Periscelis (Periscelis) winnertzii Egger, 1862
|
publication ID |
https://doi.org/ 10.37520/aemnp.2022.018 |
|
publication LSID |
lsid:zoobank.org:pub:1C001FE0-4D51-46DE-849A-D66BB9A05B47 |
|
DOI |
https://doi.org/10.5281/zenodo.10552617 |
|
persistent identifier |
https://treatment.plazi.org/id/A11387B0-FF89-FFDF-FF5C-25BEFDC9FC8B |
|
treatment provided by |
Felipe |
|
scientific name |
Periscelis (Periscelis) winnertzii Egger, 1862 |
| status |
|
Periscelis (Periscelis) winnertzii Egger, 1862 View in CoL View at ENA
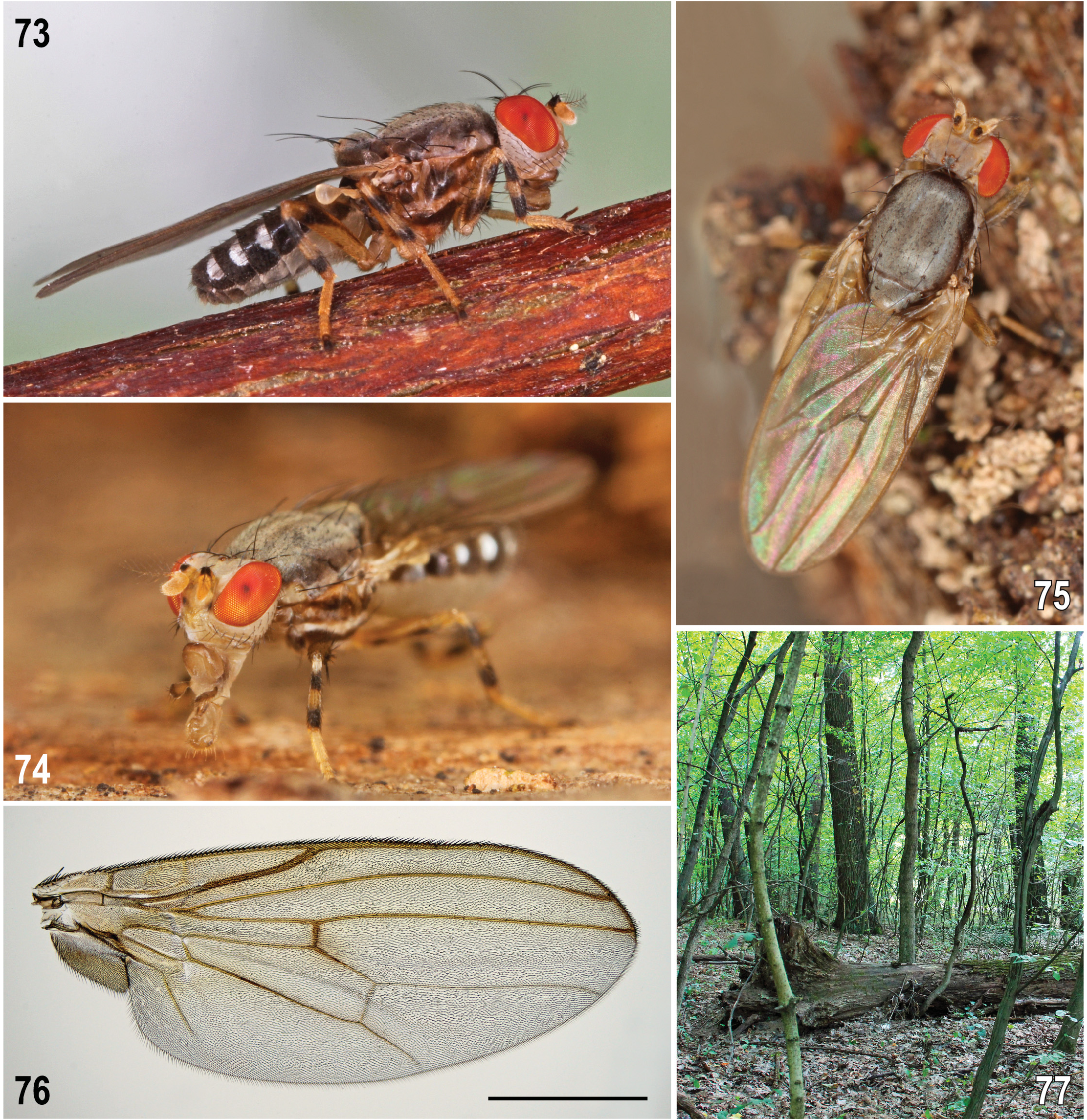
( Figs 1–5 View Figs 1–7 , 8–43 View Figs 8–12 View Figs 13–21 View Fig View Figs 23–29 View Figs 30–34 View Figs 35–43 )
Periscelis Winertzii Egger, 1862: 780 View in CoL (description, error due to misspelling of the name Winnertz).
Periscelis winertzii: MATHIS & RUNG (2011) : 358 (catalogue; in part, misspelling).
Periscelis Winnertzii: SCHINER (1864) View in CoL : 272 (revision, key, emendation of name).
Periscelis Winnertzi: BECKER (1905) View in CoL : 217 (catalog; misspelling).
Microperiscelis Winnertzi: OLDENBERG (1914) :37 (generic combination; in part?, misspelling); SḖGUY (1934): 394 (key; in part?, misspelling).
Periscelis (Microperiscelis) Winnertzi: DUDA (1934) View in CoL : 11 (revision; in part?, misspelling)
Periscelis (Microperiscelis) winnertzi: PAPP (1984) View in CoL : 234 (catalogue; in part, misspelling).
Parclioscena Winnertzii: ENDERLEIN (1936) : 177 (generic combination).
Periscelis (Periscelis) fugax Roháček &Andrade, 2017:234 View Cited Treatment (description, illustr.), syn. nov.
Type material. Periscelis winnertzii Egger. LECTOTYPE (here designated): sex unknown (probably female), labelled: “Winnertzii [handwritten], det. Schiner” [printed] (pale brown label); “ Austria [handwritten], Alte Sammlung” [printed]; “Type” [printed] (red label); “ Periscelis (Periscelis) winnertzii Egger, 1862 , sex?, J. Roháček det.2018“ and “ LECTOTYPUS, Periscelis Winertzii Egger, 1862 , J. Roháček des. 2018” (red label) (see Figs 3, 4 View Figs 1–7 ). The specimen is heavily damaged by Anthrenus larvae (their setae are visible on specimen, cf. Fig. 2 View Figs 1–7 ), with ventral part of head, ventral and some left lateral sclerites of thorax and entire abdomen missing ( Figs 1, 2 View Figs 1–7 ) but left fore leg is glued on label “ Austria, Alte Sammlung” ( NHMW, examined).
Periscelis fugax Roháček &Andrade. HOLOTYPE: ♂, labelled:“PORTUGAL: Porto: Valongo, Valongo, 41°09′33.4″N, 8°29′05.6″W, 50–100 m, R. Andrade leg.”, “ 10.x.2011, sweeping over bark of Quercus trees with sap runs”, “ Holotypus J, Periscelis (P.) fugax sp.n., J. Roháček & R.Andrade det. 2016” (red label) and “ Periscelis (Periscelis) winnertzii Egger, 1862 ,♂, J. Roháček det. 2022” ( SMOC, intact,examined, Fig. 10 View Figs 8–12 ). PARATYPES: 9♂♂ 10♀♀, same data as for holotype (4♂♂ 4♀♀ including 1 ♂ 1 ♀ with genit. prep. SMOC; 2 ♂♂ 2 ♀♀ NMPC; 3 ♂♂ 4 ♀♀ in RAP); 1 ♀ with same data but collected 26.ix.2011 ( RAP); 6 ♂♂ with same data but collected 1.x.2011 (3 ♂♂ SMOC, 3 ♂♂ /1 ♂ genit. prep./ RAP); 7 ♂♂ 2 ♀♀ with same data but collected 4.x.2011 (4 ♂♂ SMOC, 3 ♀♀ / 1 ♀ genit. prep./ RAP); 8 ♂♂ 4 ♀♀ with same data but collected 14.x.2011 (2 ♂♂ 2 ♀♀ /1 ♂ 1 ♀ genit. prep./ SMOC, 6 ♂♂ 2 ♀♀ RAP); PORTUGAL: Bragança: Bragança,Parâmio,Parque Natural de Montesinho, 41°53‘54.0‘‘N, 6°51‘16.3‘‘W, 780 m, 21.vi.2015, sweeping over bark of Quercus trees with sap runs, 1 ♂, R. Andrade leg. ( RAP); Portalegre: Marvão, Santa Maria de Marvão, 39°23‘50.2‘‘N, 7°21‘52.3‘‘W, 616 m, 21.ix.2014, sweeping over bark of Quercus pyrenaica trees with sap runs, 1 ♂ 1♀,Ana Gonçalves leg.( ARGC);all specimens in SMOC and NMPC dried from ethanol and mounted on pinned triangular cards, those in RAP and ARGC retained in ethanol. CZECH REPUBLIC: C. Bohemia: Roztoky,Tiché údolí, Roztocký háj (5852), 50°8‘47.5‘‘N, 14°23‘10.1‘‘E, beer trap, 2.–10.ix.2009, 1 ♂, J. Preisler leg. ( SMLC); Český kras PLA, Na Voskopě res., 49°54‘25‘‘N, 14°04‘05‘‘E, beer trap, oak-hornbeam forest, 16.ix.–2.x.2016, 1 ♀, P. Heřman leg. ( JMB); S Moravia: Podyjí NP, Liščí skála, 48°49‘52‘‘N, 15°56‘35‘‘E, 410 m, Quercetum, Malaise trap, 3.viii.–9.ix.2004, 1 ♀, 9.ix.–28.x.2004, 6 ♂♂ 7 ♀♀, M. Barták & Š. Kubík leg. (2 ♂♂ 4 ♀♀ MBP; 2 ♂♂ 2 ♀♀ /1♂ genit. prep./ SMOC; 1 ♀ JMB); Podyjí NP, Fládnická chata, 48°48‘42‘‘N, 15°58‘03‘‘E, 360 m, forest, 9.ix.–28.x.2004, Malaise trap, 2 ♂♂, M. Barták & Š. Kubík leg. ( MBP); Podyjí NP,Havrarníky, 48°48‘52‘‘N, 15°59‘48‘‘E, 330 m, forest--steppe, 1.–24.vii.2002, Malaise trap, 1 ♀, O. Meixnerová leg. ( MBP); Podyjí NP, Vraní skála, 48°51‘03‘‘N, 15°53‘42‘‘E, 390 m, mixed wood, 8.vii.–28.x.2003, Malaise trap, 1 ♂, O. Meixnerová leg. ( JMB); all dried from ethanol and mounted on triangular cards.All paratypes with yellow label “ Paratypus ♂ (or ♀), Periscelis (P.) fugax sp.n., J. Roháček & R. Andrade det. 2016” and most of them also with white label “ Periscelis (Periscelis) winnertzii Egger, 1862 , ♂ (or ♀), J. Roháček det. 2022”.
Other material examined. SWITZERLAND: TI 701.168 113.372, Losone: Arcegno, Collina di Maia, Castagneto con querce, 419 m, prd. 28, vino bianca [white wine trap], ARC 2, 23.viii.–7.xi.2017, 13 ♂♂ 13 ♀♀, L.Pollini P. & M.Abderhalden leg. (all dried from ethanol, SMOC); 1 ♀ ( SMOC, body after DNA extraction preserved in a pinned microvial in glycerine, with blue label: JR 34, ON637245 View Materials ); same locality data and method but prd. 8, 9.–23.x.2015, 1 ♂, L. Pollini P. & M. Abderhalden leg. ( MCSN). SLOVAKIA: S. Slovakia: Cerová vrchovina PLA, Hajnáčka-Gortva 0.9 km E, Steblová skala res., 240 m, 48°14‘51‘‘N, 19°58‘12‘‘E, beer trap, 27.ix.–1.xi.2017, 1 ♀, J. Roháček, J. Ševčík & M. Tkoč leg. ( SMOC); Cerová vrchovina PLA, Hajnáčka-Buková 0.4 km NNE, 48°13′39″N, 19°58′25″E, 375 m, beer trap, 12.ix.–11.x.2018, 3 ♂♂ 1♀, J. Roháček, J. Ševčík & M.Tkoč leg.( SMOC, 1 ♂ genit. prep.); Cerová vrchovina PLA, Gemerský Jablonec-Vodokáš 0.5 km N, 320 m, 48°12‘47‘‘N, 19°59‘30‘‘E, beer trap, 7.–27.ix.2017, 3 ♀♀, 27.ix.–1. xi.2017, 1 ♀; same locality, Malaise trap, 27.ix.–1.xi.2017, 1 ♀; all J. Roháček, J. Ševčík & M. Tkoč leg. ( SMOC); Cerová vrchovina PLA: Jestice 1.3 km SSE, Hradisko Mt., 48°12’31”N, 20°03’39”E, 255 m, wine traps on Quercus cerris , 19.vii.–17.viii.2022, 4 ♂♂ 4 ♀♀, 17.viii.–13. ix.2022, 2 ♀♀, 13.ix.–26.x.2022, 2 ♀♀, J. Roháček leg. (2 ♂♂ 2 ♀♀ NMPC, 2 ♂♂ 6 ♀♀ SMOC). HUNGARY: Szokolya: Királyrét, Szénpatak, [larva] 23.v.1996, reared from a wound of Quercus tree, [adult ♀ emerged] 13.vii.1996, [L. Papp leg.], 1♀ with puparium originally glued to a card, now preserved in glycerine in a pinned microvial ( HNHM); Szokolya, Vasfazék-v., Magas Tax alatt, 450 m, fekete tölgyfaseb, kifolyó nedvéről, 13.ix.1997, 1 ♂ 6 ♀♀, L. & J. Papp leg. ( HNHM, ♂ with terminalia lost). CROATIA: Oprtalj, 45.403 N, 13.842 E, 430 m, Swissino baited trap nr. bee hive, 10.ix.–14.x.2019, 1♂; Benčani, 45.283 N, 13.740 E, 269 m, Swissino baited trap nr. bee hive, 10.ix.–15.x.2019, 1 ♂, both B. Sladonia leg. ( SMOC).
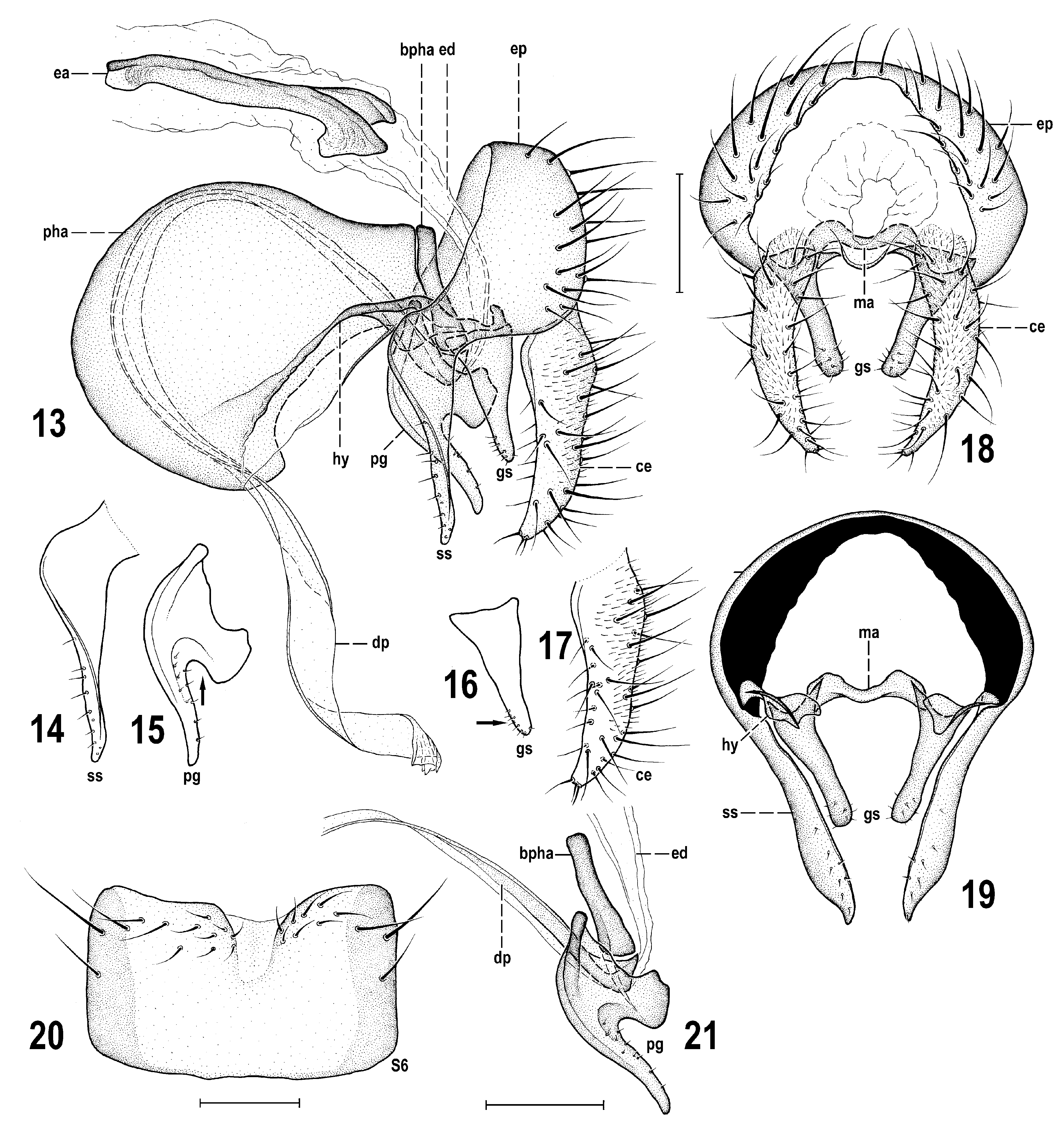
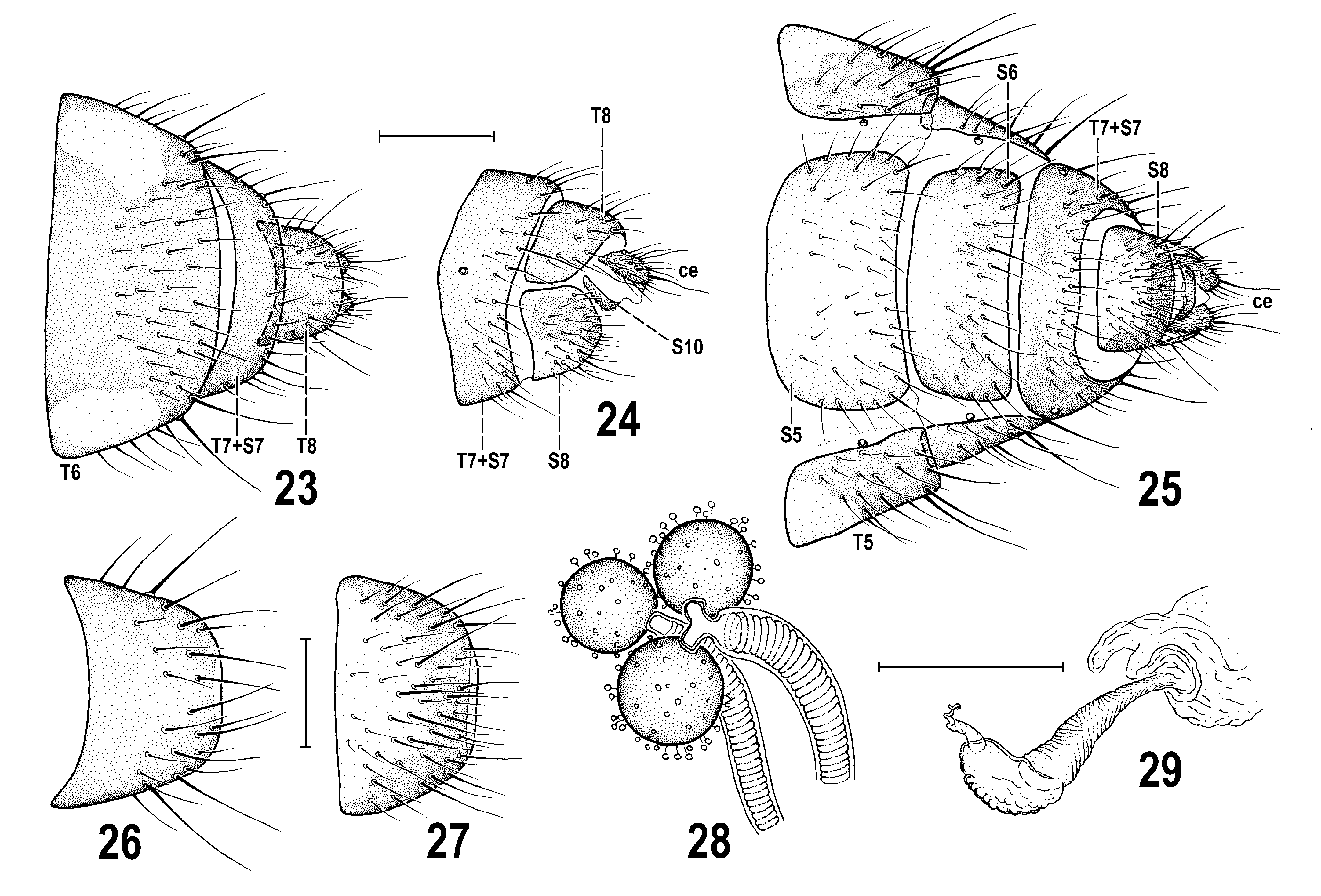
Diagnosis. A rather large Periscelis (s. str.) species ( Figs 10 View Figs 8–12 , 22 View Fig ) with body ca 2.4–3.8 mm long, sexually dichroic face ( Figs 8, 9 View Figs 8–12 ) and wing with cross-vein dm-cu developed but attenuated (or interrupted) by spurious vein in the middle. It is most similar to its closest relative P. laszloi sp. nov. (described below) but differs from the latter by a distinctly smaller black spot on antennal pedicel (not extended ventrally at anterior margin of its external side, see Figs 10, 11 View Figs 8–12 ); mesonotum usually with a distinct pair of brown microtomentose elongate spots or vittae medially ( Fig. 12 View Figs 8–12 ); largely to entirely yellow scutellum ( Fig. 12 View Figs 8–12 ); wing with more extensive dark pattern ( Figs 5 View Figs 1–7 , 10 View Figs 8–12 ); suboblong male S6 with brown lateral pigmentation and narrow medial depression ( Fig. 20 View Figs 13–21 ); surstylus with apex (albeit slender) blunt at tip ( Figs 13, 14 View Figs 13–21 ); gonostylus smaller and gradually tapering towards simple apex ( Figs 13, 16 View Figs 13–21 ); postgonite with dilated basal part being expanded posteroventrally (see Fig. 15 View Figs 13–21 , arrow); female T8 brown pigmented ( Fig. 26 View Figs 23–29 ); female S8 darkened brown at lateral and posterior margins ( Fig. 27 View Figs 23–29 ).
Redescription. The species has been described in detail as Periscelis fugax by ROHÁĆEK & ANDRADE (2017: 234–240). Consequently, it is unnecessary to redescribe P. winnertzii here and only illustrations of diagnostic characters and, particularly, of structures of the male and female terminalia are presented to facilitate its safe identification ( Figs 5 View Figs 1–7 , 8–29 View Figs 8–12 View Figs 13–21 View Fig View Figs 23–29 ). However, some new information on the variability of some external morphological characters are given below.
Variability. While the extent of the black spot on the pedicel seems to be relatively stable, the colouration of scutum and scutellum can vary. Although the scutellum is usually largely yellow and contrasting with adjacent part of scutum, it can be rarely ochreous or even brown darkened, particularly basally, exceptionally (1 ♀ from Switzerland, 1 ♂ from Slovakia) largely brown with only apex yellowish, thus most resembling that of P. laszloi sp. nov. Also the brown microtomentose vittae on mesonotum seem to be variable, both in length and distinctness. Moreover, immature adults and specimens faded due to preservation in alcohol or long stored in collections can have these colour characters obscured and sometimes cannot be identified safely. For these aberrant and/or faded specimens it is recommended to study structures of the male or female terminalia for safe identification.
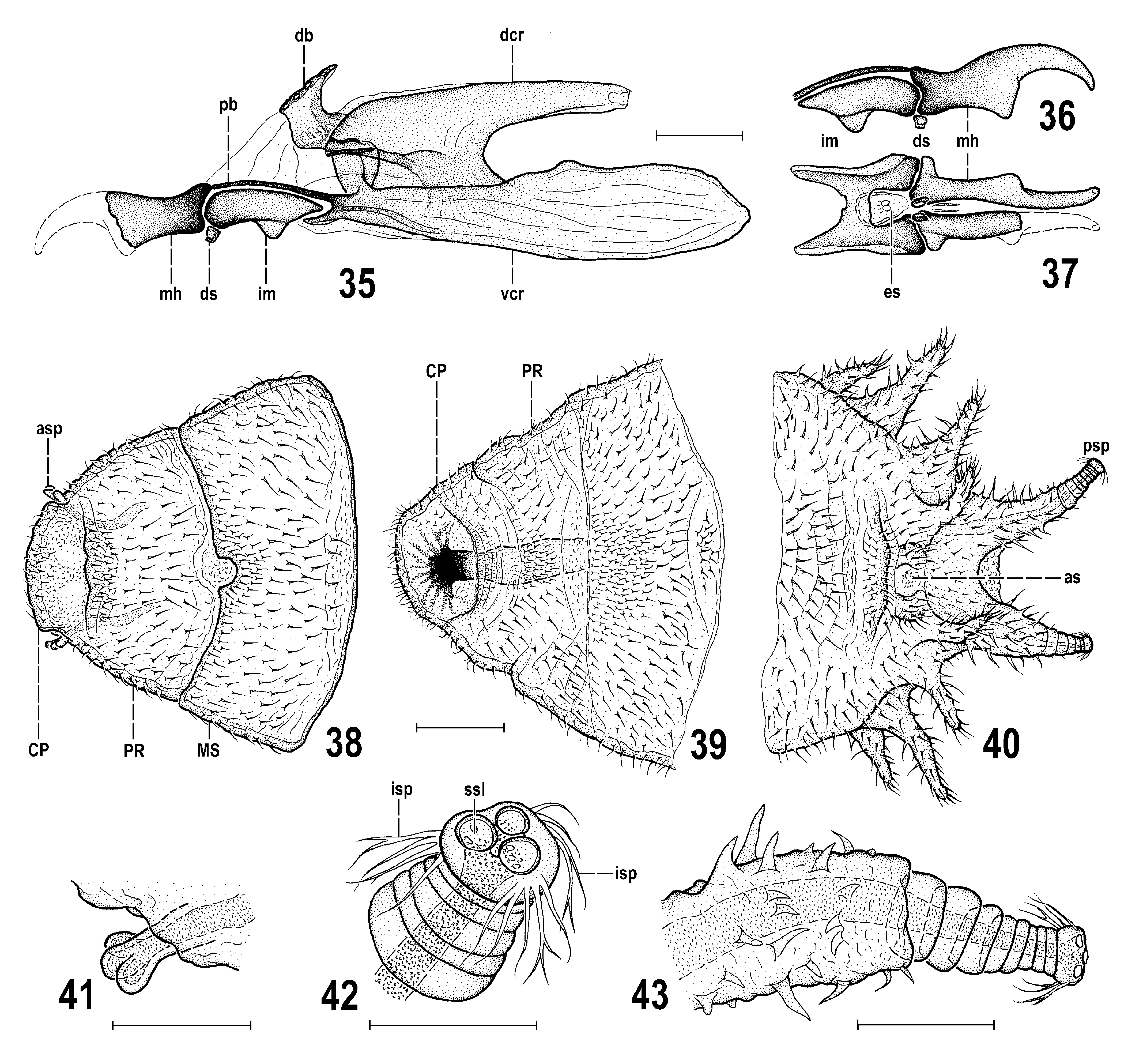
Preimaginal stages. Larva. Cephalopharyngeal (= head) skeleton of 3rd instar larva ( Figs 35–37 View Figs 35–43 ) of saprophagous type, as known in the majority of other acalyptrate larvae, most resembling that of Periscelis (Myodris) annulata , described by PAPP (1988, 1995) and MATHIS & PAPP (1998). The paired mouthhooks (= mandibles) relatively large and long compared to other parts of skeleton ( Fig. 36 View Figs 35–43 , mh); each laterally flattened, with simple, slightly bent distal hook, in the middle projecting ventrally as a blunt tooth (less strongly than in P. annulata ), posteriorly widened (more distinctly laterally than dorsoventrally, cf. Fig. 37 View Figs 35–43 ) and in lateral view somewhat incised ( Figs 35, 36 View Figs 35–43 ), all simply pigmented, gradually becoming paler distally. Dental sclerites ( Figs 35, 36 View Figs 35–43 , ds) situated below mouthhooks at their posterior margin, small, rounded block-shaped, with slightly projecting ventral ends. No accessory oral sclerites. Intermediate (= hypostomal) sclerite ( Figs 35–37 View Figs 35–43 , im) situated between mouthhooks and pharyngeal sclerite and clearly separate from both of them, strikingly short (markedly shorter than mouthhook and also shorter than that of P. annulata ) and relatively broad, H-shaped in ventral view ( Fig. 37 View Figs 35–43 ), with connecting bridge-shaped part between lateral rods expanded ventrally (but less than in P. annulata ). Epistomal plate flat ( Fig. 37 View Figs 35–43 , es), elongately rounded subtriangular, pale-pigmented, between anterior arms of intermediate sclerite, with minute, closely attached oval?perforations in the middle (see Fig. 37 View Figs 35–43 ). Pharyngeal sclerite ( Fig. 35 View Figs 35–43 ) elongate and relatively low. Its paired anterior projections (parastomal bars, pb) very slender, slighly bent and situated just above intermediate sclerite, each distally connected to posterodorsal end of mouthhook. Dorsal (dcr) and ventral (vcr) cornua of pharyngeal sclerite well developed. Dorsal cornu shorter, darker, only slightly tapered posteriorly and its end blunt ( Fig. 35 View Figs 35–43 ) in contrast to that of P. annulata . Dorsal cornua anterodorsally connected by rather unusual dorsal bridge (db) which is strikingly projecting dorsally and posteriorly, with dorsal side distinctly perforated (see Fig. 35 View Figs 35–43 ). Also anteroventral part of dorsal cornu with distinctive structure and pigmentation. Ventral cornua longer and more robust (mainly posteriorly) than dorsal cornua, pale brown pigmented and posteriorly weakly sclerotized, longitudinally striated. Ventral trough, connecting ventral cornua, entirely membranous and very finely striated. Dorsal margin of posterior half of ventral cornu without dorsal apodeme, only slighly bulging ( Fig. 35 View Figs 35–43 ).
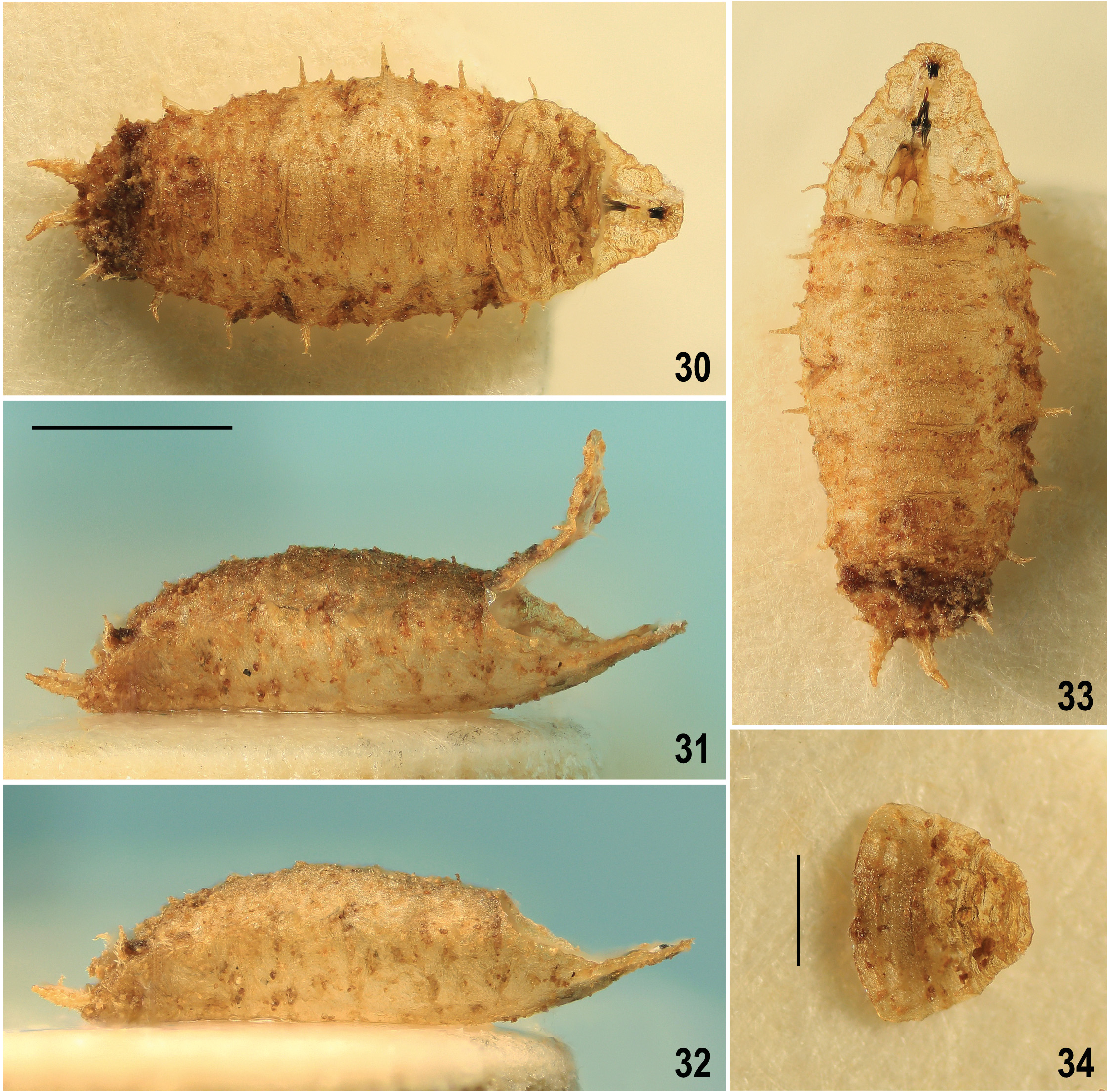
Puparium ( Figs 30–34 View Figs 30–34 ) rather spindle-shaped than barrel-shaped, moderately elongate, distinctly wider than high but not dorsoventrally flattened, with more tapered anterior end and less narrowed and rounded posterior end (apart from larval posterior digitiform processes). Measurements (based on single female puparium examined): length 2.98 mm, maximum width 1.23 mm, maximum height 0.81 mm, maximum length of lateral process 0.13 mm, length of caudal process with posterior spiracle 0.28 mm. Integument of (empty) puparium ochreous yellow to ochreous brown (darker posteriorly), all surface finely and densely spinulose apart from being sparsely and finely transversely wrinkled. All thoracic and abdominal segments with lateral digitiform and spinose processes (= secondarily sclerotized fleshy processes of larva) and smaller bulbous projections ( Figs 30, 33 View Figs 30–34 ). Thus, larval characters are rather well preserved on puparium but modified by sclerotization of puparium. Segmentation of body more or less visible ( Figs 30, 31 View Figs 30–34 ). Anterior end of puparium ( Figs 34 View Figs 30–34 , 38, 39 View Figs 35–43 ) distinctly tapered but dorsoventrally flattened, equalling to thoracic (plus integrated cephalic) segments of larva. First visible segment (= cephalic + prothoracic part) laterally with (larval) anterior spiracles; each spiracle very small, short ( Figs 38, 41 View Figs 35–43 ), terminated in 3 bulbous papillae (hence different from that of larva of P. annulata having 4 or 5 papillae). First visible segment distinctly narrower but longer than 2nd segment, anteromedially simple, rounded, dorsally ( Fig. 38 View Figs 35–43 ) and ventrally (cf. Fig. 39 View Figs 35–43 ) only very finely wrinkled but densely spinose, with finest spinulae anteromedially, largest spinulae laterally. Dorsal side of first visible segment with cephalic part ( Fig. 38 View Figs 35–43 , CP) flat, spinulose only at anterior margin and anteromedially, and with a pair of plain rounded areas; prothoracic part ( Fig. 38 View Figs 35–43 , PR) with minute spinulae restricted to anterior submarginal area, otherwise with longer spines. Ventral side of first visible segment having in cephalic part distinctive dark palmately branched pigmentation ( Fig. 39 View Figs 35–43 ) obviously representing remnants of larval oral ridges; its prothoracic part characterized by fine transverse wrinkles, finely spinulose anteromedially and sparsely spinose in other areas except for the posteromedial part being finely tuberculate. Second (mesothoracic) segment dorsally ( Fig. 38 View Figs 35–43 ) sculptured and spinulose similarly as is foregoing segment but distinguished by medial semicircular incision of anterior margin. Third (metathoracic) segment provided with lateral digitiform and spinose processes similarly as all following (abdominal) segments. Cephalopharyngeal skeleton of 3rd-instar larva situated inside of anterior part of puparium, affixed to its ventral wall (see Fig. 33 View Figs 30–34 ). Posterior end (= caudal segment) of puparium more convex and posteriorly rounded ( Figs 30, 33 View Figs 30–34 ) and bearing several digitiform processes, posteriorly, laterally and also ventrally ( Fig. 40 View Figs 35–43 ); posterior pair of processes longest and carrying posterior spiracles (see below). Dorsal side of caudal segment medially more tuberculate and densely spinose ( Fig. 30 View Figs 30–34 ); ventral side of caudal segment anteriorly finely and sparsely transversely wrinkled but in front of (larval) anus with a more elevated and dark transverse ridge that is very finely spinulose ( Fig. 40 View Figs 35–43 ). Anus surrounded by bent spinose structures ( Fig. 40 View Figs 35–43 , as). Posterior spiracles situated on long, digitiform and strongly spinose processes ( Figs 40, 43 View Figs 35–43 ) being distally bare, ringed and originally (in larva) obviously retractable; no lateral projections on these spiracular processes (in contrast to those of P. annulata ) but ventrally, at their bases with an additional pair of smaller spinose processes ( Fig. 40 View Figs 35–43 ). Posterior spiracle small, set on short, stump-like end of posterior spiracular process ( Fig. 40 View Figs 35–43 ). Each spiracle with only 3 subcircular spiracular slits and 3 tufts of lateroclinate interspiracular hairs, some of which can be branched ( Fig. 42 View Figs 35–43 ).
Remarks. Nomenclature. EGGER (1862, p. 780) used the name “ Winertzii ” throughout the original description including the derivatio nominis in the last paragraph of his text. Thus, the incorrect spelling of this name was not typographical or typing error as given by PAPP & WITHERS (2011: 356) and repeated by ROHÁĆEK & ANDRADE (2017: 247) but an unwanted (albeit several times repeated) distortion of the name of the collector, Johann Winnertz, to whom the species was dedicated. Jean Jean (also Johannes or Johann) Winnertz (11.ii.1800 – 24.vii.1890) was a factory owner, businessman and Commercial Court President in Crefeld (W. Germany) but also a well-known dipterist (mainly studying nematocerous flies) and sponsor of natural sciences ( ENSS et al. 2020). EGGER (1862, p. 780) literally wrote “Winertz hat sie schon vor Jahren gekannt und beschrieben, aber nicht veröffentlicht. Er hat sie Herrn Dr. Schiner bei Abfassung seines grossen Dipteren-Werkes zur Verfügung gestellt, wobei sich gezeigt hat, dass sie auch in Oesterreich einheimisch ist.” [my translation: Winertz knew and described it years ago but did not publish it. He gave it to Dr. Schiner to be available when he was writing his large Diptera work, whereby it has been shown that it is also native to Austria]. Consequently, J. Egger described this species from Austrian specimens (collected by J. Winnertz?) received from J. R. Schiner so that this species could be included in Fauna Austriaca ( SCHINER 1864: 272). Apparently, J. R. Schiner only orally asked J. Egger to name the species in honour of J. Winnertz which resulted in distortion of his surname. Therefore, already SCHINER (1864) correctly emended the name of this species to P. winnertzii . Subsequently, the species’ name has been variously misspelled; moreover, in the past P. winnertzii had been placed (besides Periscelis Loew, 1858 ) in two other genera and/or subgenera, viz. Microperiscelis Oldenberg, 1914 (= junior synonym of Myodris Lioy, 1864 ) and Parclioscena Enderlein, 1936 (here as type species!), see synonymies above.
Preimaginal stages. PAPP (1998) and partly also MATHIS & PAPP (1998) mention larvae and a puparial shell of P. winnertzii collected in Hungary (see in “Biology” below). However, only one larva was reared to a female adult and really belonged to P. winnertzii . The identity of the remaining larvae is uncertain, inasmuch as both P. winnertzii and P. laszloi sp. nov. occurred syntopically on oak wounds studied by him.Although PAPP (1998: 115) wrote that these larvae will be described in a forthcoming paper, this intention failed to materialize. Therefore, only the puparium (and larval cephalopharyngeal skeleton extracted from it) of the reared female has been studied and described above with notes on differences from 3rd instar larval characters of P. (Myodris) annulata as described and illustrated by PAPP (1995) and MATHIS & PAPP (1998).
Relationships. Both P. winnertzii and its closest relative P. laszloi sp. nov. clearly belong to Periscelis (s. str.) as demonstrated by the construction of the male genitalia being very similar to that of P. annulipes Loew, 1858 , the type species of the subgenus (cf. PAPP & WITHERS 2011: fig. 1) irrespective of the fact that these two species have the posterior cross-vein (dm-cu) complete. However, as shown by ROHÁĆEK & ANDRADE (2017), their dm-cu is interrupted by a “vena spuria” (cf. Figs 5 View Figs 1–7 , 76 View Figs 73–77 ), which may indicate tendency to reduction of this cross-vein as is known in P. annulipes and some other species of the subgenus. Periscelis winnertzii and P. laszloi sp. nov. were considered a sister-pair already by ROHÁĆEK & ANDRADE (2017), albeit under different names. For more detail see below under P. laszloi sp. nov.
Biology. Previously EGGER (1862) wrote that this species occurs like P. annulata on sap running from poplars, oaks and horse chestnuts. However, this information is probably somewhat simplified or generalized – in fact P. winnertzii (and also P. laszloi sp. nov.) are usually associated with sap runs on oaks of various species (for habitat see Figs 6, 7 View Figs 1–7 ). PAPP (1998) collected 28 adults on black oozing wounds of two oak trees but this series in fact included both true P. winnertzii and P. laszloi sp. nov. as found by their revision. PAPP (1998) also reported on 13 (1 first instar, 3 second instar, 9 third instar) larvae and 1 pupal shell (empty puparium) collected from wounds of oak tree. These could probably also belong to both species but one 3rd instar larva that was collected on 23 May 1997 and reared in a vial with sap and wet corky bark in lab at 20℃, pupated on 17 June and emerged on 13 July was a female of true P. winnertzii (its empty puparium has been described above). Because both species occurred here on the same locality, habitat (wounds on oak trees) and time (September) they probably have also very similar phenology. According to his observations PAPP (1998: 118) believes that adults emerge in mid-July to early September, mate and lay eggs up to the beginning of September. Larvae develop around wounds under bark moistened by tree sap and reach at least to the 2nd instar during September. The species overwinter as 3rd instar larvae because they were caught in May (of the next year). Thus, P. winnertzii (and surely also P. laszloi ) has only one generation per year. We now know at least four localities where both species occurred syntopically, viz. in an oak-chestnut forest in Switzerland, in an oak forest in Hungary and in two thermophilous oak ( Quercus cerris ) forests in Slovakia. The most efficient method to collect adults apparently are traps baited by wine, vinegar or beer (thus simulating fermenting tree sap), particularly those hanging higher (some 5 m) in canopies (cf. BĀCHLI 1997; PAPP 1998; Pollini Paltrinieri, personal communication 2021). The majority of the known specimens of P. winnertzii were collected in August–October, but there are some exceptional records from June and July.
Distribution. Periscelis winnertzii seems to be widespread in Europe. Because it was formerly mixed with its closest relative P. laszloi sp. nov. (= winnertzii auctt.), the reliable old records are only those by EGGER (1862) and SCHINER (1864) from Austria (both based on type specimens). Further reliable records are those recorded recently (after ROHÁĆEK & ANDRADE 2017) under P. fugax and those revised here. Currently, P. winnertzii is known from the following countries: Portugal (ROHÁĆEK et al. 2016, as P. sp. cf. winnertzii ; ROHÁĆEK & ANDRADE 2017, as P. fugax ); Spain (CARLES- TOLRÁ et al., 2018, as P. fugax ), Great Britain: England ( CHANDLER 2017, HELLQVIST 2020, both as P. fugax ), France ( WITHERS 2017, CHANDLER 2017, both as P. fugax ), Switzerland (material examined), Czech Republic: Bohemia and Moravia ( MÁCA et al. 2005; MÁCA 2009; ROHÁĆEK & ANDRADE 2017, as P. fugax ), Slovakia (new), Hungary (new), Croatia (new), Albania (DE BREE 2022, as P. fugax ). Periscelis winnertzii has been confirmed to occur sympatrically with P. laszloi sp. nov. in the following countries: Portugal, Spain, Great Britain, Switzerland, Slovakia and Hungary.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Periscelis (Periscelis) winnertzii Egger, 1862
| Roháček, Jindřich 2022 |
Periscelis winertzii:
| MATHIS W. N. & RUNG A. 2011: 358 |
Periscelis (Microperiscelis) winnertzi:
| PAPP L. 1984: 234 |
Parclioscena Winnertzii:
| ENDERLEIN G. 1936: 177 |
Periscelis (Microperiscelis) Winnertzi:
| DUDA O. 1934: 11 |
Microperiscelis Winnertzi: OLDENBERG (1914)
| OLDENBERG L. 1914: 37 |
Periscelis Winnertzi:
| BECKER T. 1905: 217 |
Periscelis Winnertzii:
| SCHINER J. R. 1864: 272 |
Periscelis Winertzii Egger, 1862: 780
| EGGER J. 1862: 780 |