Pseudotrapezium aff. groenlandicum Spath, 1936
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3859.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:24FCAAE1-AB7C-4FAD-8698-D0C9F12400EC |
|
DOI |
https://doi.org/10.5281/zenodo.5228265 |
|
persistent identifier |
https://treatment.plazi.org/id/A2311D4D-9F31-E30F-04E6-FD9FFDFD2F57 |
|
treatment provided by |
Felipe |
|
scientific name |
Pseudotrapezium aff. groenlandicum Spath, 1936 |
| status |
|
Pseudotrapezium aff. groenlandicum Spath, 1936
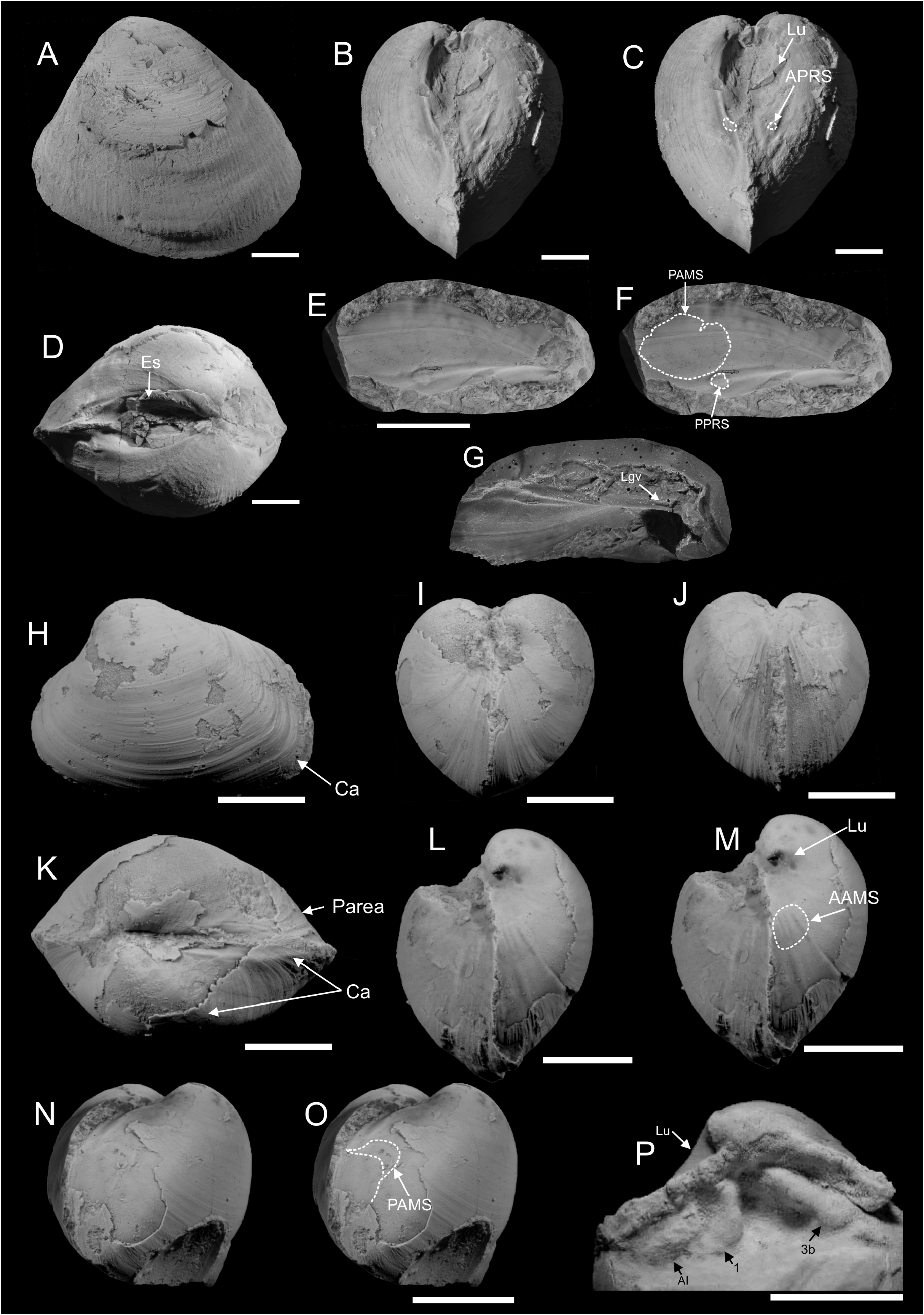
( Figure 16 H–P View FIGURE 16 )
1936 aff. Pseudotrapezium groenlandicum sp. nov. —Spath, p. 125, pl. 49, fig. 7a–c.
? 1982 aff. Hartwellia (Hartwellia) groenlandica (Spath) —Fürsich, p. 89.
2011 Arcticid—Hammer et al., fig. 7m, tab. 2.
Material examined. 820 specimens, mostly articulated or semi-articulated shells and internal moulds. See Appendix 1 for list of specimens.
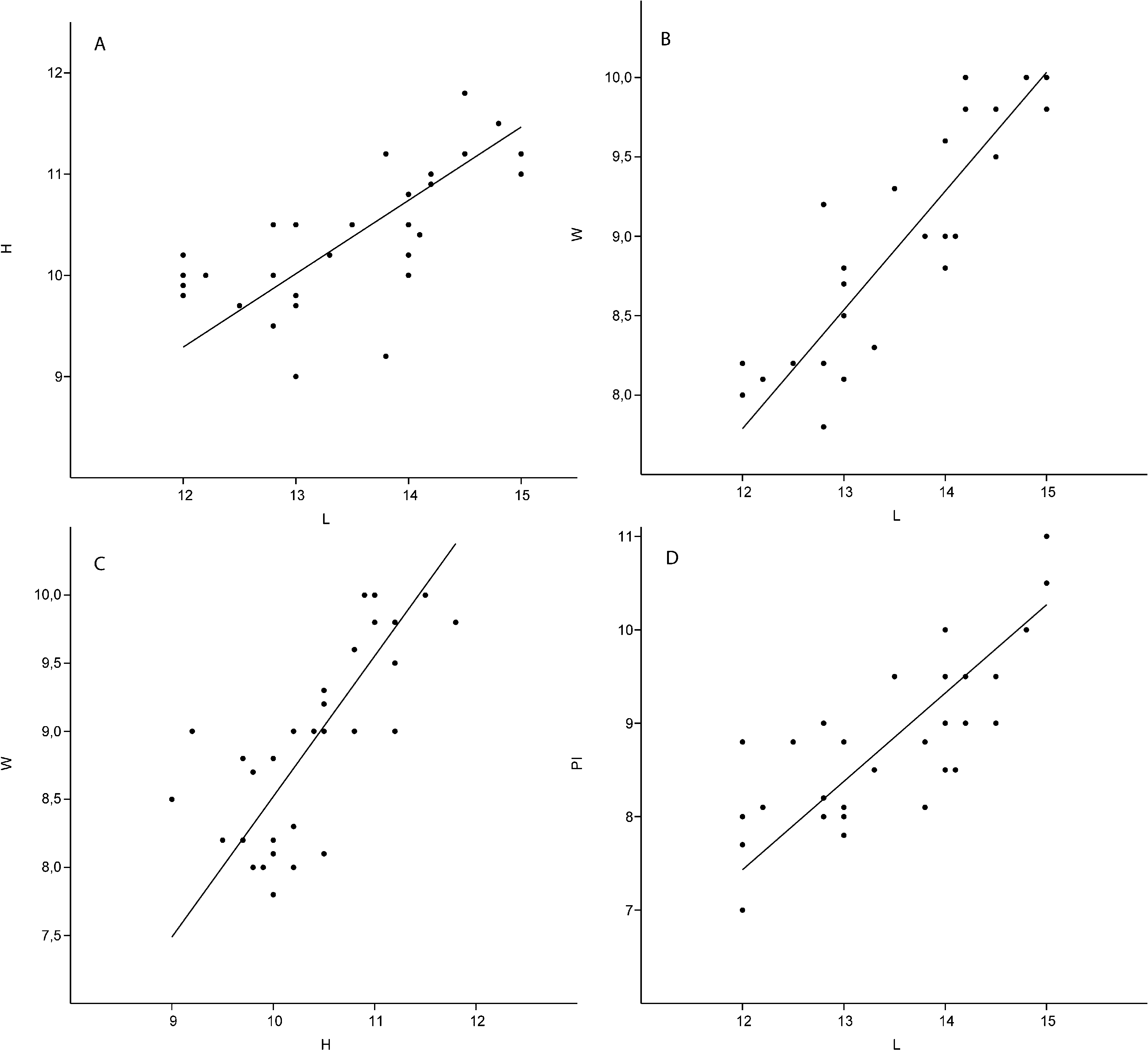
Dimensions. 12–15 mm in length, 9.8–11 mm in height, 8–10 mm in width. See Figure 18 A–D View FIGURE 18 and Appendix 2L for details.
Description. Small, moderately inflated with very thin shell. Beaks prosogyrate, very strongly incurved. Lunule moderately deep. Anterior margin projecting, arcuate, passing into broadly arched ventral margin. Posterior area with two carinae, posteroventral extremity slightly rostrate where intersected by first carina, passing into truncated oblique posterior margin. Second carina weak, parallel to the posterodorsal margin. Posterodorsal margin straight. External ornament composed of very weak commarginal growth lines. Hinge plate large and thick; lateral tooth AI developed, separated from 1; 3a absent. 1 and 3b separated, diverging from umbo; 1 strong and vertical; 3b thick, anterodorsally inclined. Angle between 1 and 3b ca. 70°. Left valve dentition unknown. Anterior adductor muscle scar weak, elongated along pallial line, around twice as long as wide, not detached. Posterior adductor muscle scar weak, rounded. Pallial line weak, entire.
Remarks. We compare our specimens with Pseudotrapezium groenlandicum Spath, 1936 , from Milne Land, East Greenland ( Spath 1936) due to their thick hinge plate, similar cardinal dentition and external shape. However, the Svalbard specimens are much smaller and more thin shelled than the Greenland material, so we are not entirely sure they represent the same species and leave them in open nomenclature. Fürsich (1982) moved P. groenlandicum into the genus Hartwellia Kitchin, 1926 , and redescribed it as Hartwellia (H.) groenlandica . Hartwellia was considered by Cox (1944) to be a synonym of Pronoella Fischer, 1887 . Including P. groenlandicum into Pronoella is not justified by the hinge of our Svalbard specimens. Both Pronoella and Pseudotrapezium have relatively thick hinge plates and a similar external shape (e.g. Casey 1952), but AI and 1 are connected in Pronoella into a single elongate denticle and separated in Pseudotrapezium ( Benecke 1905) , showing the genera are not the same.
Occurrence. P s eudotrapezium groenlandicum : Lower Volgian–Lower Ryazanian of Milne Land, East Greenland ( Spath 1936; Fürsich 1982). Pseudotrapezium aff. groenlandicum : seeps 2, 3, 5, 8, 9 and 12 (Upper Volgian–uppermost Ryazanian), Slottsmøya Member, Svalbard ( Tab. 1 View TABLE 1 ).
Palaeoecology. We assume that P. aff. groenlandicum was a burrower, feeding on organic-rich sediment layer while resting in the shallow subsurface. The lack of a pallial sinus suggests that P. aff. groenlandicum possessed very short siphons, similar to its extant relative Arctica islandica ( Linnaeus, 1767). Arctica islandica has very short siphons and positions its posterior extremity at the sediment-water interface for feeding on organic matter from the sediment surface ( Morton 2011). As it does so it remains relatively motionless, circulating water only by means of ciliary movement ( Brand & Taylor 1974). It can rebury itself into deeper sediment layers, where it remains isolated from seawater for up to seven days when it is not feeding, and respires anaerobically ( Taylor 1976). The clustering of very large numbers of P. aff. groenlandicum specimens in seep 9 is difficult to explain. No association between arcticids and chemosymbiotic bacteria has been noted to date, so we do not envisage any specific trophic link between P. aff. groenlandicum and the seep environment.
Subclass Anomalodesmata Dall, 1889
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.