Cretaxinus hurumi, Hryniewicz & Little & Nakrem, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3859.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:24FCAAE1-AB7C-4FAD-8698-D0C9F12400EC |
|
DOI |
https://doi.org/10.5281/zenodo.4929787 |
|
persistent identifier |
https://treatment.plazi.org/id/A2311D4D-9F3D-E30D-04E6-FEC7FE46286E |
|
treatment provided by |
Felipe |
|
scientific name |
Cretaxinus hurumi |
| status |
sp. nov. |
Cretaxinus hurumi sp. nov.
( Figures 13 K–N View FIGURE 13 , 15 View FIGURE 15 , 16 A–G View FIGURE 16 )
2011 Thyasira sp. —Hammer et al., fig. 7a–c, tab. 2.
Etymology. After Jørn H. Hurum, leader of 2004–2012 Svalbard expeditions of the Natural History Museum, University of Oslo.
Type locality. Seep 9, Knorringfjellet, Spitsbergen, 78°18’49.9”N 16°10’58.9”E.
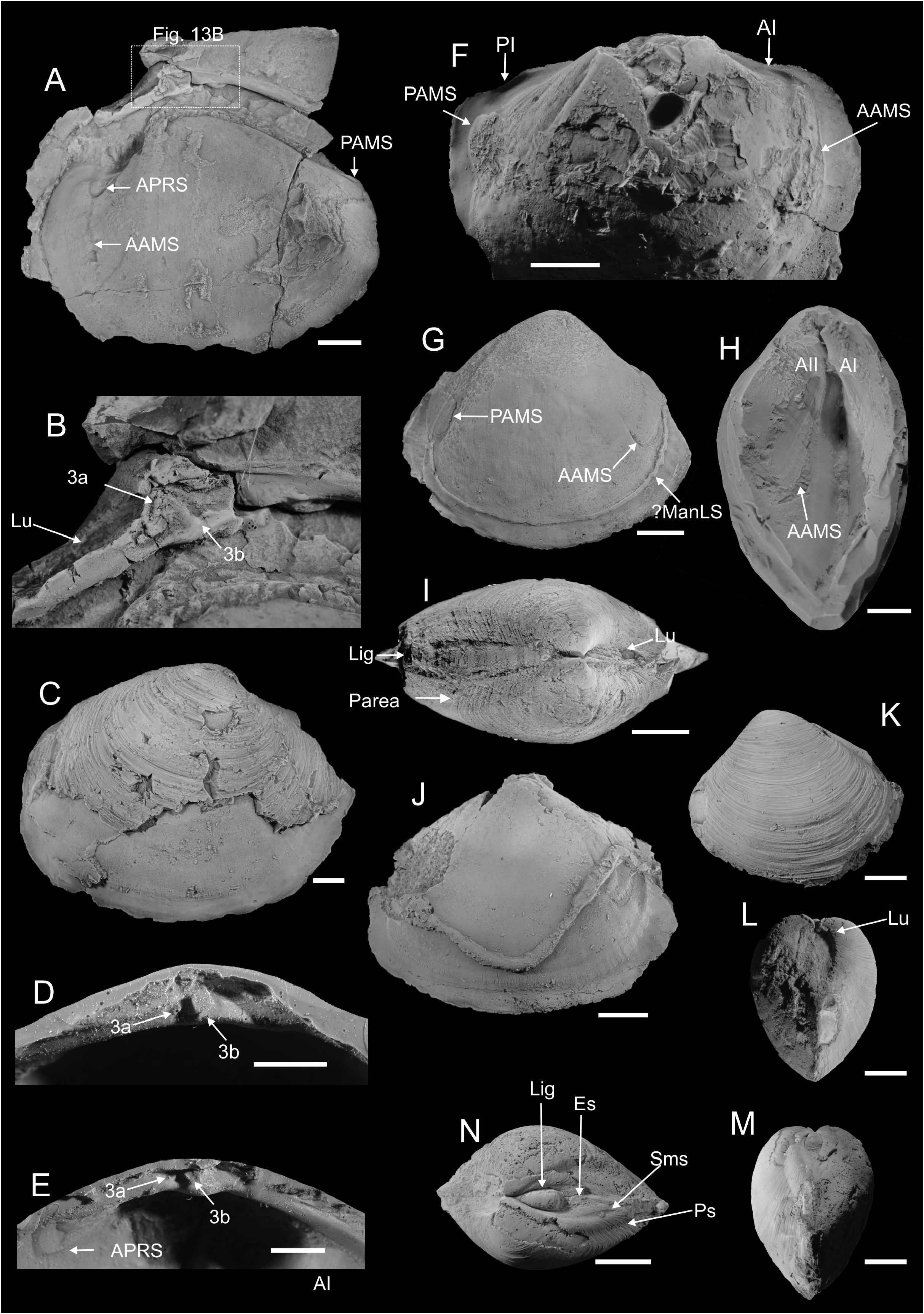
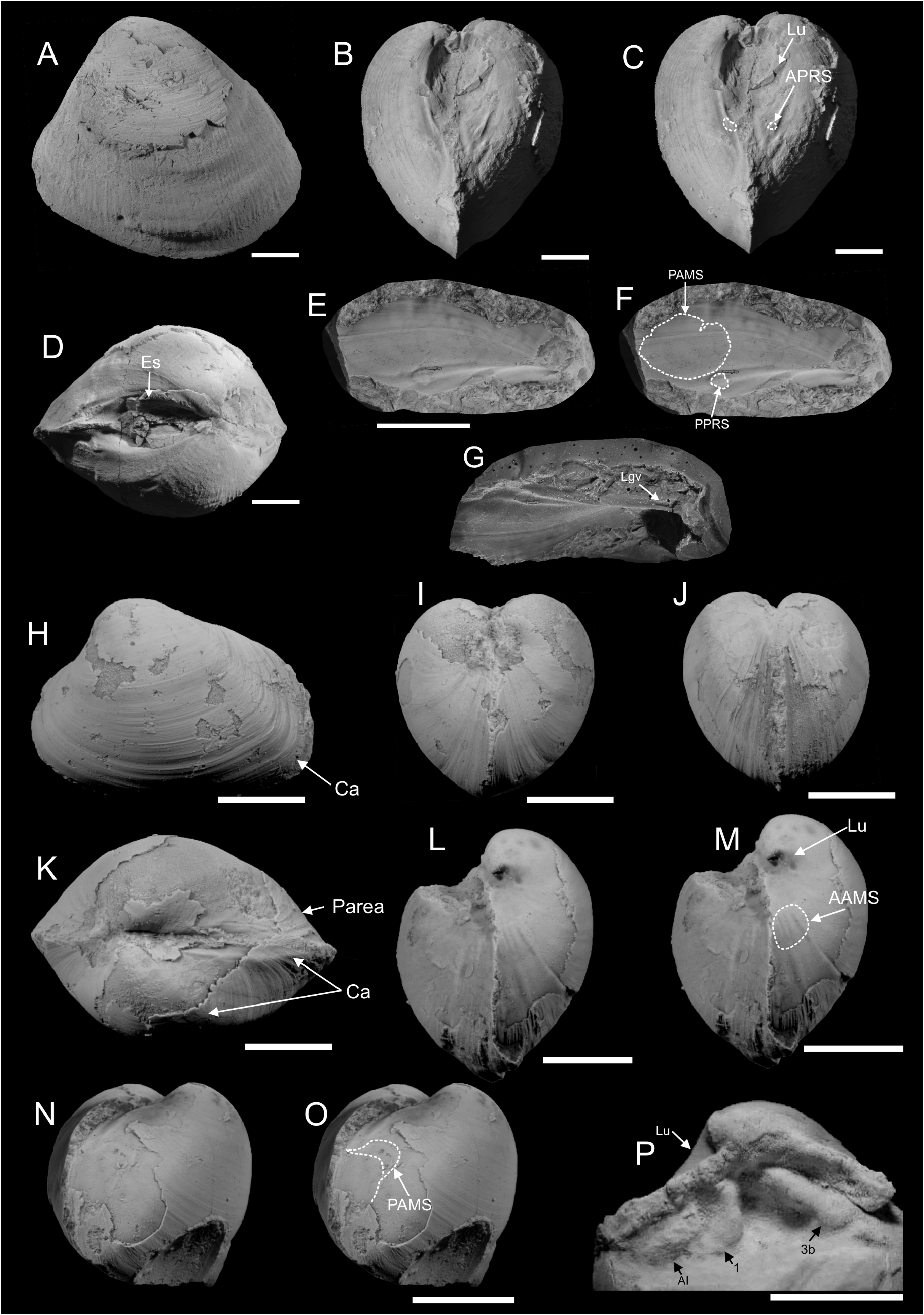
Type mterial. Holotype: PMO 217.277 ; an internal mould with shell partially preserved, showing a triangular outline, external ornament, a sulcated posterior margin and a thick, short, external ligament . Paratypes: PMO 217.172 ; an almost complete internal mould showing outline and anterior adductor muscle scar . PMO 217.175 ; a fragment of an internal mould and silicone rubber cast showing posterior adductor muscle scar and posterior pedal retractor. The silicone rubber cast shows the cardinal area with an elongated resilifer . PMO 217.540 ; complete internal mould showing the triangular outline, well impressed rounded posterior adductor muscle scar and deep escutcheon . PMO 225.128 ; an almost complete internal mould showing anterior adductor muscle scar and crosssection through the external ligament and ligament nymphs . PMO 225.136 ; an internal mould showing very weak anterior adductor muscle scar .
Material examined. 56 specimens, all articulated or semi-articulated internal moulds with variable amounts of shell preserved. See Appendix 1 for list of specimens.
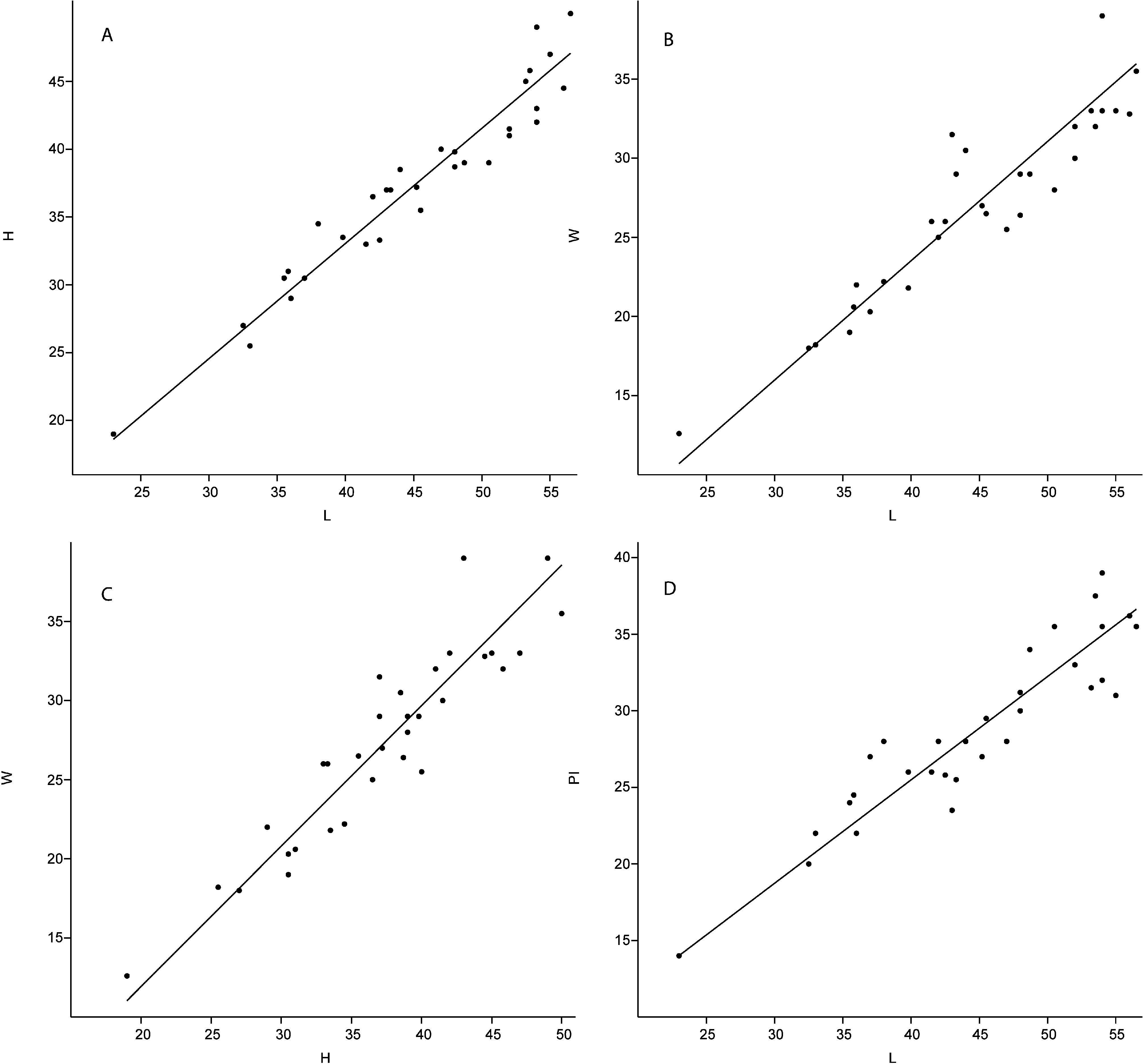
Dimensions. 23–56.5 mm in length, 12.6–50 mm in height, 19–39 mm in width. See Figure 17 A–D View FIGURE 17 and Appendix 2K for details.
Diagnosis. As for the genus.
Description. Shell large, subtriangular in outline, up to 56.5 mm long, 50 mm high, and 39 mm wide. Average H/L ratio ≈ 0.85, W/L ≈ 0.75, and W/H ≈ 0.88. Shell less than 0.5 mm thick, covered with commarginal growth lines. Umbonal angle usually larger among smaller specimens, which have concave anterodorsal and posterodorsal margins. Beaks incurved, prosogyrate, not prominent, positioned closer towards the anterior, with average Pl/L ≈ 0.67. Umbonal angle slightly acute. Lunule large, deep, heart-shaped. Anterior margin tightly rounded, more angular in larger specimens. Ventral margin curved, with curvature deepest close to mid-line in smaller specimens, progressively displaced towards posterior during growth. Curvature shows some intraspecific variation, from deep and prominent, to shallow and gentle. Posterior extremity weakly pointed, posterodorsal margin sloping, with very weak sulcus. Smaller specimens usually have slightly concave posterodorsal margin, which is more straight in larger specimens. Escutcheon large and deep. Ligament external, thick, short, occupying 1/3 of escutcheon. Hinge plate narrow. Cardinal area with single, elongate groove, probably representing a ligament groove. Lateral dentition not observed. Anterior adductor muscle scar very weak and small, elongated along pallial line, with straight ventral margin and irregular dorsal margin; well impressed in anterior part, fading towards posterior so length of anterior adductor muscle scar cannot be fully ascertained. Anterior pedal retractor scar small and weak, circular, separated from the anterior adductor muscle scar by narrow margin, visible in one specimen only. Posterior adductor muscle scar larger than anterior adductor muscle scar, circular, deeply impressed, displaced towards hinge plate. Posterior pedal retractor small, circular, approximately same size as anterior pedal retractor scar; positioned close to hinge plate and separated from posterior adductor muscle scar by narrow distance. Pallial line entire, weak, marked by pallial muscle scars in some specimens. Internal shell surface covered with fine radial ornament, probably representing traces of descending pallial muscles.
Remarks. Cretaxinus hurumi gen. et sp. nov. is the oldest thyasirid species known to date. The slightly younger Valanginian to Hauterivian thyasirid is Lucina ? rouyana d’Orbigny, 1844, from the shelf deposits of Europe and possible seep sites of Grodziszcze beds in the Carpathians ( Ascher 1906, p. 164, pl. XIV, fig. 9a–c; Kiel et al. 2008a; Kaim et al. 2013), which has more of a typical Thyasira shape. The Albian Lucina ? sculpta Phillips, 1829, from Southern England ( Woods 1907, p. 153, pl. 24, fig. 7–9) has a very distinct shape reminiscent of the genus Axinus Sowerby, 1821 ( Taylor et al. 2007). Thyasira tanabei Kiel, Amano & Jenkins, 2008 (a) is known from Albian to Campanian hydrocarbon seeps of Hokkaido, Japan. Thyasira tanabei has a large anterior adductor muscle scar, unlike C. hurumi , but the lack of information about the ligament of T. tanabei makes more detailed comparison difficult. Thyasira sp. from the Cenomanian Kanajirasawa seep of Hokkaido ( Kiel et al. 2008a) is known from a single, partially preserved specimen only and, therefore, is also difficult to compare to C. hurumi . Various Campanian thyasirid species from the Western Interior Seaway ( Kauffman 1967) differ in shape from C. hurumi , having flattened posterior areas, narrow escutcheons, deeper sulci and more ovate shapes, reminiscent of Recent Thyasira species.
Occurrence. Seep 9 (uppermost Ryazanian), Slottsmøya Member, Svalbard ( Tab. 1 View TABLE 1 ). Known only from the type locality .
Palaeoecology. We infer that Cretaxinus hurumi was a chemosymbiotic and possibly seep-restricted Mesozoic thyasirid. This is supported by the large shell size of C. hurumi . Among modern thyasirids chemosymbiosis is present mainly in species with two gill demibranchs ( Dufour 2005) and these usually have large shells, up to around 10 mm for the genera Axinus , Thyasira and Parathyasira , but can reach up to 110 mm in Conchocele (e.g. Payne & Allen 1991; Kamenev et al. 2001; Oliver & Killeen 2002). Large shells provide enough space in the mantle cavity for large symbiont-bearing gills ( Taylor & Glover 2010). In contrast, thyasirids with a single gill demibranch are usually asymbiotic ( Dufour 2005; Taylor & Glover 2010). These asymbiotic species are much smaller, with sizes of only a few millimeters ( Payne & Allen 1991; Oliver & Levin 2006). Cretaxinus hurumi shells are up to 56.5 mm long, and this is very large for the family, which strongly suggests the species had hypertrophied gills suitable for symbiosis with chemoautotrophic bacteria. Smaller chemosymbiotic thyasirids (≈ 10 mm in length) are known from seep environments (e.g. Dando et al. 2004), but also occur in non-seep settings with high redox potential, such as organic-rich sediments in fjords ( Dando & Spiro 1993), pulpmill effluents ( Dando & Southward 1986) and in the vicinity of offshore hydrocarbon production sites ( Oliver & Killeen 2002). However, the only Recent thyasirid genus which attains sizes comparable to C. hurumi is Conchocele , and this is closely associated with seeps (Kamenev et. al 2001; Okutani 2002; cf. Weaver 1942; Coan et al. 2000). Another line of evidence indicating that C. hurumi was both chemosymbiotic and seep-restricted comes from absence of the species in contemporary ‘normal’ marine sediments on Svalbard (e.g. Sokolov & Bodylevsky 1931; Weir 1933; Birkenmajer et al. 1982), despite being relatively abundant in the hydrocarbon seeps ( Hammer et al. 2011).
Thyasirids are burrowers (e.g. Dando & Southward 1986; Oliver & Killeen 2002). Chemosymbiotic species dig into fine grained sediment to a depth a few times the length of the shell ( Pearson 1972). In these burrows they use a vermiform foot to construct a three dimensional network of downward directed tunnels reaching up to 30 times the length of the shell, which serve as conduits for sulfide-rich pore waters from deeper interstitial levels ( Dando & Southward 1986; Zuschin et al. 2001; Oliver & Killeen 2002; Dando et al. 2004; Taylor & Glover 2010). However, they are usually unable to tolerate high sulfide concentrations.
Chemosymbiotic thyasirids are also capable of feeding on photosynthetic organic matter if conditions for chemosynthesis become unfavourable ( Dando & Spiro 1993). Cretaxinus hurumi was probably a relatively deep burrower, based on similar lines of evidence to the lucinid Tehamatea rasmusseni .
Order Venerida Gray, 1854
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.