata .
We do consider ‘ clarki ’ to be an ecotype of E. crispata characterized by darker coloration, green diverticula in the foot, and reduced parapodial undulation. We have sampled this ecotype from low-light and low-flow habitats including mangrove lagoons, borrow pits, and shaded rock overhangs in shallow, protected areas throughout the Caribbean. In contrast, the light-colored, highly ruffled crispata ecotype predominates in high-flow, high-light habitats such as drainage channels and subtidal reefs. The recurring association of clarki features with a particular habitat is consistent with either phenotypic plasticity during development, or local selection favoring the clarki ecotype. The lighter coloration and higher degree of parapodial ruffling may protect chloroplasts in diverticula lining the dorsum of the typical crispata morph from excess light, prolonging plastid function. The onshore Florida populations of E. crispata show coloration consistent with low-light adaptation (overall green to purple body coloration, including in the foot), possibly due to the greater turbidity of coastal waters compared to the rest of the Caribbean. Further study is needed to determine whether phenotypic plasticity or local selection produces the observed differences in external morphology between ‘ clarki’ ecotypes and typical E. crispata specimens. Indeed, given the ability to study diet via DNA barcoding of plastid DNA ( Middlebrooks et al. 2014), E. crispata may provide a valuable system with which to investigate local adaptation, which is rare among marine herbivores ( Sotka 2005).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Elysia crispata Mörch, 1863
| Krug, Patrick J., Vendetti, Jann E. & Valdés, Ángel 2016 |
Elysia crispata Mörch
| Krug 2015: 990 |
| Redfern 2013: 282 |
| Zamora-Silva 2012: 366 |
| Valdes 2006: 62 |
| Collin 2005: 690 |
| Redfern 2001: 162 |
| Espinosa 2001: 44 |
| Clark 1994: 904 |
Tridachia ornata
| White 1952: 118 |
Tridachia crispata
| Hess 1994: 161 |
| Jensen 1983: 6 |
| Ev 1980: 75 |
| Thompson 1977: 131 |
| Bandel 1976: 96 |
| Ev 1976: 128 |
| Ev 1974: 509 |
| Er 1970: 50 |
| Ev 1967: 33 |
| Ev 1963: 23 |
| Ev 1962: 461 |
| Ev 1960: 153 |
| Engel 1927: 115 |
Elysia (Tridachia) crispata var. schiadura Mörch 1863 : 40
| Morch 1863: 40 |
Elysia schrammi Ørsted & Mörch in Mörch 1863 : 41
| Morch 1863: 41 |