Sobolevitaenia whittingtoni, Mariaux & Georgiev, 2018
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.440 |
|
publication LSID |
lsid:zoobank.org:pub:DB80A42B-5C53-455B-86A4-2003D6F03522 |
|
DOI |
https://doi.org/10.5281/zenodo.3846846 |
|
persistent identifier |
https://treatment.plazi.org/id/0B872F99-8193-4EC4-AB17-3A3A0FD00D3F |
|
taxon LSID |
lsid:zoobank.org:act:0B872F99-8193-4EC4-AB17-3A3A0FD00D3F |
|
treatment provided by |
Valdenar |
|
scientific name |
Sobolevitaenia whittingtoni |
| status |
sp. nov. |
Sobolevitaenia whittingtoni sp. nov.
urn:lsid:zoobank.org:act:0B872F99-8193-4EC4-AB17-3A3A0FD00D3F
Figs 1–6 View Figs 1–6 , Table 1 View Table 1
Etymology
The species is dedicated to our colleague and friend, the late Professor Ian Whittington, principal research scientist at the South Australian Museum, Parasites Department.
Material examined
Holotype
AUSTRALIA: Victoria, Geelong , 38°8′ S, 144°22′ E, 22 Apr. 2001, Ian Beveridge leg. (AHC 36460).
GoogleMapsParatypes
AUSTRALIA: six specs, same data as for holotype (AHC 36461–36466).
Type host
Corvus mellori Matthews, 1912 ( Passeriformes , Corvidae ).
Intensity
About 55 specimens in a single host, together with S. beveridgei sp. nov.
Description
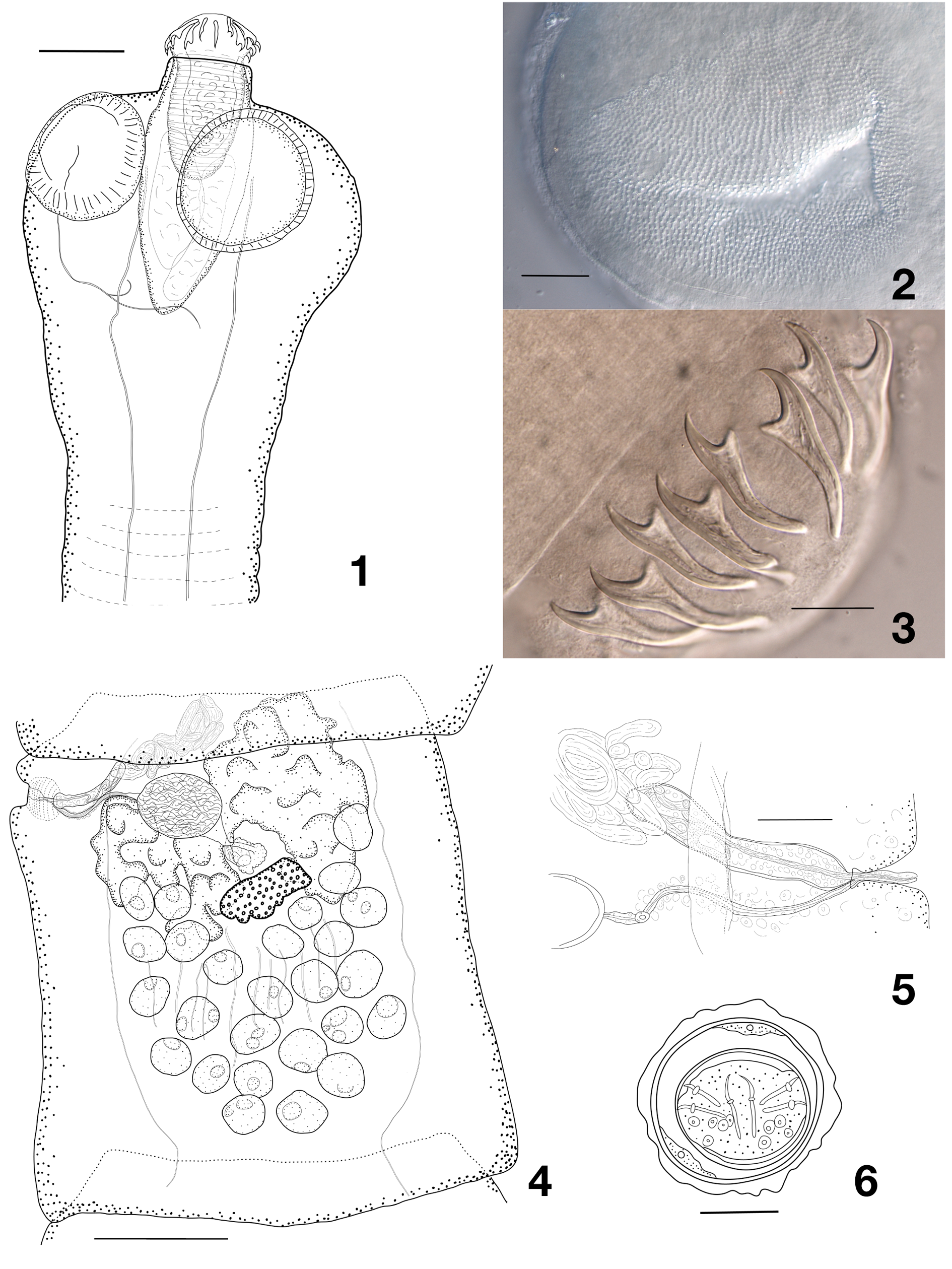
Body of medium size, with length 40–45 mm and maximum width 890–1050 (985, n = 6) at level of early gravid proglottides. Most complete specimens consisting of 110–115 proglottides. Proglottides craspedote, initially up to twice as wide as long, progressively becoming longer, as long as wide at mature stage and longer than wide at late mature or pregravid stage; gravid proglottides elongating significantly and becoming up to twice longer than wide. Scolex rounded, not delineated from neck, with diameter 260–425 (355, n = 7) ( Fig. 1 View Figs 1–6 ). Suckers rounded 116–169 (144, n = 28) in diameter; internal surface of suckers armed with large triangular spine-like microtriches, 2–3 µm long, arranged in dense regular rows ( Fig. 2 View Figs 1–6 ); microtriches only partly preserved in some specimens. Rostellar apparatus musculo-glandular, with strongly developed glandular tissue within rostellar sac. Rostellar sac well-delineated, tapering posteriorly, extending beyond level of posterior margin of suckers, 275–340 × 110–157 (304 × 128, n = 7). Rostellum with expanded anterior part bearing rostellar hooks, slightly constricted behind hooks, 172–210 × 81–109 (191 × 95, n = 7), with thick muscular walls and moderately developed glandular tissue inside it. Rostellar hooks ( Fig. 3 View Figs 1–6 ) in 2 regular rows, 19 (in one worm)–20 (n = 6) in number, robust, handle showing a discrete concave depression at guard level, extremity of handle slightly to markedly bent toward guard. Anterior hooks 42–50 (46, n = 22), posterior hooks 38–44.5 (40.5, n = 21). Neck 125–250 (209, n = 4) wide; proglottization distinct at 325–400 (367, n = 3) from posterior margin of suckers. Genital pores lateral, situated in anterior 20–25% of length of lateral proglottis margin, irregularly alternating in very short series, e.g., 2, 1, 1, 1, 2, 1, 2, 1, 1, 1, 1, 1, 2, 2, 1, 1, 1, 2, 1, 1, 1, 2, 1, 1, 1; no more than three consecutive pores observed on a single side. Ventral osmoregulatory canals 11.5–44 (23.5, n = 21) wide, connected posteriorly in each proglottis by transverse anastomosis. Dorsal osmoregulatory canals 1.5–6 (3.5, n = 22) wide. Genital ducts passing between osmoregulatory canals. Genital atrium well marked, infundibular or sink-shaped, with slight muscular sphincter, up to about 30–40 deep and 40–50 in diameter. Genital papilla present, often conspicuous.
Testes 20–30 (24, n = 43) in number, in single layer, in one continuous posterior field, extending laterally up to level anterior to vitellarium, not overlapping osmoregulatory canals and often overlapping posterior lobes of ovary ( Fig. 4 View Figs 1–6 ). External vas deferens 16–22 in diameter (18.5, n = 20), highly convoluted in antero-poral part of median field, sometimes slightly overlapping cirrus-sac. Cirrus-sac elongate, 133–173 × 23–31 (153 × 27, n = 42), slightly oblique, often bent anteriorly in proximal part, extending beyond osmoregulatory canals; with slightly-expressed bulb and pipette-like tapering porally and with rounded antiporal end; filled with large cells, more numerous in distal part ( Fig. 5 View Figs 1–6 ). Internal vas deferens forming several (at least 5–6) coils. Cirrus cylindrical, unarmed, 7 in diameter.
Vitellarium central, slightly antiporal to seminal receptacle, lobate, mostly oval and transversely elongated, variable in shape. Ovary transversely elongated, bi-alate, antiporal wing larger than poral wing; occupying all width of anterior third of median field, with lateral branches often overlapping longitudinal osmoregulatory canals; highly lobed, lobes mostly elongate. Mehlis’ gland subglobular, 50–62 (55, n = 14) in diameter, anterior to vitellarium. Empty seminal receptacle round, becoming oval when filled, reaching up to 200 × 115, dorsal and between ovary wings or over poral wing, closely posterior to proximal extremity of cirrus-sac. Vagina opened posteriorly to male pore, mostly straight and transverse, parallel to cirrus-sac, sometimes bent posteriorly; lumen of copulatory part thick-walled, surrounded by sleeve of loose, large cells; no vaginal sphincter; conductive part of vagina very short, thin.
Uterus starts its development in late mature proglottides as reticulum situated ventrally to testes and female gonads and occupying entire median field, crossing osmoregulatory canals and lateral parts of it situated in lateral fields. Developing eggs situated in rows mostly by one, rarely in groups of 2– 4 eggs along canals of reticulum. Uterine walls persistent even in most developed gravid proglottides, though sometimes become very transparent and difficult for observation. Outer shell of eggs with irregular shape, sometimes not distinct ( Fig. 6 View Figs 1–6 ). Embryophore oval, 37–50 (42, n = 31) in diameter, thick. Oncosphere round to slightly oval, 30–40 (33, n = 28) in diameter, thick. Embryonic hooks subequal, 11–12 long.
Remarks
The presence of spines on suckers, the topology of genital organs in mature proglottides, the pattern of the uterine development and the thick embryophore of eggs unambiguously link our material to the genus Sobolevitaenia Spasskaya & Makarenko, 1965 erected for two species found in Motacillidae in Russia ( Spasskaya & Makarenko 1965). This genus comprises about 15 species found in various passeriform birds, mostly Motacillidae and Turdidae , although Corvidae are also known as hosts ( Table 1 View Table 1 ). Spasskaya & Spasskii (1977) listed seven species while Kugi (2000) reported 12 species in the genus. Hosts are all from Northern Hemisphere ( Spasskaya & Spasskii 1977; Kugi 2000), except S. korochirei ( Voser & Vaucher, 1988) that has been found in Paraguay ( Voser & Vaucher 1988). Bona (1994), however, noted the probable cosmopolitan distribution of this genus.
Sobolevitaenia spp. have a homogeneous morphology, including about 20 hooks on two rows for most of them. In order to facilitate comparisons and because metrical reports are highly variable for most species (see e.g., Spasskaya & Spasskii 1977), values from original descriptions are reported as much as possible in Table 1 View Table 1 . Most known species show rostellar hooks that are of different size than observed in our material. Furthermore many of them also have a smaller number of testes and (or) cirrus-sacs of different sizes. These characters allow the easy differentiation of our material from the majority of the known Sobolevitaenia spp. (see Table 1 View Table 1 ). A few remain however more difficult to distinguish:
– Sobolevitaenia iola ( Lincicome, 1939) , a North American taxon originally reported from Turdus migratorius Linnaeus, 1766 (Turdidae) , but also reported from corvids Cyanocitta cristata (Linnaeus, 1758) in Canada ( Cooper & Crites 1974). Although resembling our material, S. iola has fewer testes (13–17), slightly shorter hooks (32 long on average) and an armed cirrus ( Lincicome 1939).
– Sobolevitaenia japonensis Kugi, 2000 from Turdus naumanni Temminck, 1820 in Japan has both a smaller rostellar sac (178–180 × 63–65) and cirrus-sac (96–120 × 1518) as well as spinose cirrus ( Kugi 2000).
– Sobolevitaenia spinosocapite ( Joyeux & Baer, 1955) from various passerines in France, shows a longer cirrus-sac (180–250). Furthermore, Joyeux & Baer (1955) report up to 30 rostellar hooks in this species. Minute spines are mentioned not only on the suckers but on the whole scolex. Joyeux & Baer (1955) also reported this taxon from a corvid, Garrulus glandarius (Linnaeus, 1758) , but with noticeably different measurements, including longer (50) and more numerous rostellar hooks (25–28).
– Sobolevitaenia unicoronata ( Fuhrmann, 1908) from a number of Turdus spp., has hooks of different shape; the length of hooks is similar on both crowns and therefore slightly longer (48) for the second row than those in our material and the double row of hooks is indistinct. Fuhrmann (1908) reported hooks in a single row but Clerc (1911) found them in 2 rows and this point remains contentious. Mettrick’s (1958), and numerous other reports of this species in the Palaearctic probably refer to different taxa ( Spasskaya & Spasskii 1977).
Two other species initially described from Greenland by Krabbe (1869) possibly belonging to Sobolevitaenia resemble our material, but their exact generic assignations remain unsettled. However, both S. (?) borealis ( Krabbe, 1869) and S. (?) trigonocephalum ( Krabbe, 1869) have much shorter rostellar hooks and fewer testes. Furthermore S. (?) borealis has an armed cirrus ( Baer 1956). We conclude that our material differs from all described species of the genus and we recognise it as a new species, Sobolevitaenia whittingtoni sp. nov.
We should, however, consider that the definition of Sobolevitaenia , based essentially on the presence of spines on the suckers, makes the exact content of this genus uncertain as these structures are easily lost or overlooked. The diversity of the genus is thus probably greater than presently known and, possibly, some taxa currently recognised as members of the genus Monopylidium Fuhrmann, 1899 should be reexamined as possible further species of Sobolevitaenia .
The little raven, Corvus mellori , is closely related to C. coronoides Vigors & Horsfield, 1827 . Both are endemic to South and Eastern Australia ( Lepage 2017). They are omnivorous and feed predominantly on insects and other soil arthropods among which the intermediate host of S. whittingtoni is likely to be found. The cestode fauna of Australian corvids is very poorly known: besides a single full identification of Raillietina corvina (Fuhrmann, 1905) in C. orru Bonaparte, 1850 , Mawson et al. (1986) only mention a Hymenolepis sp. in C. mellori , a Davainea sp. in C. coronoides and a few unidentified cestodes. We know seven species of these birds from Australia ( Lepage 2017), which must certainly host a much wider diversity of tapeworms.
Table 1 (continued on next page). Comparison of Sobolevitaenia whitingtoni sp. nov. with the other species of the genus Sobolevitaenia. Notes: * in the original description, the species is found in a second host species, with slight variations in measurements; ** synonym of S. anthusi (Spasskaya, 1958) according to Spasskaya & Spasskii (1977), *** additional measurements in parentheses from Dubinina (1953) in Matevosyan (1963).
| Species | Host family | Type locality | Scolex diameter | Suckers diameter | Rostellar sac | Rostellum | Hooks number | Anterior hooks length | Posterior hooks length | Testes number | Cirrus sac | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. whittingtoni sp. nov. | Corvidae | Australia | 260–425 | 116–169 | 275–340 × 110–157 | 172–210 × 81–109 | 19–20 | 42–50 | 38–44.5 | 20–30 | 133–173 ×23–31 | New data |
| S. anthusi (Spasskaya, 1958) (Type species) | Motacillidae | Palaearctic | 168 | 72 | 227 × 74 | 129 × 72 | 20 | 31–33 | 28–31 | 16–25 | 179–184 × 56–67 | Spasskaya & Spasskii (1977) |
| S. iola ( Lincicome, 1939) | Corvidae and others | USA | 244–444 | 92–127 | 102–239 × – | – × 46–97 | 17–20 | 26–43 | 26–43 | 13–17 | 122–224 x 26–40 | Lincicome (1939) |
| S. japonensis Kugi, 2000 | Turdidae | Japan | 250–280 | 118–120 | 178–180 × 63–65 | 128–130 × 63–75 | 20 | 48–50 | 43–45 | 26–35 | 98–120 × 15–17.5 | Kugi (2000) |
| S. korochirei (Voser & Vaucher, 1988)* | Turdidae | Paraguay | 293–410 | 82–166 | – | 148–229 × 64–112 | 20–22 | 44–56 | 37–49 | 14–17 | 76–114 | Voser & Vaucher (1988) |
| S. moldavica (Shumilo & Spassakaja, 1975) | Turdidae | Moldova | 330 | 145 × 126 | – | 180–207 × 84–90 | 20 | 45–47 | 40–42 | 12–20 | 160–195 × 17–22 | Kugi (2000) |
| S. oitaensis Kugi, 1996 | Turdidae | Japan | 480–550 | 200–250 | 370 × 160 | 190 × 60 | 22 | 53 | 43 | 16–17 | 100–120 × 25 | Kugi (1996) |
| S. orientalis Spaskii & Konovalov 1969** | Motacillidae | Russia | 225 | 115 | 115 × 100 | 170 × 79 | 20 | 33–34 | 31 | 14–18 | 180–225 ×39–42 | Spasskaya & Spasskii (1977) |
| S. similis Spasskii & Konovalov, 1969 | Motacillidae | Russia | 205 | 80 × 95 | 140 × 74 | 125 × 70 | 20 | 31 | 25 | 24–28 | 285–300 × 42–57 | Spasskaya & Spasskii (1977) |
| S. sobolevi Spasskaya & Makarenko, 1965 | Motacillidae | Russia | 200 | 155 × 82 | 173 × 82 | 109 × 45 | 20 | 19–20 | 19–20 | 20 | 238 × 45 | Spasskaya & Makarenko (1965) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |