Nucinella svalbardensis, Hryniewicz & Little & Nakrem, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3859.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:24FCAAE1-AB7C-4FAD-8698-D0C9F12400EC |
|
DOI |
https://doi.org/10.5281/zenodo.4929756 |
|
persistent identifier |
https://treatment.plazi.org/id/A2311D4D-9F12-E329-04E6-FBC5FE5A2E56 |
|
treatment provided by |
Felipe |
|
scientific name |
Nucinella svalbardensis |
| status |
sp. nov. |
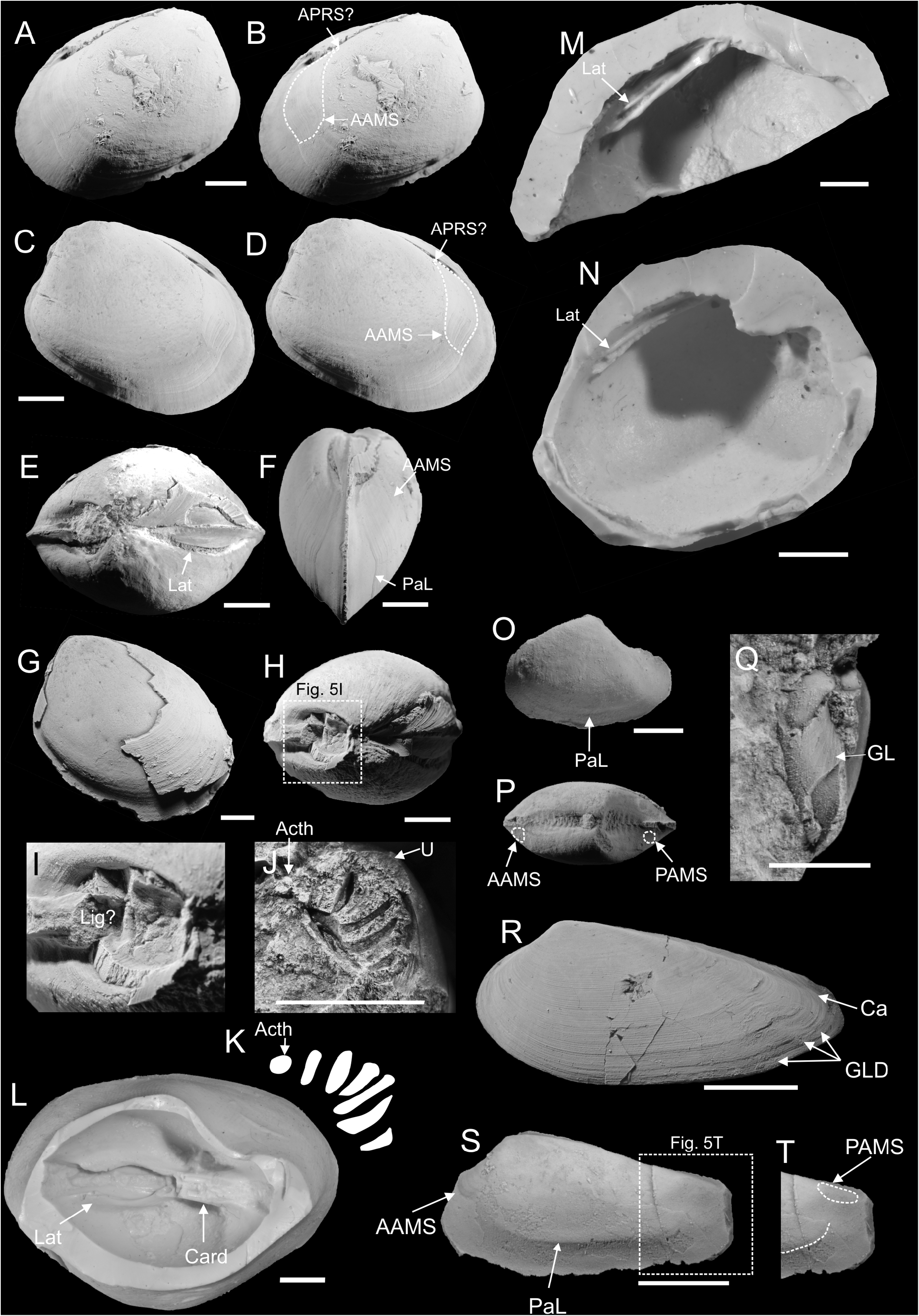
Nucinella svalbardensis sp. nov.
( Figure 5 A–N View FIGURE 5 )
2011 Nucinella sp. —Hammer et al., fig. 7f–g, tab. 2.
Etymology. After the archipelago of Svalbard.
Type locality. Seep 9, Knorringfjellet, Spitsbergen, 78°18’49.9’’N 16°10’58.9’’E.
Type material. Holotype: PMO 217.171 ; a well preserved articulated internal mould showing external shape and anterior muscle scar arrangement . Paratypes: PMO 217.217 ; an articulated internal mould and silicone rubber casts showing lateral teeth morphology . PMO 224.978 ; an articulated internal mould with shell partially preserved, showing external ornament of commarginal growth lines . PMO 224.981 ; an articulated internal mould and silicone rubber cast showing shape of cardinal teeth, as well as lateral teeth length . PMO 225.020 ; an articulated internal mould and silicone rubber casts showing lateral teeth length . PMO 225.042 ; an articulated internal mould with shell partially preserved showing the shape of the posteriormost cardinal teeth .
Material examined. 98 specimens; mostly articulated or semiarticulated shells or moulds. See Appendix 1 for list of specimens.
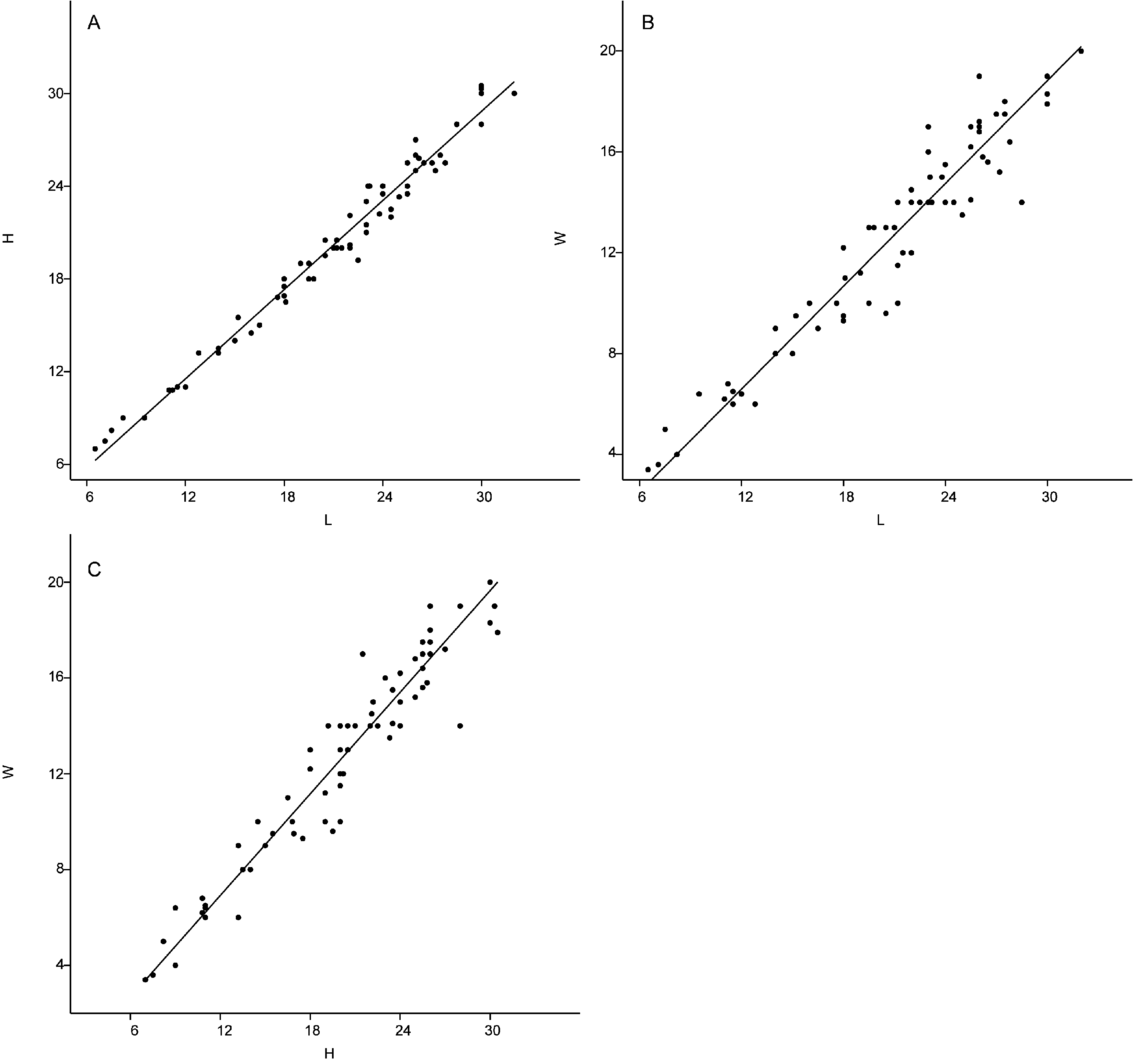
Dimensions. 3.5–23 mm in length, 1.2–9.1 mm in height, 1–8.2 mm in width. See Figure 6A–C View FIGURE 6 and Appendix 2B for details.
Diagnosis. A very large species of Nucinella with rounded posteroventral margin, and sabre-shaped lateral teeth, two in the right valve, one in the left.
Description. Shell very large, moderately inflated, equivalve, inequilateral, covered with commarginal growth lines. Umbo opisthogyrate, weakly projecting above dorsal margin, situated around ¼ of shell length. Anterodorsal margin weakly and evenly convex, passing smoothly into broadly arched anterior margin. Curvature of anterior margin even and symmetrical, with extremity around half distance from dorsal to ventral sides. Ventral margin weakly convex, roughly parallel to dorsal margin. Posterior extremity asymmetrically rounded, with ventral part of arch smoother than dorsal. Posterodorsal margin weakly concave, hosting deep ligament pit. Anterior adductor muscle scar elongated, attached to, and slightly projecting over, pallial line. Ventral part of anterior adductor muscle scar convex. Dorsal part of anterior adductor muscle scar narrow and long, connected with anterior part of lateral tooth; elongation possibly representing anterior pedal retractor scar, merged with anterior adductor muscle scar. Entire surface of anterior adductor muscle scar covered with commarginal lines. Surface of internal moulds covered with fine radial striae. Pallial line entire. Cardinal teeth diverse in shape; specimen measuring ca. 27 mm in length has eight teeth in left valve and seven in the right valve. Innermost cardinal teeth of right valve strong and long. Teeth get progressively smaller towards anterior and posterior extremities of hinge plate. Anteriormost cardinal tooth small, button-shaped. Lateral teeth long, thickest close to their anterior extremities, giving them characteristic sabre-like shape. In all specimens with investigated lateral teeth two laterals of right valve form a socket hosting single lateral of left valve. The socket is deepest close to anteriormost margin. Ligament external, dorsal to cardinal teeth. Detailed shape unknown.
Remarks. Nucinella svalbardensis sp. nov. can be distinguished from Nucinella gigantea Amano, Jenkins & Hikida, 2007 , from the Cenomanian to Campanian hydrocarbon seeps of Hokkaido (Amano et al. 2007; Kiel et al. 2008a) by its rounded posteroventral margin, less projecting beaks and greater shell inflation. The cardinal dentition of N. svalbardensis is similar to that of N. gigantea . The slightly larger number of teeth in N. svalbardensis compared to N. gigantea can be explained by the larger size of the former species, since the number of cardinal teeth is a function of specimen size, as shown by La Perna (2004) in other species of Nucinella . According to Amano et al. (2007) a single lateral is present in the both left and right valve of N. gigantea , unlike in N. svalbardensis . A possible fossil nucinellid from the Triassic (Norian) seeps in Oregon ( Peckmann et al. 2011) has been only tentatively identified and comparison with N. svalbardensis is at the present not possible. Nucinella svalbardensis can be distinguished from non-seep Mesozoic species by its much larger size. The Hettangian Nuculina liasina Bistram, 1903 , of Val Solda ( Bistram 1903) is much smaller than N. svalbardensis , reaching only a couple of millimetres in length. Nucinella birkelundi Clausen & Wignall, 1990 , from the Kimmeridgian of Southern England ( Clausen & Wignall 1990), is much smaller, in addition to being much higher, being 1.5 times high as long.
Occurrence. Seeps 1, 2, 3, 5, 9, 12, 13, 14 and 15 (Upper Volgian–uppermost Ryazanian), Slottsmøya Member, Svalbard ( Tab. 1 View TABLE 1 ).
Palaeoecology. There are a number of reasons to suggest that Nucinella svalbardensis was a chemosymbiotic shallow burrower. First, chemosymbiosis was suggested by McLeod et al. (2010) for the Recent species Nucinella maoriana ( Hedley, 1904) because of the presence of light organic carbon in its tissues. Second, the presence of bacterial structures in the gills of Nucinella owenensis Oliver & Taylor, 2012 , led Oliver & Taylor (2012) to infer that chemosymbiosis may be common within the family, although some species are known to be active deposit feeders ( Oliver & Taylor 2012). Third, large species of Nucinella are common in fossil hydrocarbon seep deposits, suggesting a chemosymbiotic relationship (Amano et al. 2007; Kiel et al. 2008a; Hammer et al. 2011; Peckmann et al. 2011); N. svalbardensis , being up to 32 mm in length, is the largest species of Nucinella currently known. However, some of the non-seep deep marine Nucinella species are also quite large, being up to 25 mm long ( Thiele & Jaeckel 1931; Matsukuma et al. 1982; La Perna 2005). Hence, a relationship between size and seepassociation in Nucinella is not straightforward.
In addition to seeps, Nucinella can be found in a range of other shallow to deep marine modern environments ( Allen & Sanders 1969; Matsukuma et al. 1982; Okutani & Iwasaki 2003; La Perna 2004), including organic-rich sediments in fjords ( McLeod et al. 2010), and at oxygen-minimum zones ( Oliver & Taylor 2012). As a fossil it occurs in a range of post-Triassic marine deposits (i.e. Wood 1851; Bistram 1903; Vokes 1956; Clausen & Wignall 1990; Studencka et al. 1998; Harries & Little 1999; Schneider 2008).
Order Nuculoida Dall, 1889
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.