Periscelis (Periscelis) laszloi, Roháček, 2022
|
publication ID |
https://doi.org/ 10.37520/aemnp.2022.018 |
|
publication LSID |
lsid:zoobank.org:pub:1C001FE0-4D51-46DE-849A-D66BB9A05B47 |
|
DOI |
https://doi.org/10.5281/zenodo.10552619 |
|
persistent identifier |
https://treatment.plazi.org/id/A11387B0-FF81-FFC0-FF73-26C8FDA2F97A |
|
treatment provided by |
Felipe |
|
scientific name |
Periscelis (Periscelis) laszloi |
| status |
sp. nov. |
Periscelis (Periscelis) laszloi View in CoL sp. nov.
( Figs 44–76 View Figs 44–48 View Figs 49–61 View Figs 62–69 View Figs 70–72 View Figs 73–77 )
Periscelis winnertzii: PAPP & WITHERS (2011) View in CoL : 354 (revision, illustr.)
Periscelis (Periscelis) winnertzii: ROHÁĆEK & ANDRADE (2017) View in CoL : 244 View Cited Treatment (diagnosis, illustr.)
Type material. HOLOTYPE: ♂, labelled: “ Szokolya , Vasfazék-v ., Magas Tax alatt, 450 m, Papp László és János ”, “fekete tölgyfaseb, kifolyó nedvéről” (obverse), “ 13 Sep 1997 ” (reverse, handwritten), “Hungarian Natural History Museum, Diptera Coll. Budapest” (blue label) and “Holotypus J, Periscelis (P.) laszloi sp.n., J. Roháček det. 2022” (red label) ( HNMH, intact, examined, Fig. 44 View Figs 44–48 ).
PARATYPES: 3♂♂ 12♀♀, same data as for holotype (2♂♂ 2♀♀ genit. prep., 2 ♂♂ 2 ♀♀ captured in copula) (all HNMH). [fekete tölgyfaseb, kifolyó nedvéről = on black sap runs from wound of oak].Other paratypes: PORTUGAL: PORTO: Vila Nova de Gaia,Avintes, Parque Biológico de Gaia, 41°06′00.0″N, 8°33′35.3″W, 50 m, 29.vi.2011, sweeping over bark of Quercus trees with sap runs, 1♂, R.Andrade leg. (dried from ethanol, genit. prep., SMOC); Valongo, Valongo, 41°09′33.4″N, 8°29′05.6″W, 50–100 m, sweeping over bark of Quercus trees with sap runs, 4.x.2011, 1 ♂ 1♀, R.Andrade leg. (in glycerine,♂ genit.prep., SMOC). SWITZERLAND: TI 701.168 113.371, Losone: Arcegno, Collina di Maia, Castagneto con querce, 417 m, prd. 32B, Finestra [window trap], ARC 2, 20.xi.–22.xii.2017, 2 ♂♂ 1 ♀ ( SMOC, 1 ♂ genit. prep.); same locality but TI 701.298 113.204, 365 m, prd. 3, SLAM trap UP [Malaise type trap in tree canopy],ARC 1, 7.–18.viii.2015, 1♂ ( MCSN); same locality but TI 701.013 113.741, 411 m, prd. 7, VINO Gialla [trap with yellow wine], ARC 3, 24.ix.–9.x.2015, 1 ♂ 9 ♀♀ ( MCSN); same locality but TI 701.168 113.372, prd. 8, VINO Bianca [trap with white wine], ARC 2, 9.–23.x.2015, 4♂♂ 15♀♀ (2♂♂ 11♀♀ MCSN, 1♂ 2♀♀ NMPC,1♂ 2 ♀♀ SMOC); same locality but TI 701.011 113.773, prd.6, BIRRA Bianca [trap with pale beer], ARC 3, 8.–24.ix.2015, 1 ♂ 9 ♀♀ ( MCSN), all L. Pollini P.& M.Abderhalden leg. HUNGARY: W.Hungary:Kőszegi TK: Kőszek, Hétforrás patak fölött, 10.vii.2002, 1 intersex ( PHOTO), L. Papp leg. (det. L. Papp 2002 as P. winnertzii ) (intact, HNHM); N. Hungary, Bükk Mts: Varbó env., Fonagy sági-tó, 48°08′56″N 20°35′21″E, 250 m, on oak tree bark in deciduous forest, 9.viii.2018, 1 ♀, J. Roháček leg. ( PHOTO) ( SMOC). SLOVAKIA: C. Slovakia: Muránska Dlhá Lúka 2 km SE, 48°42′12″N, 20°05′51″E, 360 m, beer trap in hornbeam forest, 3.ix.–27.x.2012, 1 ♀, J. Roháček & J. Ševčík leg. (dried from ethanol, SMOC); Muránska planina NP: Muránska Lehota 3.7 km E, above Javorníčková dolina, 48°43′15″N, 19°59′56″E, 780 m, sweeping undergrowth of oak-linden forest, 13.viii.2015, 1 ♀, J. Roháček leg. ( PHOTO) (genit. prep., SMOC); Muránska planina NP:Šarkanica res., 48°42′45″N, 19°59′19″E, 580 m, protein trap, 9.viii.–5.ix.2017, 1 ♀; same locality, Malaise trap, 6.–27.ix.2017, 1 ♂, J. Roháček, J. Ševčík & M. Tkoč leg. (both dried from ethanol, NMPC); S. Slovakia: Cerová vrchovina PLA: Hajnáčka-Buková 0.4 km NNE, 48°13′39″N, 19°58′25″E, 375 m, beer trap, 12.ix.–11.x.2018, 1 ♂, J. Roháček, J. Ševčík & M. Tkoč leg. (dried from ethanol, genit. prep., SMOC); Cerová vrchovina PLA: Jestice 1.3 km SSE, Hradisko Mt., 48°12′31″N, 20°03′39″E, 255 m, wine traps on Quercus cerris , 19.vii.–17.viii.2022, 1 ♂ 1 ♀, 13.ix.–26.x.2022, 1 ♀, J. Roháček leg. (dried from ethanol, SMOC). SWEDEN: Ög: Boxhom k:n: Björneberg, WGS84: 58.196997 / 14.911511, inäga, 28.vi.–23. vii.2019, 1♂, 31.viii.–19.x.2019, 1♀, Niklas Johansson leg. (dried from ethanol, ♀ with right wing glued to card) ( NHRS, ♂ no. 000104579, ♀ no. 000104337). According to HELLQUIST (2020, as P. winnertzii ) these specimens were collected in a Malaise trap.
Other material examined (excluded from type series). SWITZERLAND: TI 701.168 113.371, Losone: Arcegno, Collina di Maia, Castagneto con querce, 417 m, prd. 32B, Finestra [window trap], ARC 2, 20.xi.–22.xii.2017, 1 ♀, used for mo1ecular study ( SMOC, body after DNA extraction preserved in a pinned microvial in glycerine, with blue label: JR 35, OM314933 View Materials ). HUNGARY: Kapornak [= Nagy Kapornak], without further data, 3 ♂♂, heavily damaged, all Aradi det. as „ Microperiscelis Winnertzi ” ( HNHM, all genit. prep.); Badacsony, without further data, 1 ♂, heavily damaged, Thalhammer leg., Aradi det. as „ Microperiscelis Winnertzi ” ( HNHM, genit. prep.). Note. There are 2 other specimens from Badacsony in HNHM but they are headless and without abdomen, hence not safely identifiable. GREAT BRITAIN: ENGLAND: Herefordshire, Little Doward, 9.viii.–20.ix.2021, 1 ♀, flight interception trap in large cavity of live beech, K.N.A. Alexander leg., P. J. Chandler det. ( PCB, not examined) (P.J. Chandler, personal communication 2022).
Diagnosis. Slightly larger on average (2.66–4.17 mm) and more robust than P. winnertzii , with similar body colouration, wing venation and chaetotaxy. It distinctly differs from the latter species by: pedicel with black spot larger, extended laterally towards its ventral side ( Figs 44, 48 View Figs 44–48 , 72 View Figs 70–72 ); mesonotum more uniformly grey microtomentose, with brown medial spots small, indistinct to absent ( Figs 47 View Figs 44–48 , 75 View Figs 73–77 ); acrostichal setulae more numerous than in P. winnertzii , in 10–12 rows on suture; scutellum normally darker, with yellow colour reduced ( Figs 47 View Figs 44–48 , 75 View Figs 73–77 ); wing with brown infuscation reduced, particularly at apex, between r-m and dm-cu and on A 1 ( Figs 44 View Figs 44–48 , 76 View Figs 73–77 ); male pregenital sternum (S6) more or less widened posteriorly, without posteromedial depression but with acutely projecting anterior corners ( Figs 60, 61 View Figs 49–61 ); surstylus distally more slender and acutely pointed ( Fig. 50 View Figs 49–61 ); gonostylus with apex distinctly curved ( Fig. 51 View Figs 49–61 ); postgonite with basal part not expanded ventrally ( Figs 58, 59 View Figs 49–61 ); female T8 wider, posteromedially pale pigmented ( Fig. 66 View Figs 62–69 ); female S8 also wider, less densely setose, only laterally dark-pigmented ( Fig. 67 View Figs 62–69 ); spermathecae ( Fig. 69 View Figs 62–69 ) larger, with diameter more than 3.5 times larger than diameter of duct. For other (smaller) differences see description below.
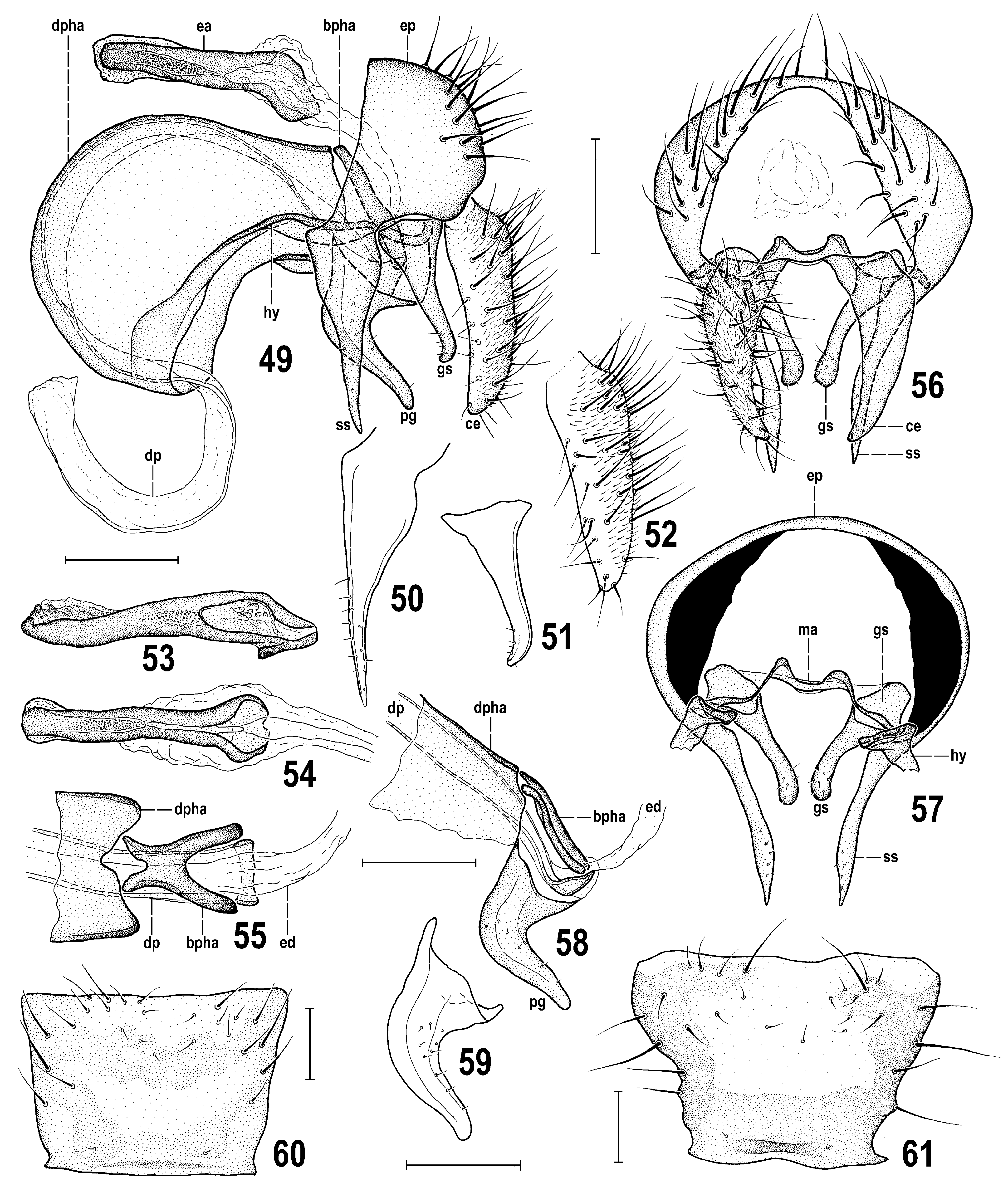
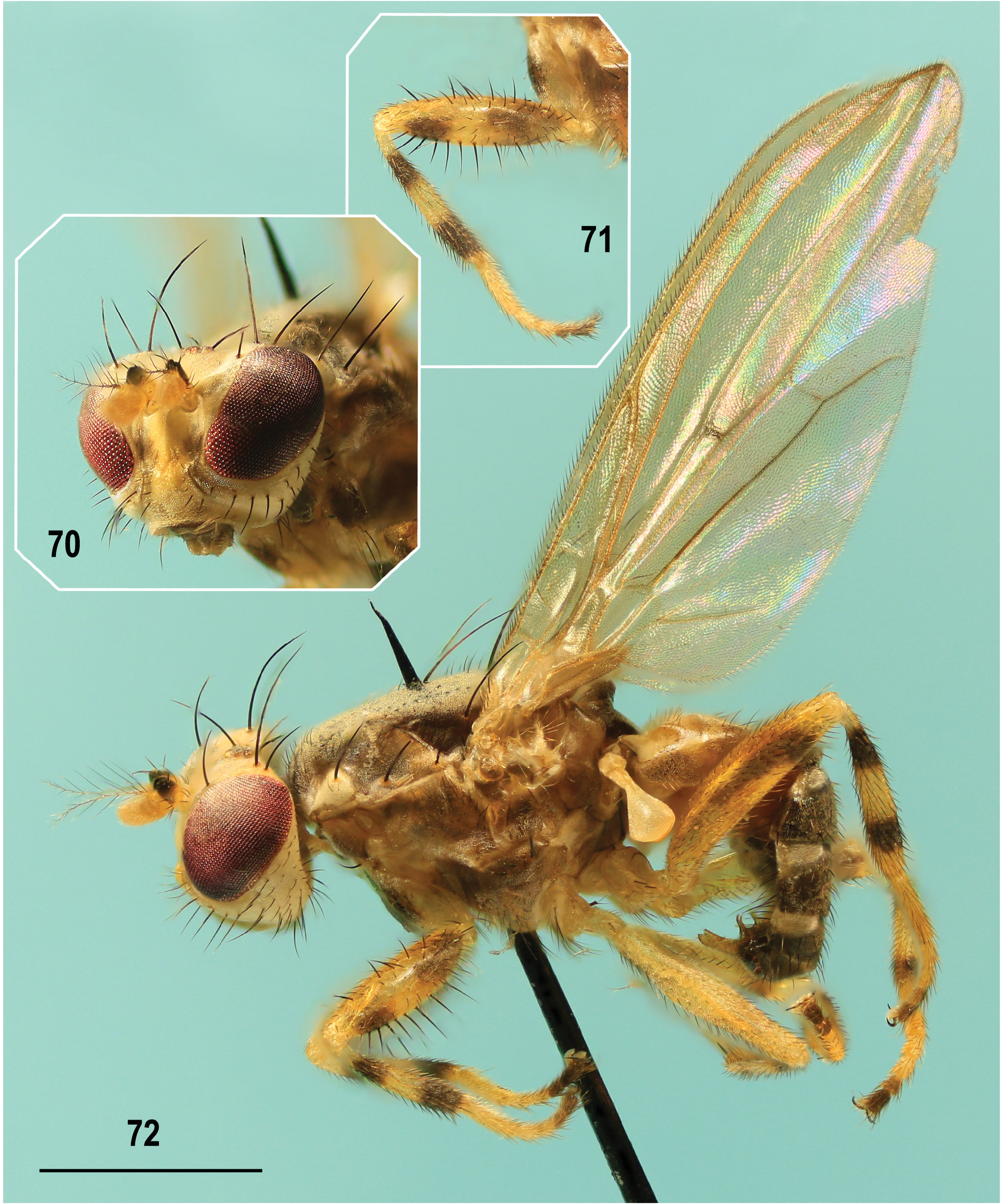
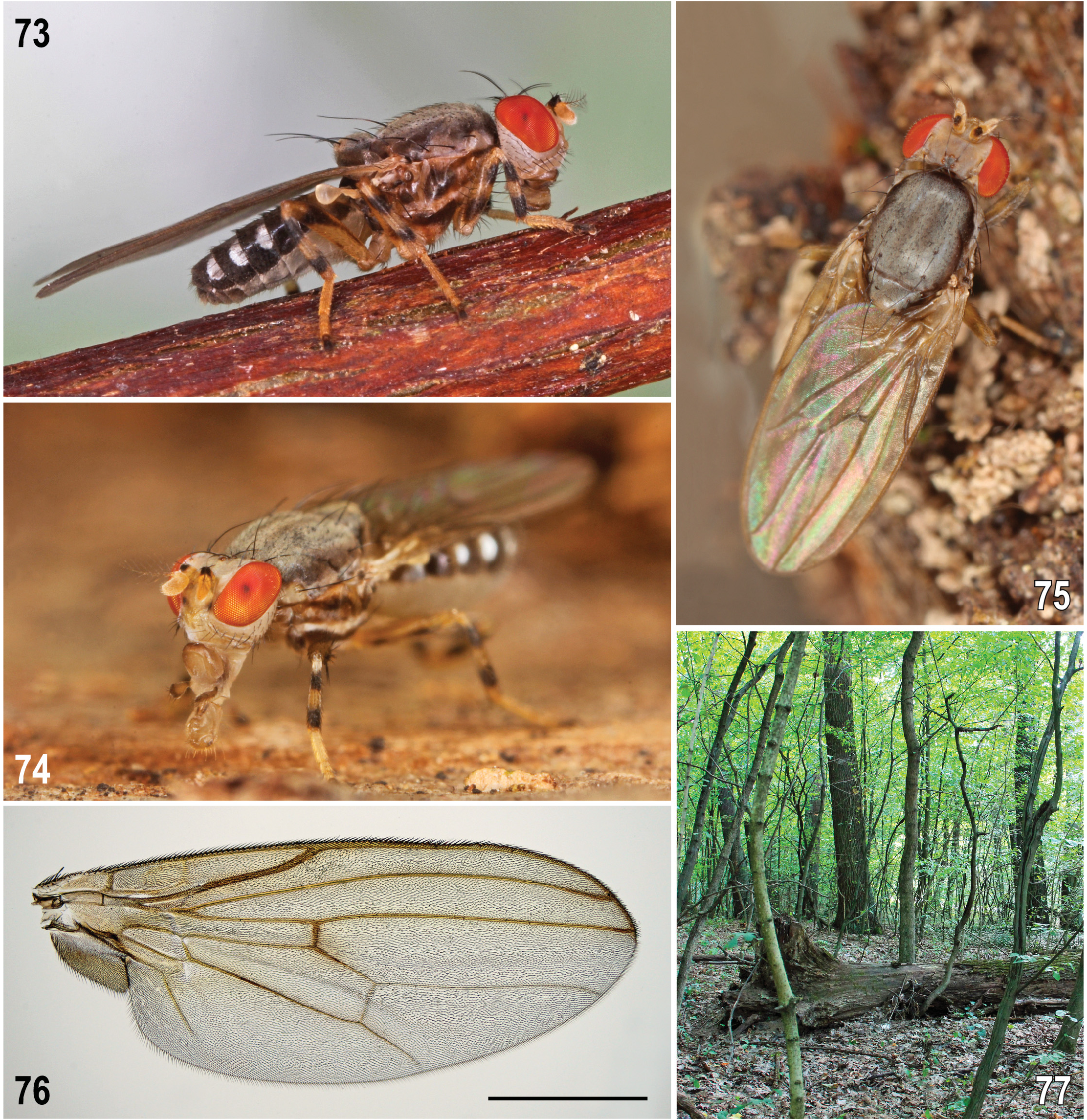
Description. Male. Total body length 2.66–3.81 (holotype 3.30) mm. General colour brown, dull, grey (with silvery blue tinge) microtomentose; some parts of head, thorax and all legs yellow to whitish variegated; abdomen with silvery white microtomentose lateral maculae on terga T2–T5 ( Figs 44, 47 View Figs 44–48 , 72 View Figs 70–72 ). Head distinctly longer ventrally than dorsally in profile ( Fig. 44 View Figs 44–48 ); face medially keel-like, protruding below antennae and relatively pale-pigmented (ochreous to whitish) but with some parts darkened ( Fig. 45 View Figs 44–48 ), largely microtomentose and dull. Eye elongately suboval, ellipsoid to slightly ovoid with longest diameter oblique and 1.6–1.7 times as long as shortest. Postgena expanded towards occiput (mainly ventrally) due to eye shape and position; eye brightly red in living adults (cf. Figs 73–75 View Figs 73–77 ), with sparse and short whitish interfacetal microsetulae. Occiput distinctly concave, brown to pale brown, only marginally yellow to (ventrally) whitish yellow; concave brownish area of occiput with relatively large patch of silvery microtomentum on each side. Frons without microsetae, broad (ca 1.7 times as wide as high), very slightly narrowed anteriorly; medially slightly but distinctly depressed against orbits and ochreous or pale brown; lateral parts of frons (mainly orbits) yellow to (anteriorly) whitish yellow and whitish microtomentose; ocellar triangle at posterior margin of frons, small but somewhat elevated, brown to blackish brown (darkest part of frons); ocelli relatively large, arranged in equilateral triangle. Face ( Fig. 45 View Figs 44–48 ) distinctly bicolourous: ventrally brown to blackish brown, dorsally yellow to pale yellow including narrow medial keel-like carina [thus face dorsally distinctly paler than in P. winnertzii where carina is normally brownish], only in Swedish specimen distinctly darkened laterally. Dark ventral part of face protruding above mouthedge and medially shining tuberculate but ventrolateral areas with distinct silvery, slightly bluish, microtomentum. Facial sensilla absent but ventrolateral part of face (below anterior end of gena) with fine inclinate setae. Gena relatively low, with only anterior corner brown, otherwise pale, largely dirty white and whitish microtomentose but its ventral margin more (anteriorly) or less darkened (ochreous to pale brown); postgena and adjacent part of occiput pale yellow to dirty white. Antennae very slightly divergent and largely yellow, 1st flagellomere whitish yellow, but pedicel with dull blackish anterodorsal spot extended anterolaterally more ventrally that in P. winnertzii , see Fig. 44 View Figs 44–48 , in extreme (specimen from Sweden) covering its entire outer side. Pedicel somewhat expanded dorsally, setose at anterior margin, with dorsal setae longer than those lateral. 1st flagellomere elongate, with dorsal margin straight to slightly concave and apex with short whitish pilosity; arista dirty yellow, somewhat longer than antenna, long-pectinate (longest dorsal rays about as long as 1st flagellomere), dorsally with 4–5, ventrally usually with 3 long brown rays in addition to shorter rays basally and in its distal half. Mouthparts ochreous to pale brown; clypeus usually dark brown; palpus slightly clavate, pale brown to brown, with a number of short dark setulae.
Cephalic chaetotaxy ( Figs 44, 45 View Figs 44–48 ): all macrosetae blackish brown; pvt well developed (but shortest of frontal setae), strongly divergent, arising behind and between posterior ocelli; vti robust and very long (longest cephalic seta, almost as long as eye longest diameter), upright, very slightly inclinate; vte and oc subequal in length, strong but much shorter than vti; vte lateroclinate; oc proclinate, subparallel to slightly divergent, inserted outside ocellar triangle; only 1 reclinate ors, shorter than vte and situated in middle of orbit; 3–5 microsetulae in front of ors; no vibrissa or pseudovibrissa but with 3–5 short ventro-proclinate setae on ventral side of vibrissal angle and anterior part of gena; 4 or 5 smaller inclinate setae also on lateroventral margin of face; gena posteriorly to vibrissal part with a series of 6–7 thicker and longer ventroclinate peristomal setae; no true genal seta; posteriorly extended postgena and occiput behind eye with a number of short setae; also posteroventral angle of occiput with a number of setae, 1 or (less often) 2 longer than others; postocular setulae behind posterodorsal margin of eye numerous, dorsally in single, ventrally in 2 to 3 rows.
Thorax ( Figs 44, 47 View Figs 44–48 , 72 View Figs 70–72 ) slightly narrower than head, brown to dark brown but laterally pale (yellow to whitish) variegated, densely microtomentose and mostly dull (except for some parts of pleural sclerites). Mesonotum (cf. Fig. 47 View Figs 44–48 ) dark brown, dorsally almost uniformly densely bluish grey microtomentose (at most dorsomedially with some brownish microtomentum) but laterally with a narrow dark brown microtomentose stripe. Scutellum normally concolourous with mesonotum, thus largely brown with greyish microtomentum but with apex often ochreous to yellow, more rarely (as in holotype, intersex (see below) and some other specimens, particularly those faded in ethanol) also medially ochreous yellow. Humeral callus (postpronotal lobe) white to whitish yellow; notopleural area also lighter than adjacent mesonotum, pale brown to yellowish around posterior npl ( Figs 44 View Figs 44–48 , 72 View Figs 70–72 ). Pleural part of thorax generally paler than mesonotum, less microtomentose and subshining, with narrow ochreous to yellow longitudinal stripe dorsally and yellow to white (anteriorly) and whitish microtomentose band in the middle, ranging from base of fore coxa to haltere ( Fig. 44 View Figs 44–48 ). Sternopleuron (katepisternum) dorsally brown but its ventral corner lighter brown to ochreous yellow. Mediotergite brown. Scutellum basally wider than long, rounded trapezoidal; subscutellum small but distinct and dark brown.
Thoracic chaetotaxy ( Figs 44, 47 View Figs 44–48 ): macrosetae and setulae blackish brown to black; ac setulae more numerous than in P. winnertzii , in 10–12 rows on suture but with only 4 rows reaching almost to scutellum, prescutellar ac setulae enlarged (more so than in P. winnertzii ); 2 very long and strong postsutural dc, the anterior about two-thirds of the more robust posterior, 12–15 dc setulae in front of anterior dc but no or only 1 setula between dc setae; 1 strong hu (postpronotal) seta plus 5 or 6 setulae on whitish humeral callus; 2 strong npl, anterior as long as or slightly longer than hu, posterior distinctly shorter; 1 sa (as long as anterior npl) and 1 slightly shorter to subequal pa; 2 sc, apical as long as posterior dc, laterobasal distinctly shorter than anterior dc; 1 pair of fine setulae between apical sc (sometimes absent); 1 short but distinct ppl, sometimes with 1 ppl setula in addition; anepisternum with a number of short setulae in posterodorsal half; 2 stpl (katepisternal) setae, anterior always shorter, some setulae between stpl, numerous setulae on disc and 4 longer but fine setae on ventral corner of katepisternum.
Wing ( Fig. 76 View Figs 73–77 ) relatively broad, with pale brown membrane darker infuscated in some small parts; veins ochreous to dark brown. Wing pattern less distinct than in P. winnertzii : infuscation around apices of R 2+3 and R
4+5 reduced, more distinct only around r-m and along M between r-m and dm-cu; also alula is darkened. Veins are distinctly darkened in all these fumose parts and also in distal two-thirds of R 1, almost entire R 4+5 and basal parts of M and CuA 1; in contrast to P. winnertzii A 1 is not so much darkened. C entire, without breaks, uniformly setulose and reaching to apex of R 4+5. Sc short, seemingly ending free in subcostal cell but its apex upcurved to C as a somewhat darkened venal fold. R 1 short but robust, slightly bent to C; R 2+3 long, running more distantly from R 4+5 than in P. winnertzii and with upcurved apex ending closer to apex of R 4+5 than M. R 4+5 slightly sinuate (not straight) and ending at wing apex. Distal part of M apically recurved, diverging from R 4+5. M normally reaching wing margin (1 ♂ from Hungary and 1 ♀ from Slovakia aberrant, with M on one wing ending far from it). Cross-vein r-m situated in distal half of dm cell; cross-vein dm-cu distinct but attenuated or interrupted by spurious vein. CuA 1 apically slightly bent, ending just in front of wing margin. Cells bm and cup closed, veins of cup somewhat attenuated. A 1 distinct but ending far from wing margin. Alula well developed, darkened, with marginal ciliation as long as that of anal lobe of wing. Wing measurements: length 2.90–4.01 (holotype 3.93) mm, width 1.11–1.49 (holotype 1.45) mm, Cs 3: Cs 4 = 0.53–0.61, r-m/dm-cu: dm-cu = 1.44–2.50. Haltere relatively large, yellowish; knob usually with slightly darker apex.
Legs ( Figs 44 View Figs 44–48 , 71, 72 View Figs 70–72 ) yellow or yellowish white and brown variegated on fore coxa (with posterodorsal and ventral part pale yellow) and all femora, tibiae and tarsi; setae brown but setulae pale, often ochreous. f 1 and f 2 have two incomplete (dorsally interrupted) brown rings, the basal paler, the subapical darker (thus with knees and dorsal side of femur yellow); while f 3 has anterodorsally (or only anteriorly) longitudinal brown spot being distally dilated and darkened to form an almost complete ring subapically, leaving knee and all remaining parts of femur yellow. Thus, f 3 is generally paler (more yellow) than that of P. winnertzii . Tibiae with two brown rings: a proximal below knee and a distal subapically but t 1 has distal ring longer, almost reaching tibial apex. Tarsi largely yellowish white to (fore tarsus) white, each with 2 distal segments brown (apical segment darker). Chaetotaxy: f 1 ( Fig. 71 View Figs 70–72 ) with a series of 8–11 (more on the average than in P. winnertzii ) long and thicker posteroventral setae and with a double row of shorter and finer upright posterodorsal setae (4 or 5 in more dorsal row thicker); f 2 posteroventrally with a row of numerous fine setae (longest about three-fourths of maximum width of femur); t 1 with 1 short (slightly longer than other tibial setulae) posterodorsal subapical seta; t 2 with 1 distinct and thicker ventroapical seta (slightly shorter than maximum width of tibia); remaining parts of legs simply shortly setulose.
Abdomen relatively broad, of subovoid outline in dorsal view, dorsally largely brown to dark brown, with some parts ochreous to yellow, ventrally pale brown. T1 + T2 as long as T3 + T4, with distinct boundary between T1 and T2. T1 dorsally ochreous yellow, only laterally brown; T2–T5 broad and transverse, bent laterally onto ventral side, gradually becoming narrower posteriorly and each brown, with a blackish brown transverse band in front of posterior margin, T3–T5 with yellowish white, silvery microtomentose spot on each side ( Figs 44 View Figs 44–48 , 72 View Figs 70–72 ). All preabdominal terga shortly setose, with longest and thickest setae in posterolateral corners. Preabdominal sterna (S1–S5) relatively large (hence membrane between terga and sterna narrow), broad and more or less transverse. S1 undescribed, probably short and pale; S2 transversely suboblong, almost completely yellow to pale ochreous; S3–S4 subequal, of similar, slightly transversely trapezoidal, brown, darker laterally; S5 narrower than S4, slightly transversely suboblong, with corners rounded. S2–S5 with scattered short fine setosity.Abdominal spiracles (1–6) in membrane close to lateral margins of terga.
Postabdomen: T6 relatively large, although narrower and somewhat shorter than T5, transverse but distinctly tapered posteriorly, setose similarly and also bearing lateral silvery spots as have T3–T5 ( Fig. 44 View Figs 44–48 ). S6 (= pregenital sternum, Figs 60, 61 View Figs 49–61 ) narrower and generally paler than S5, more or less widened posteriorly, with anterior corners acutely projecting and always without posteromedial depression, typically with brown pattern along margins ( Fig. 61 View Figs 49–61 ) and sparse fine setae in posterior half (longest laterally); rarely S6 is less widened posteriorly and with more irregular pigmentation (see Fig. 60 View Figs 49–61 ); dorsal pregenital synsclerite (a fusion of T7 and S7 and, possibly, also T8 and S8) relatively short, arch-shaped, symmetrical and laterally reaching far onto ventral side of postabdomen, dark brown and shortly setose in posterior half and also embedding 7th spiracles in its lateral parts.
Genitalia ( Figs 49–59 View Figs 49–61 ). Epandrium relatively small, dark brown, wider than high, in form of an arch-like sclerite, with large anal opening ( Fig. 56 View Figs 49–61 ), setose only in posterior third, anteriorly projecting ventrally to form slender and long surstylus ( Figs 49, 50, 57 View Figs 49–61 ) on each side. Cerci large and robust, longer than heigth of epandrium without surstylus ( Figs 49, 52 View Figs 49–61 ), relatively distant from each other ( Fig. 56 View Figs 49–61 ). Each cercus elongate, with apex tapered and slightly incurved but not acute ( Figs 49, 56 View Figs 49–61 ) and rather uniformly setose, mainly posteriorly (distinctly more densely than in P. winnertzii ); micropubescence restricted to posterior and posterolateral surface ( Fig. 52 View Figs 49–61 ). Surstylus ( Figs 49, 50 View Figs 49–61 ) proximally wider (with more or less angular anterodorsal corner), distally tapered, slender, with microsetulae in distal third, largely at outer side, and with apex acute in lateral view ( Fig. 50 View Figs 49–61 ), somewhat lanceolate in anterior view ( Fig. 57 View Figs 49–61 ) but more slender than that of P. winnertzii . Gonostylus ( Figs 49, 51, 57 View Figs 49–61 ) distinctly shorter than surstylus, basally wider, distally tapered and rod-like (in distal two-thirds), with apex curved anteriorly and with a group of microsetulae in distal fourth. Gonostyli are dorsomedially posteriorly movably attached to medandrium ( Figs 56, 57 View Figs 49–61 , ma); the latter reduced to a small transverse, slightly arched and bare sclerite. Hypandrium ( Fig. 49 View Figs 49–61 , hy) frame-like, bare, relatively symmetrical and slender, but dorsally fused to ventral parts of enlarged pocket-shaped anterior part of phallapodeme and its sides projecting posteriorly to reach medandrium. Pregonites not developed. Aedeagal complex ( Figs 49, 58 View Figs 49–61 ) more voluminous than epandrium due to enlarged phallapodeme. Phallapodeme ( Fig. 49 View Figs 49–61 , pha) composed of two sclerites: anterior (distal) sclerite strongly expanded and pocket-shaped, arched anterodorsally and ventrolaterally fused with hypandrium ( Fig. 49 View Figs 49–61 , dpha); posterior sclerite short, more heavily sclerotized, forked (shallowly anterodorsally, deeply posteroventrally (see Figs 55, 58 View Figs 49–61 , bpha) and attached to posterior end of anterior sclerite. Aedeagus simple, undivided (no separate phallophore), basally very slightly dilated and darker-pigmented ( Figs 55, 58 View Figs 49–61 ), otherwise formed by a very long, ribbon-shaped, submembranous distiphallus being proximally hidden in a pocket-shaped part of phallapodeme and distally projecting from its ventral part (see Fig. 49 View Figs 49–61 ); apex of distiphallus somewhat flattened and widened, simple to somewhat denticulate on tip. Postgonite ( Figs 58, 59 View Figs 49–61 , pg) relatively stout, much larger and thicker than gonostylus, with proximal part dilated but not expanded posteroventrally, knee-like bent in the middle, and distally slender, digitiform (but more robust than in P. winnertzii ) and with several microsetulae at posterior margin of outer side. Ejacapodeme large but smaller than phallapodeme, generally rod-like but its shape and (particularly) thickness somewhat variable (cf. Figs 49, 53 View Figs 49–61 ), with both ends dilated, proximal end more widened and somewhat forked at insertion of ejaculatory duct ( Fig. 54 View Figs 49–61 ).
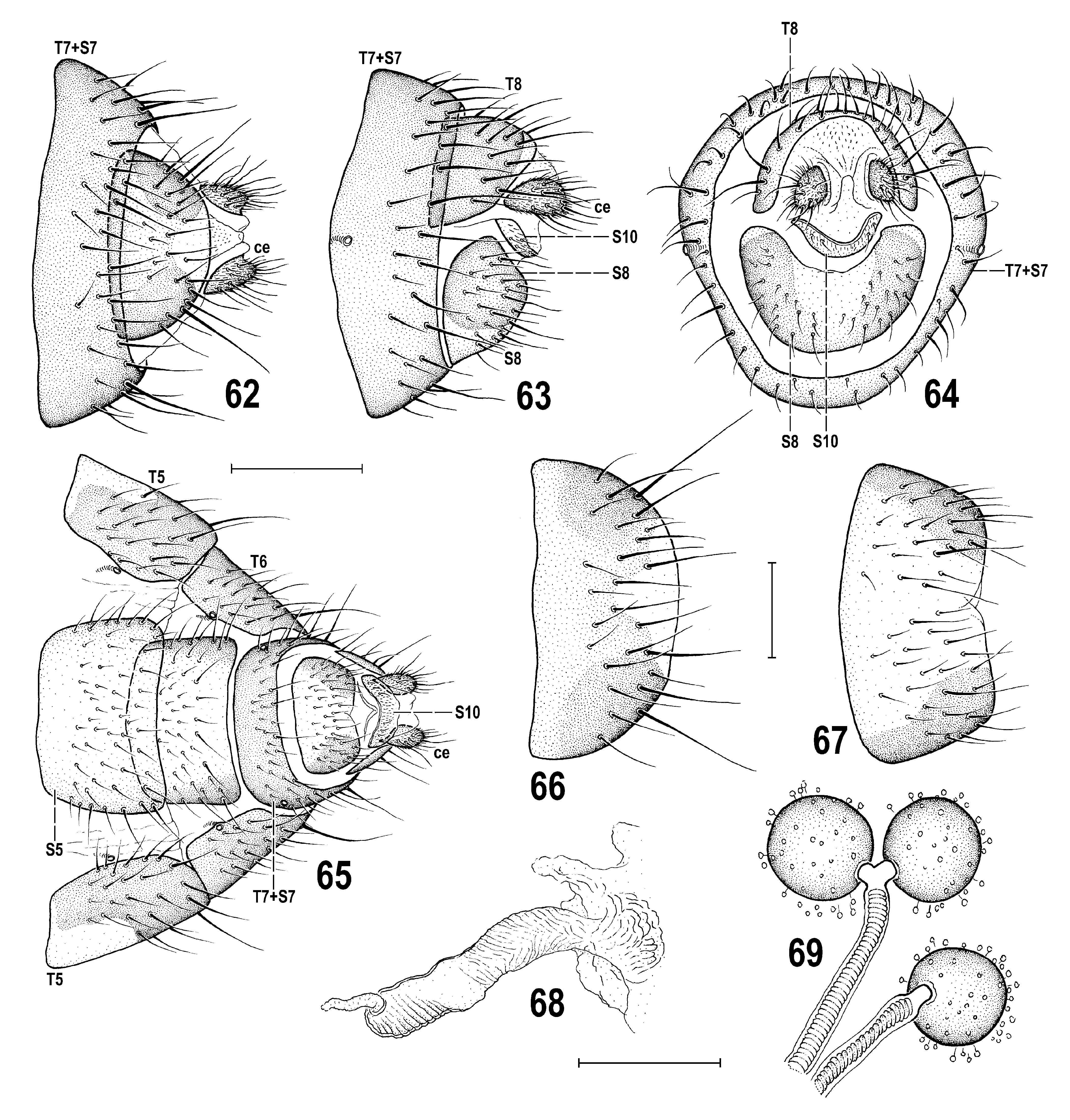
Female. Similar to male unless mentioned otherwise. Total body length 3.02–4.17 mm. Head with lighter colouration of frons and, particularly, face. Frons somewhat paler, medially ochreous, laterally and anteriorly yellow to white, only ocellar triangle brownish ( Fig. 47 View Figs 44–48 ); face dorsally almost entirely yellow to whitish yellow ( Fig. 46 View Figs 44–48 ), at most darkened pale brownish near vibrissal angle; ventrally, including shining protruding tuberculate part, dirty ochreous and below the latter densely whitish microtomentose; parafacialia with distinct small elongate brown spot above vibrissal angle (this is reduced or pale in P. winnertzii ); gena whitish yellow to (posteriorly) white ( Fig. 48 View Figs 44–48 ), with vibrissal angle and bases of peristomal setae somewhat darkened and ventral marginal line ochreous. f 2 posteroventrally without long row of setae, only subapically with 4 or 5 longer setae. Wing measurements: length 3.30–4.25 mm, width 1.29–1.63 mm, Cs 3: Cs 4 = 0.52–0.65, rm/dm-cu: dm-cu = 1.67–1.94. An aberrant specimen with supernumerary r-r cross-vein between R 2+3 and R 4+5 near apex of left wing has been found among specimens from Switzerland. Abdomen wider than in male, broadly ovoid. Preabdominal terga T3–T5 more transverse and brown (dorsomedially paler than laterally), without darker transverse bands (darkening of posterior margins is caused by overlap of sclerites) but with silvery lateral spots as in male ( Fig. 73 View Figs 73–77 ). T5 distinctly narrowed posteriorly ( Fig. 65 View Figs 62–69 ) in contrast to foregoing terga. Preabdominal sterna with similar colouration and setosity as in male but S2–S3 more transverse; S3–S5 subequal or becoming very slightly wider posteriorly, laterally less distinctly darkened and all transversely suboblong, with corners more or less rounded.
Postabdomen ( Figs 62–65 View Figs 62–69 ) broad anteriorly, strongly tapered posteriorly. T6 large, not shorter than T5 but smaller and very strongly tapered posteriorly, with lateral part bent ventrally, brown but laterobasally bearing white and silvery microtomentose spots (visible on Fig. 73 View Figs 73–77 but not on Fig. 65 View Figs 62–69 because they are situated dorsolaterally), finely setose in posterior half and laterally. S6 simple ( Fig. 65 View Figs 62–69 ), transversely suboblong, smaller than S5 but distinctly longer and less transverse than that of P. winnertzii , ochreous but posterolaterally more or less darkened, with 3 pairs of longer setae at posterior margin besides short setosity. 6th spiracle situated in margin of T6 ( Fig. 65 View Figs 62–69 ), which is more bent ventrally than that of P. winnertzii . T7 and S7 fused to tergosternum T7+S7 forming a complete ring ( Figs 64, 65 View Figs 62–69 ), uniformly brown and ventrally more densely setose than dorsally, with longest setae at posterior margin laterodorsally. 7th spiracle situated laterally, inside T7+S7 ( Fig. 63 View Figs 62–69 ). T8 forming a bent, subcircular to crescent-shaped sclerite ( Figs 62, 64, 66 View Figs 62–69 ), markedly wider than that of P. winnertzii and posteromedially pale pigmented, relatively long setose in posterior two-thirds (longest setae posterolaterally). S8 ( Figs 67, 65 View Figs 62–69 ) of size similar to T8 but round trapezoidal, narrower posteriorly, setose on entire disc (less densely than that of P. winnertzii ), largely yellowish, dark-pigmented only laterally. Genital chamber elongate, membranous; ventral receptacle ( Fig. 68 View Figs 62–69 ) submembranous, digitiform, distally not dilated, finely striated both proximally and distally (without tuberculate surface /known in P. winnertzii / subterminally), and with terminal projection simple, rather finger-like, in contrast to being tail-like and twisted in P. winnertzii . Spermathecae (1+2) globular ( Fig. 69 View Figs 62–69 ) as in P. winnertzii , but larger compared to ducts, blackish brown and heavily sclerotized; spermathecal ducts long, with internal spiral structure; duct fork connecting 2 spermathecae very short. T10 (supraanal plate) practically absent or entirely membranous ( Fig. 62 View Figs 62–69 ). S10 (subanal plate) reduced to short, crescent-shaped, weakly sclerotized and pale-pigmented sclerite ( Figs 64, 65 View Figs 62–69 ), somewhat micropubescent and with 2 or 3 posterolateral setulae. Cercus ( Figs 62, 63 View Figs 62–69 ) small, short, subovoid, with numerous (but not long) fine setae apart from dense micropubescence; lateral setae usually slightly longer than longest apical seta.
Intersex. A peculiar adult specimen from Hungary, obviously an intersex, has been examined ( Fig. 72 View Figs 70–72 ). Its terminalia are distinctly male, with all characters (including the apically curved gonostylus, visible on Fig. 72 View Figs 70–72 ) identical to those of a typical male. Also the mid femur is of a male, with a long row of posteroventral setae. However, its head has frons and face coloured as in female, including characteristically pale lower face ( Fig. 70 View Figs 70–72 ) and there are no dark transverse bands on preabdominal terga (as in female). Thus, the dimorphic male and female characters seem to have mosaic distribution on body. Moreover, it has an unusually pale (ochreous with yellow apex) scutellum, resembling more that of P. winnertzii than that of typical P. laszloi . Because this interesting intersex specimen undoubtedly belongs to P. laszloi sp. nov., it has been included in the type series as a paratype.
Etymology. The species is named in honour of the late Laszló Papp (1946–2021), an eminent Hungarian dipterist and my friend, who essentially contributed to the knowledge of the family Periscelididae and many other families of Acalyptratae.
Remarks. As noted above, Periscelis (P.) laszloi sp. nov. is identical with the species previously interpreted by PAPP & WITHERS (2011) as P. winnertzii . This erroneous concept of P. winnertzii was followed by ROHÁĆEK & ANDRADE (2017) who described a closely allied sister-species, P. fugax , being recognized here as a synononym of true P. winnertzii Egger (see above). However, the series identified by L. Papp in HNHM included in fact both species ( P. laszloi sp. nov. and true P. winnertzii ). He apparently had not differentiated them because the only male of true P. winnertzii in the collection of HNHM has lost its terminalia.
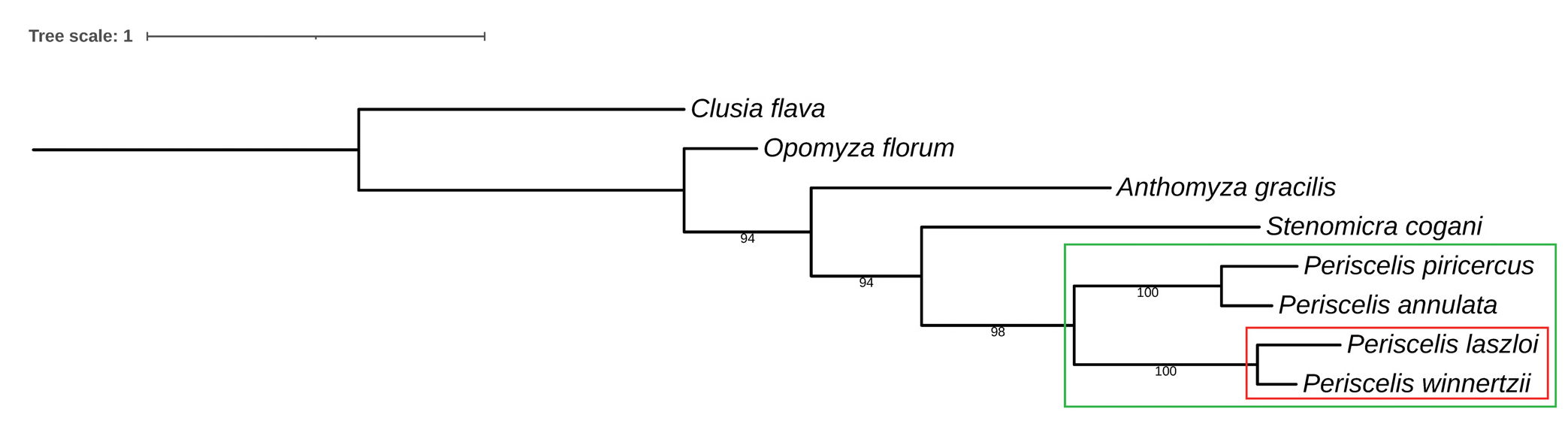
Relationships. As given above, P. laszloi sp. nov. is the closest relative of P. winnertzii Egger, 1862 . Their sister--species relationships seems to be demonstrated by close similarity of external features and, particularly, of characters of their male and female terminalia. Particularly, the gonostylus with microsetulae restricted to the apical part and the male cercus with shortly setose apex can be considered putative synapomorphies of this pair. Also the sexual dichroism of the lower face (dark brown in male, yellow in female) can be another synapomorphy of these species. No synapomorphy can be currently found in the female postabdomen because female terminalia remain unknown in other congeners. However, the close alliance of both species is also supported by similarity of their barcoding region of COI gene (see Tab. 2 View Table 2 and Fig. 78 View Fig ). This sister-pair belongs to the subgenus Periscelis (s. str.) which has been elevated to genus by PAPP & WITHERS (2011) but this act has not been accepted by MATHIS & RUNG (2011) or ROHÁĆEK & ANDRADE (2017).
Biology. PAPP (1998, as P. winnertzii ) studied the biology and behaviour of the species in Hungary. Although he did not distinguish between species under study ( P. laszloi and P. winnertzii were mixed in his series of adults) it seems that they have very similar biology, including the univoltine life-history (for detail see above under P. winnertzii ). Although preferentially associated with sap runs on oak ( Quercus robur , Q. petraea ) trees in warm forests ( Fig. 77 View Figs 73–77 ) including Quercus cerris in Slovakia ( Figs 6, 7 View Figs 1–7 ), Q. pyrenaica and other Quercus species in Portugal (ROHÁĆEK et al. 2016, fig. 11), the data obtained from material examined indicate that P. laszloi can similarly live also on other trees. Adults were also collected in oak-chestnut (Querceto-Castanetum) forest, hornbeam (Carpinetum) forest, mixed submontane deciduous forest with prevailing beech and, according to HELLQUIST (2020, as P. winnertzii ), a pair of Swedish specimens was even captured in a Malaise trap installed among uprooted aspen tree trunks. As in P. winnertzii adults of P. laszloi are attracted to wine and beer and can be most easily collected by mean of traps with this bait installed in tree canopy; interestingly, in Slovakia, one female was also captured into a protein (meat-baited, with ethanol as preservation medium) trap. Adults occur from July to November (most commonly in August to October) but two specimens were captured already in late June.
Distribution. Because previous records of “ Periscelis winnertzii ” have not all been revised, data on distribution of Periscelis laszloi sp. nov. remain rather fragmentary. Only as and when the two species have been separated by ROHÁĆEK & ANDRADE (2017) subsequent authors recorded this species reliably but under the name P. winnertzii .
Thus, P. laszloi has hitherto been only confirmed in Portugal ( ROHÁĆEK & ANDRADE 2017, as P. winnertzii , see also type material), Spain (CARLES- TOLRÁ 2018, as P. winnertzii = a revised previous record by CARLES- TOLRÁ & PAGOLA- CARTE 2013), Switzerland ( POLLINI PALTRINIERI & ROHÁĆEK 2022, as P. winnertzii and type material), Slovakia ( ROHÁĆEK 2013, ROHÁĆEK & ANDRADE 2017, both as P. winnertzii , and type material), Hungary ( ROHÁĆEK & ANDRADE 2017, as P. winnertzii , and type material) but also in Great Britain ( England, P. J. Chandler, personal communication 2022, see above), southern Sweden (HELLQUIST 2020, as P. winnertzii ) and southern Finland ( HAARTO & WINQVIST 2014, as P. winnertzii ). The identity of the specimen from the latter country, viz. from N: Raasepori, Dragsvik has been verified by comparison of its photograph and COI sequence in BOLD system, see https://www.boldsystems.org/index.php/Public_BarcodeCluster?clusteruri=BOLD:ACE1527. A record of P. winnertzii from Poland (Breslau = Wrocław) revised by PAPP & WITHERS (2011) is uncertain because these authors have not recognized both species. This also is true for some of the previous records of P. winnertzii from Great Britain, France, The Netherlands, and Germany (summarized by MATHIS & RUNG 2011). Hitherto, P. laszloi has not been ascertained in the Czech Republic because all specimens recorded by MÁCA et al. (2005) proved to belong to true P. winnertzii (= P. fugax , see above), thus the latter species is listed correctly in MÁCA (2009). Obviously, P. laszloi is widespread in southern and temperate Europe, and, consequently, it is expected to be recorded from other countries in future, particularly by means of wine and beer baited traps installed in canopies of deciduous trees.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Periscelis (Periscelis) laszloi
| Roháček, Jindřich 2022 |
Periscelis winnertzii:
| PAPP L. & WITHERS P. 2011: 354 |