Elysia velutinus Pruvot-Fol, 1947
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4148.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:91353147-FDA8-45CC-A8F1-1DE801C835A6 |
|
DOI |
https://doi.org/10.5281/zenodo.5664187 |
|
persistent identifier |
https://treatment.plazi.org/id/A04A7E6D-9C7B-FFBA-46C9-F9B3FCFF1E8C |
|
treatment provided by |
Plazi |
|
scientific name |
Elysia velutinus Pruvot-Fol, 1947 |
| status |
|
Elysia velutinus Pruvot-Fol, 1947 View in CoL
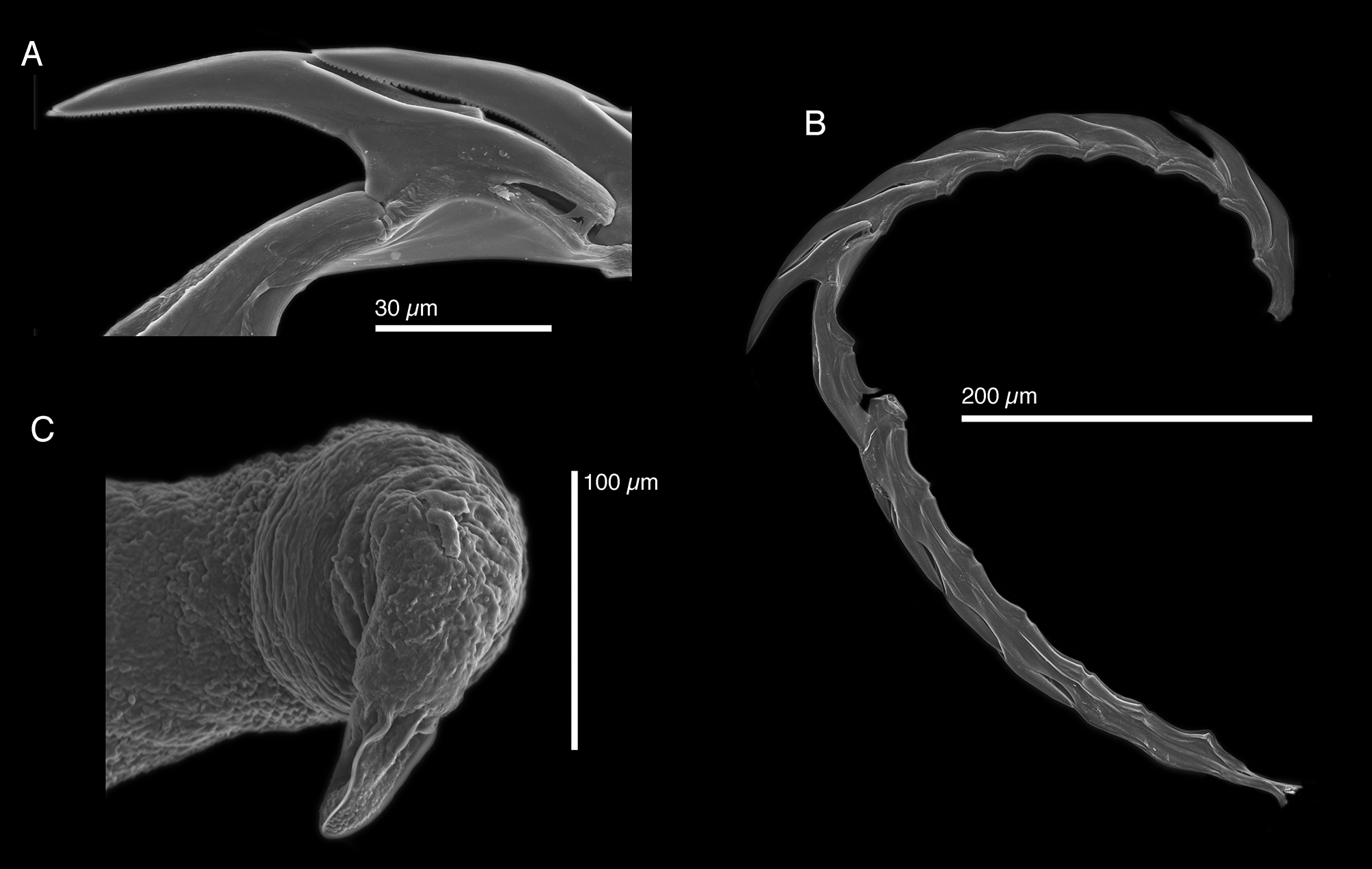
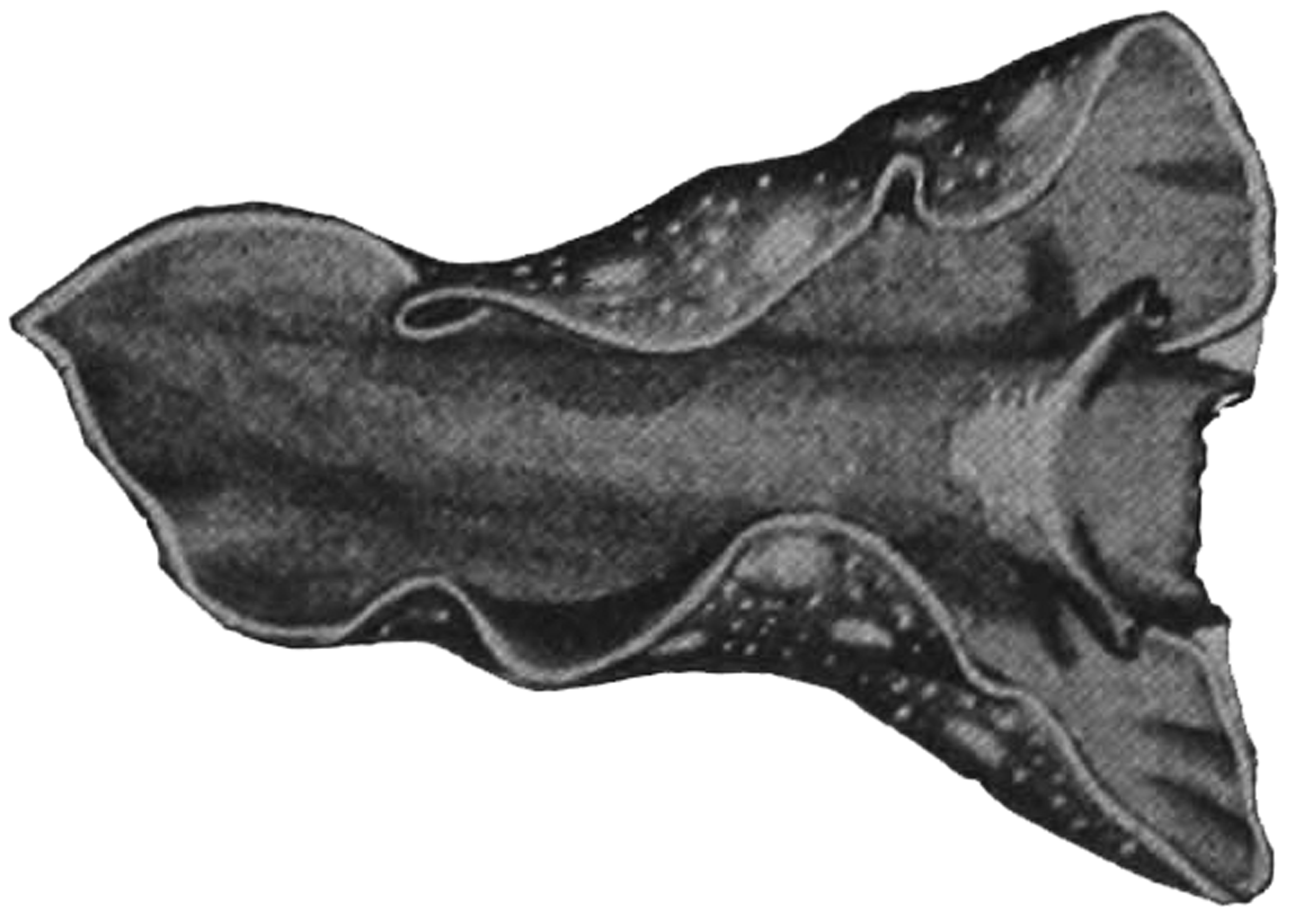
( Figs. 6 View FIGURE 6 N, 27–29)
“ Elysia crispa ” [not available] Verrill 1900: 547, pl. 66, fig. 4 (error for Elysia crispata ).
Elysia verrilli Pruvot-Fol 1946: 39 View in CoL [non Thiele, 1931] (Type locality: Bailey Bay, Bermuda) — new name for “ Elysia crispa ” sensu Verrill (1900) .
Elysia velutinus Pruvot-Fol 1947: 115 View in CoL (Type locality: Bailey Bay, Bermuda) — replacement name for Elysia verrilli Pruvot-Fol, 1946 View in CoL [non Thiele, 1931].
Elysia pruvotfolae Er. Marcus 1957: 415 View in CoL (Type locality: Bailey Bay, Bermuda) — replacement name for Elysia verrilli View in CoL Pruvot- Fol, 1946 [non Thiele, 1931].
Elysia papillosa View in CoL [non Verrill, 1901] — Ev. Marcus & Er. Marcus 1963: 21 –22, fig. 29.
Elysia tuca Ev. Marcus & Er. Marcus, 1967: 29 View in CoL –31, figs. 28–32 (Type locality: Soldier Key, Biscayne Bay, Florida) — Er. Marcus & Ev. Marcus 1970: 46 –47, figs. 81, 84–85; Ev. Marcus & Hughes 1974: 507, figs. 15–16; Thompson 1977: 128 – 129, figs. 26c, 27a–b; Clark & Goetzfried 1978: 285, fig. 1; Ev. Marcus 1980: 70 –72, figs. 16–17, 55; Clark 1984: 90, fig. 21; Jensen & Clark 1983: 5 –6; Hess et al. 1994: 163; Clark 1994: 904; Espinosa & Ortea 2001: 44; Redfern 2001: 162 – 163, figs. 674A–B; Collin et al. 2005: 690; Espinosa et al. 2005: 56; Valdés et al. 2006: 66 –67; Krug 2009: 360–365, figs 1, 2A, 3A, 4, 6; Redfern 2013: 285, figs. 791A–B; Ortigosa et al. 2013: 65 – 66 n. syn.; Christa et al. 2014: figs. 1F, 3; Krug et al. 2015: 990 –991, figs. 3B, 4.
Type material. Elysia velutinus— untraceable, not at YPMNH; Elysia tuca— Syntype (USNM 576286).
Material examined. Punta Uva, Gandoca-Manzanillo, Costa Rica, 20 September 1999, 1 specimen ( CPIC 00148 ) ; Stocking Island , Bahamas, 23 January 2008, 1 specimen ( CPIC 00012 ), 15 Dec 2007, 2 specimens ( CPIC 00013–14 ) ; Geiger Beach , Florida, USA, 2006, 1 specimen ( LACM 178632 View Materials ) ; Southwest Flamingo Bay, Water Island, St. Thomas , U.S . Virgin Islands, March 1985, 1 specimen ( LACM 178633 View Materials ) ; Union Island, St. Vincent and the Grenadines, 1987, 1 specimen ( LACM 178634 View Materials ) ; Prince Rupert Bay, Dominica, 19 April 2008, 1 specimen ( LACM 178642 View Materials ) .
Live animal. Parapodia held together when resting. Slugs do not swim when disturbed. Although normally living in association with Halimeda spp., large specimens were occasionally found crawling on other algae in dense beds of mixed algae.
External anatomy. Overall coloration light to dark green, with spots or large patches of pigment ranging from white to tan ( Fig. 27 View FIGURE 27 ). Head with large “Y”-shaped pigment patch (usually white, sometimes tan) behind head, starting anterior of pericardium and running up to base of each rhinophore, usually extending laterally to just above small eyespots ( Fig. 27 View FIGURE 27 B–D, F). Front of head rounded and smooth, no oral lobes. Rhinophores green at base but increasingly white or tan towards tip; uniform in width along entire length, sometimes dotted with small papillae. Foot pale yellow-green. Specimens from Panama ( Fig. 27 View FIGURE 27 A–C) generally lacking papillae or white patches on parapodia; Bahamas specimens often with scattered white papillae across body, head and rhinophores, and larger patches of white concentrated along parapodial margins.
Parapodia high and thick, usually held closed to cover dorsal surface and pericardium. Edges of parapodia bow out to form one small siphonal opening about halfway along body ( Fig. 27 View FIGURE 27 B–D). Outer parapodial surface ranging from smooth to dotted with white papillae, small and rounded. Parapodia dull to dark green, with regularly spaced patches or speckles of white to tan pigment. Larger patches of white sometimes concentrated at intervals along parapodial margin ( Fig. 27 View FIGURE 27 D–F). Margin with smooth, even edge, sometimes lined along inner and/or outer edge with white to tan pigment. Inner surface of parapodia, dorsum and pericardium speckled (sometimes densely) with iridescent blue-green dots ( Fig. 27 View FIGURE 27 F–G). Posterior end of body sometimes blunt-ended, or else narrowing to form short, triangular tail. Dorsal surface sometimes pierced by egg masses of parasitic copepod, colored light bluegreen, not observed on other species ( Fig. 27 View FIGURE 27 C).
Pericardium large and rounded, pale green to white in ground color, with scattered iridescent blue-green dots ( Fig. 27 View FIGURE 27 F–G). Renopericardium short and not distinct from pericardium, giving rise to one pair of posterior dorsal vessels often densely coated with iridescent blue-green dots ( Fig. 27 View FIGURE 27 G). Each vessel forks into two main branches, one curving towards anterior end and the other running towards posterior end of body. Each branch sending off 8– 15 short lateral side branches each forking 0–3 times, and sometimes anastomosing. All branches extending only three-quarters of the way up inner parapodial surface before terminating at a distance from parapodial margin. Branching network of white reproductive ducts visible through dorsum around renopericardial complex.
Internal anatomy. Radula with 17 teeth (CPIC 000148), 9 teeth in ascending limb and 8 in descending limb ( Fig. 28 View FIGURE 28 A–B). Leading tooth elongate and narrow, with slightly curved cusp, bearing numerous minute denticles ( Fig. 28 View FIGURE 28 A). Housing depression for interlocking teeth “V”-shaped and extending ½ total tooth length ( Fig. 28 View FIGURE 28 A). Base of the tooth approximately ⅓ of total tooth length.
Penis wide and elongate with rigid musculature resistant to desiccation ( Fig. 6 View FIGURE 6 N) and tapering distally into a conical apex bearing a unique “ridged” stylet ( Fig. 28 View FIGURE 28 C). Deferent duct narrow and convoluted.
Reproduction and development. Larval biology described by Krug (2009) is summarized below. Development is lecithotrophic, with a ribbon of bright orange ECY zigzagging through egg mass, contacting every egg capsule ( Fig. 27 View FIGURE 27 H). Veligers ingest granules of ECY that enter their capsule, or absorb yolk material during development, acquiring an orange hue. Mean number of eggs per clutch for Bahamas specimens was 113.7 ± 20.2 SE (n = 7; range = 32–194), and for Florida specimens was 177.3 ± 32.7 SE (n = 19; range = 6–580). Mean egg diameter for one clutch from Bahamas was 104.8 µm (± 0.5 SE; n = 32).
Mean time to commencement of hatching was a comparable 17.7 d (± 0.3 SE; n = 3) for egg masses from Bahamas slugs, and 18.1 d (± 0.7 SE; n = 19 clutches) for Florida egg masses. Krug (2009) described extensive intra-clutch variation in time to hatching of siblings, with larvae in outermost whorl of egg mass hatching about a week earlier than siblings from innermost whorl; mean time from initial to final hatching was 8.6 d (± 3.9 SD; n = 19 clutches; range = 2–16 d). Time necessary to complete hatching scaled linearly with clutch size. Egg masses often deposited on seagrass Thalassia testudinum (this study; Jensen & Clark 1983) as well as on H. incrasatta .
Mean larval shell length at hatching varied substantially among five clutches, ranging from 261.9 µm to 284.1 µm (grand mean length = 275.8 µm ± 3.9 SE; n = 5). No intracapsular metamorphosis occurred, and less than 0.5% of larvae metamorphosed in absence of an algal substrate over a week in filtered sea water (FSW). About half of larvae were induced to metamorphose by exposure to one of three species of adult host genus Halimeda ( H. incrassata , H. monile , H. opuntia ), whereas negligible settlement occurred in response to three non-host algae ( Udotea flabellum , Caulerpa verticillata , Batophora oerstedii ). Larvae settled directly onto blades of Halimeda and metamorphosed over 2–3 d. Some larvae successfully metamorphosed after 12 d with no planktonic food. Newly metamorphosed juveniles measured 358.2 µm in length (± 30.5 SE; n = 2 clutches) when crawling.
Host ecology. Elysia velutinus feeds on various species in the genus Halimeda , and is most commonly associated with the upright branching species H. incrassata and H. monile (this study) and H. discoidea (Jensen 1983; Jensen & Clark 1983). Being large, mobile and abundant, E. velutinus is also commonly found crawling on non-host algae in the field. Clark & Busacca (1978) reported that in the laboratory, starved specimens of E. velutinus consumed Avrainvillea nigricans , Udotea sp., three species of Caulerpa ( C. racemosa , C. mexicana , C. sertularoides ), and possibly Batophora or Rhipocephalus ; however, the metric used to assess feeding was unclear, and may have been observed ingestion, growth, or maintenance of chlorophyll levels relative to starved slugs. These results do not reflect the typical host association of field-surveyed animals and are considered unreliable without further confirmation. Further, we have observed starved slugs of several species feeding on algae with which they are not associated in the field; starved animals are thus capable of feeding on non-host algae, but will not normally do so if preferred (host) species are present, and typically cannot sustain growth or long-term survival on non-host algae.
Phylogenetic relationships. Elysia velutinus was recovered as sister to an undescribed but morphologically similar species ( Elysia sp. 6) from the eastern Pacific coast of Central America with complete support ( Fig. 4 View FIGURE 4 ). No other closely related species were identified. Bayesian analyses indicated the clade ( E. velutinus + E. sp. 6) was sister to a clade of Pacific Elysia spp. that feed on other udotacean algae ( Chlorodesmis , Udotea ), including Elysia bennettae Thompson, 1973 , Elysia degeneri Ostergaard, 1955 , and two undescribed species ( E. cf. bennettae , Elysia sp. 15).
Range. Bahamas ( Redfern 2013), Barbados ( Ev. Marcus & Hughes 1974), Belize ( Clark & DeFreese 1987); Bermuda ( Verrill 1900; Ev. Marcus & Er. Marcus 1963; Clark 1984), Brazil ( Ev. Marcus 1980), Cayman Islands ( Hess et al. 1994), Costa Rica ( Espinosa & Ortea 2001), Cuba (Espinosa et al. 2005), Curaçao ( Ev. Marcus & Er. Marcus 1963; Er. Marcus & Ev. Marcus 1970; Valdés et al. 2006), Dominica (present study), Florida, USA ( Er. Marcus & Ev. Marcus 1970; Jensen & Clark 1983; Waugh & Clark 1986; Clark 1994), Jamaica ( Thompson 1977), Mexico ( Valdés et al. 2006; Ortigosa et al. 2013); Panama ( Collin et al. 2005), St. Thomas, U.S. Virgin Islands (present study). Union Island, St. Vincent and the Grenadines (present study).
Remarks. Pruvot-Fol (1946) introduced the name Elysia (Elysiopterus) verrilli Pruvot-Fol, 1946 for the specimens identified as “ Tridachia crispa Mörch ” by Verrill (1900), which she argued were different from the true Tridachia crispata . In a later note, Pruvot-Fol (1947) mentioned that Elysia verrilli was preoccupied by Elysia (Elysiella) verrilli Thiele, 1931 and therefore introduced the replacement name Elysia (Elysiopterus) velutinus Pruvot-Fol, 1947 . Unaware of Pruvot-Fol’s (1947) paper, Er. Marcus (1957) introduced the replacement name Elysia (Elysiopterus) pruvotfolae Er. Marcus, 1957 for Elysia (Elysiopterus) verrilli Pruvot-Fol, 1946 , realizing that it was preoccupied by Thiele’s name.
The characteristics of the animals described and illustrated by Verrill (1900) from Bermuda include the presence of a white spot on the head and relatively smooth parapodia ( Fig. 29 View FIGURE 29 ). All described features are consistent with the species commonly known in the Caribbean literature as Elysia tuca Ev. Marcus & Er. Marcus, 1967 , which is common in Bermuda. Clark (1984) noted for the first time that the specimens described from Bermuda by Verrill (1901) as “ E. crispa ” were indeed E. tuca . Because the name E. tuca is widely used in Caribbean literature it is desirable to maintain the usage of the name. However, under the provisions of the Code of Zoological Nomenclature (ICZN 1999: Article 23.9), a senior synonym can only be replaced automatically by a commonly used junior synonym if the former has not been used as a valid name after 1899. Because Elysia velutinus was introduced in 1947, following the Principle of Priority and the available evidence we propose to reinstate the name Elysia velutinus for this species.
The penial stylet in this species is only clearly visible under SEM (LACM 178634, CPIC 00013) as a “cuticular tube with several folds” ( Ev. Marcus 1980). Such morphology is consistent with the “three spines” morphology of the penis mentioned by Ortea et al. (2005).
| LACM |
Natural History Museum of Los Angeles County |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Elysia velutinus Pruvot-Fol, 1947
| Krug, Patrick J., Vendetti, Jann E. & Valdés, Ángel 2016 |
Elysia tuca
| Krug 2015: 990 |
| Redfern 2013: 285 |
| Ortigosa 2013: 65 |
| Valdes 2006: 66 |
| Collin 2005: 690 |
| Espinosa 2001: 44 |
| Redfern 2001: 162 |
| Hess 1994: 163 |
| Clark 1994: 904 |
| Clark 1984: 90 |
| Jensen 1983: 5 |
| Ev 1980: 70 |
| Clark 1978: 285 |
| Thompson 1977: 128 |
| Ev 1974: 507 |
| Er 1970: 46 |
| Ev 1967: 29 |
Elysia papillosa
| Ev 1963: 21 |
Elysia velutinus
| Pruvot-Fol 1947: 115 |
Elysia verrilli
| Pruvot-Fol 1946: 39 |
Elysia crispa
| Verrill 1900: 547 |