Nipponoluciola cruciata ( Motschulsky, 1854 ) Ballantyne & Kawashima & Jusoh & Suzuki, 2022
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.855.2023 |
|
publication LSID |
lsid:zoobank.org:pub:A31C64CB-6C6D-424A-A54C-F86FDD77ABAB |
|
DOI |
https://doi.org/10.5281/zenodo.7528345 |
|
persistent identifier |
https://treatment.plazi.org/id/A2478793-7756-FF8B-FE2D-FE85846FFDB5 |
|
treatment provided by |
Felipe |
|
scientific name |
Nipponoluciola cruciata ( Motschulsky, 1854 ) |
| status |
gen. et comb. nov. |
Nipponoluciola cruciata ( Motschulsky, 1854) gen. et comb. nov.
Figs 1‒3 View Fig View Fig View Fig , 5‒12 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
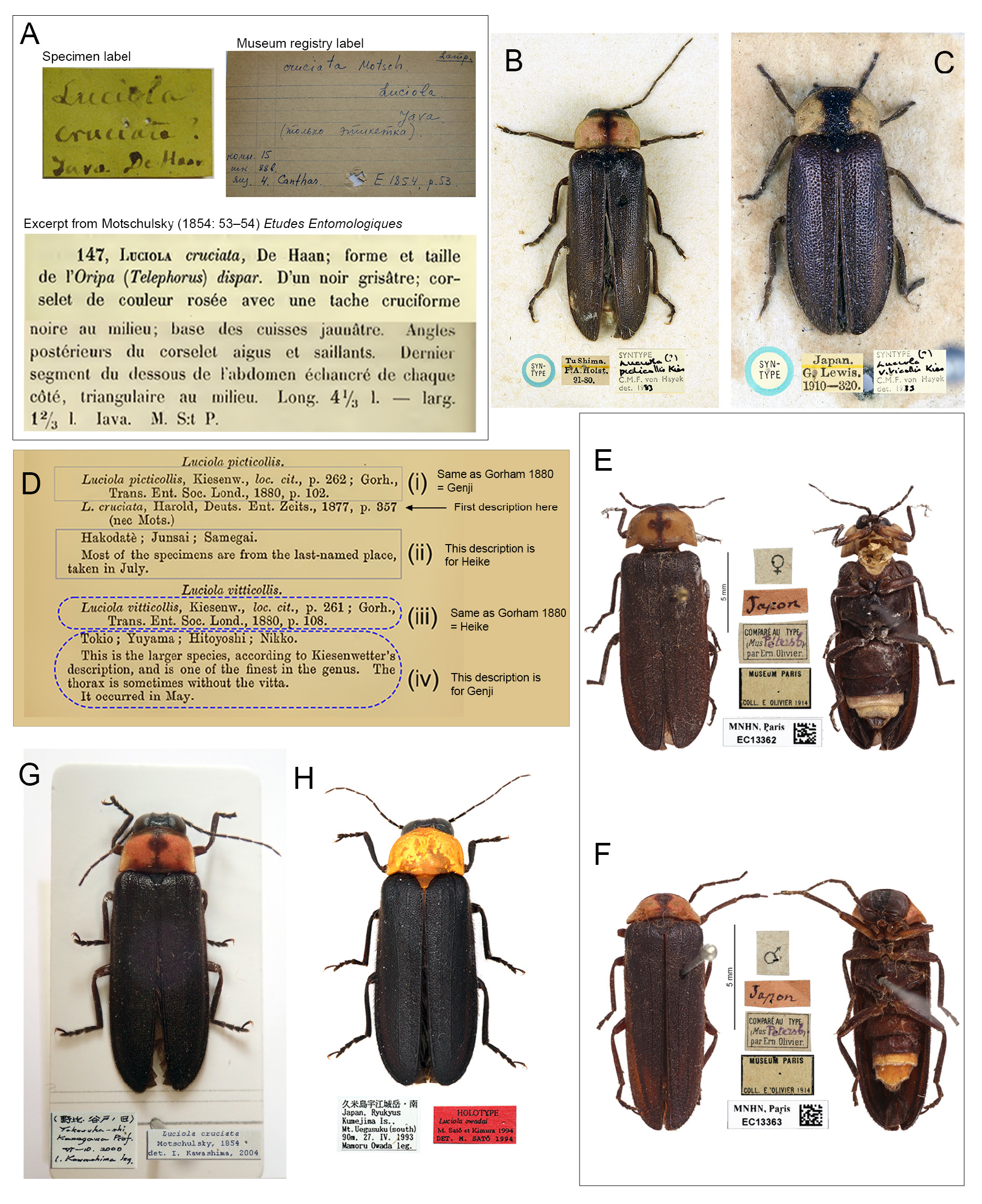
Luciola cruciata Motschulsky, 1854: 53 View in CoL .
Type specimen represented by label only, ZMMU.
Luciola picticollis Kiesenwetter, 1874: 262 View in CoL .
Series of syntype males. NHMUK. Syn. nov.
Luciola cruciata towadensis Nakane, 1987: 173 .
Luciola cruciata PARTIM View in CoL – Lacordaire 1857: 338. — Heyden 1879: 350. — Gorham 1880: 102. — Olivier 1902a: 77; 1902b: 188; 1907: 51; 1910: 41. — McDermott 1962: 24; 1966: 102. — Takakura 1977: 7. — Jeng et al. 2003: 541. — Kawashima et al. 2003: 247.
Luciola cruciata View in CoL – Motschulsky 1866: 167. — von Harold 1877: 357. — Lewis 1879: 17. — Matsumura 1918: 86, 87. — Okada 1928: 102; 1931: 134, 146. — Kanda 1935:31. — Kishida 1936: 12, 20, 21. — Hasama 1942a: 366; 1942b: 378; 1943: 23; 1944: 155. — Nakane et al. 1959: 170. — Nakane 1960: 36, 118; 1968: 5; 1970: 285; 1983: 111; 1987: 173. — Minami 1961: 21‒106, 152‒238. — Bertrand 1972: 599; 1973: 107. — Satô 1974: 133; 1978: 17; 1985: 121; 1989: 352. — Ohba 1978: 25; 1983: 24; 1984: 23; 2001: 45; 2004a: 228; 2005: 240; 2012: 13. — Yuma 1981: 57. — Ohba et al. 1994: 13. — Suzuki et al. 1996a: 191; 1996b: 682; 2004: 297. — Suzuki 1997: 1; 2001: 99. — Sawada 2000: 93. — Branham & Wenzel 2003: figs 1–2, 4–5, 8. — Takeda et al. 2006: 177. — Ballantyne & Lambkin 2009: 21; 2013: 9. — Oba et al. 2011: 771. — Ballantyne et al. 2015: 8; 2016: 204; 2019: 163. — Fu & Ballantyne 2009: 243. — Fu et al. 2009: 155; 2010: 3; 2012a: 6 View Cited Treatment ; 2012b: 14 View Cited Treatment . — Jusoh et al. 2018: 14; 2021: 1. — Kato et al. 2020: 1.
Luciola picticollis View in CoL – Von Harold 1877: 357. — Lewis 1879: 17. — Olivier 1902a: 85; 1902b: 189; 1907: 54; 1910:45. — Matsumura 1918: 87. — Okada 1931: 130. — McDermott 1966: 111 (partim). — Nakane 1983: 111.
Luciola vitticollis – Sensu Gorham 1883: 409. — Okada 1931: 146.
Luciola cruciata vitticollis – Okamoto 1924: 182–183, 226 (incorrect record from Korea). — Matsumura 1928: 59 (misidentification).
Luciola cruciata towadensis – Kawashima et al. 2003: 247 (synonymy).
Luciola cruciata cruciata View in CoL – Geisthardt & Satô 2007: 232.
non Luciola picticollis View in CoL – Gorham 1883: 409 (= lateralis View in CoL ).
non Luciola vitticollis – Sensu Gorham 1883: 409. — Okada 1931: 146 (= lateralis View in CoL ).
non Luciola cruciata var. vitticollis – Olivier 1902a: 71; 1902b: 188; 1907: 54; 1910: 42. — McDermott 1966: 111 (= lateralis View in CoL ).
non Lueiola erciata – Matsumura 1918: 86 (misspelling, typographical error).
non Luciola Cxuca’a var. vitticollis View in CoL – Matsumura 1918: 82 (misspelling, typographical error).
non Luciola cruciata vitticollis – Matsumura 1928: 59, pl. 5 fig. 15, pl. 6 figs 5–6, pl. 7 fig. 14.
non Luciola cruciata View in CoL – Thapa 2000: 115 (incorrect record).
Diagnosis
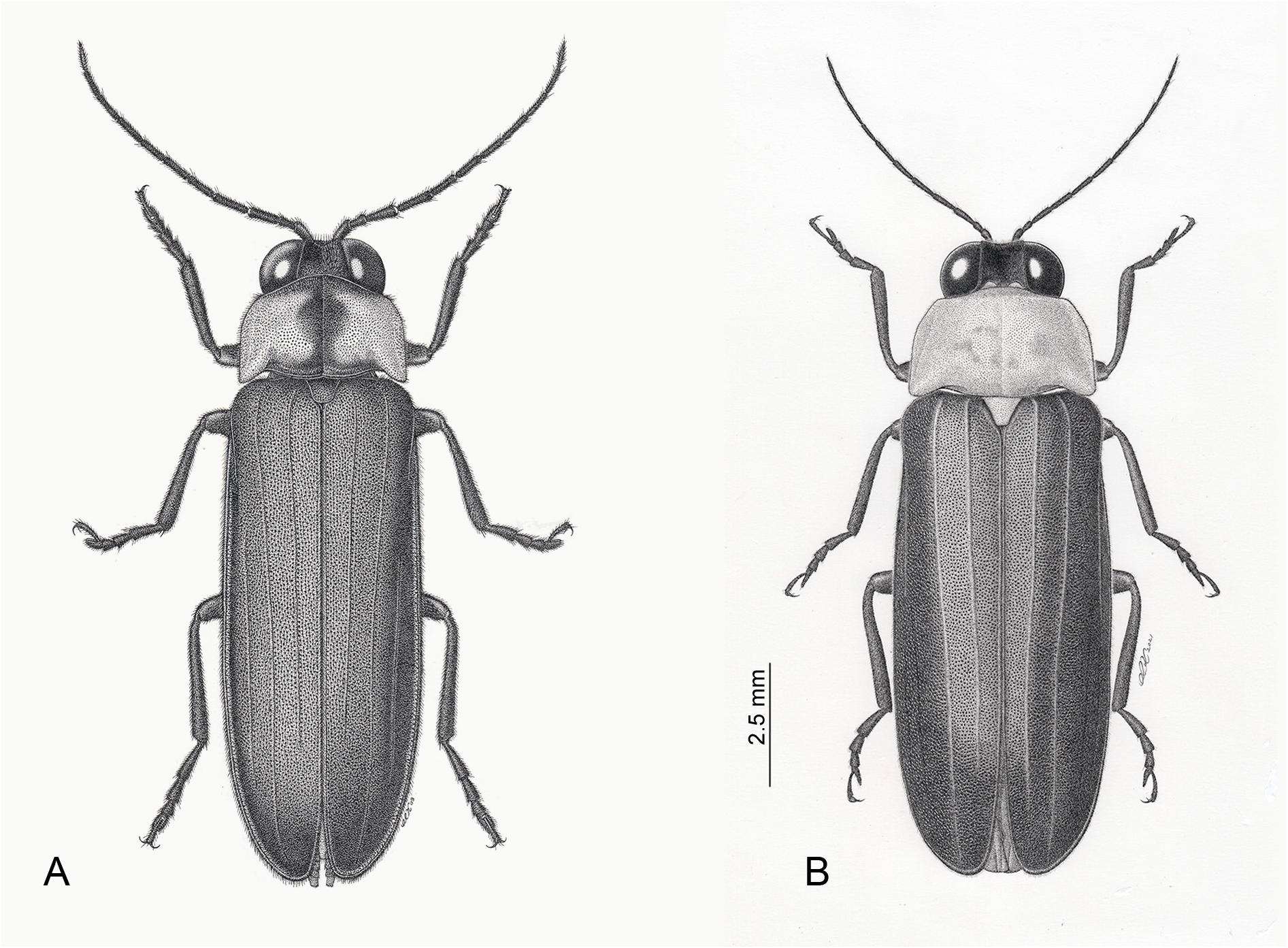
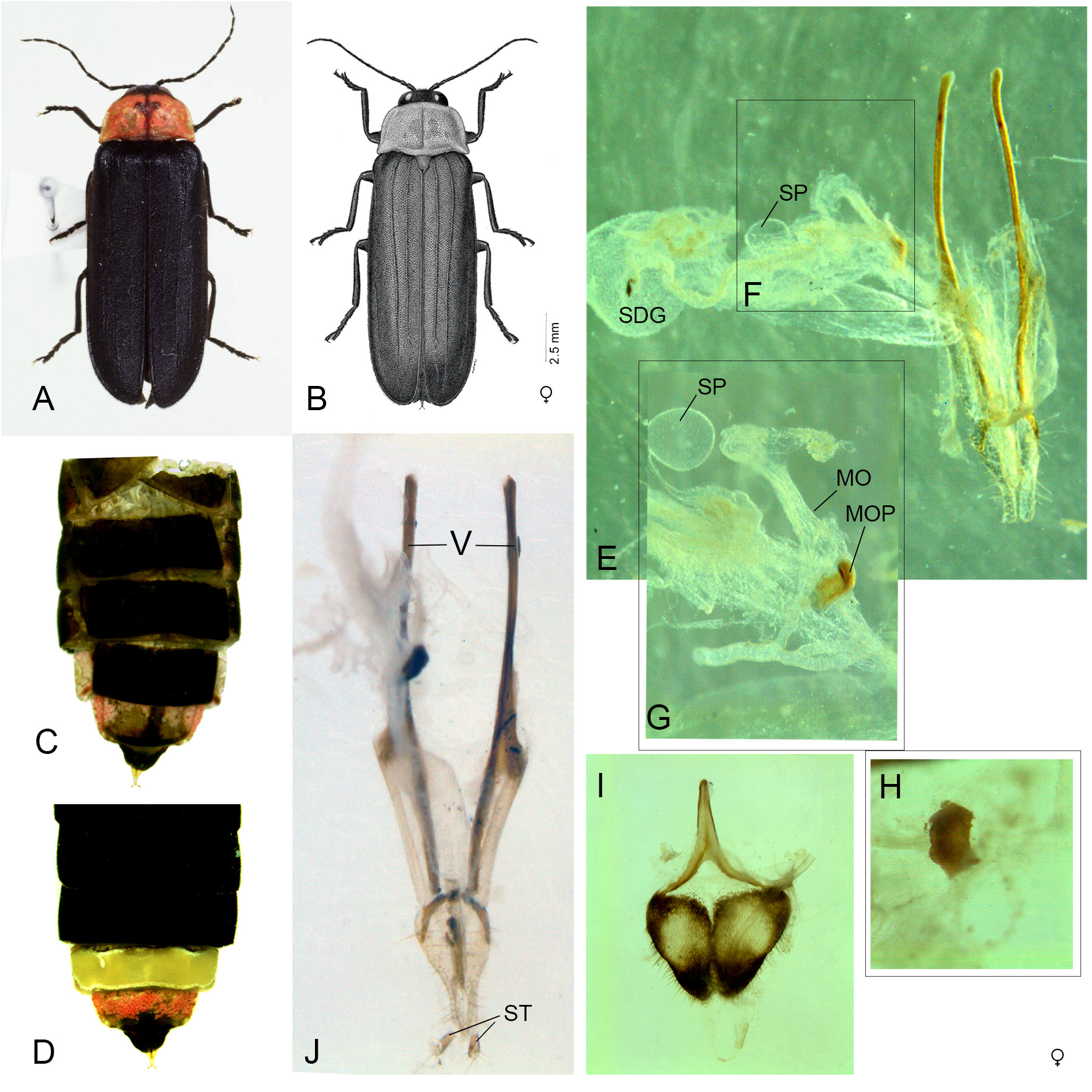
One of the most famous fireflies in Japan, distinguished most obviously from the only other species in the genus, N. owadai gen. et comb. nov., by the pinkish pronotum with median blackish markings (that of owadai is yellowish orange without dark markings), and black MS and elytra ( owadai has yellow MS and black elytra). Widely distributed throughout the main islands of Japan in contrast with owadai , which is restricted to a part of the Ryukyu islands, Kume-jima Island, and presently considered endangered in the locality. Macropterous females coloured as for males, except for white LO in V6, semitransparent anterior ⅔ of V7, with underlying pink fat body, and median posterior area black; V8 largely black.
Type material
Neotype of Luciola cruciata Motschulsky, 1854 (here designated) JAPAN • ♂; Kanagawa Pref., Yokosuka-shi, Nobi (Aza-Yatonota) ; 10 Jun. 2000; Itsuro Kawashima leg.; registration number: KPM-NK 80923 .
Remarks
This designation fulfils the requirements of the ICZN neotype designation as follows: 75.3: in the absence of a type specimen there is a need to designate a neotype to preserve the existing taxonomy; 75.3.1: having established that the type locality given in Motschulsky (1854) as Java is incorrect, we designate a specimen from mainland Japan; 75.3.2: characters differentiating the neotype from other genera in the Luciolinae , and the other species in this new genus, owadai , are given in the generic description and key to species; 75.3.3: the specimen is fully labelled (outlined below) and given an identifying number in the type depository below; 75.3.4: introductory sections of this paper outline the steps taken to establish that no type specimen could be found; 75.3.5: this paper outlines all the references to this species including the original description by Motschulsky (1854), and we believe the morphological features of this specimen are consistent with all; 75.3.6: we established that the original type specimen could not have come from Java, but Japan; the locality chosen for this neotype designation is an area of high density of the species; 75.3.7: upon publication of this paper the neotype specimen, already lodged in the Kanagawa Prefectural Museum of Natural History, Odawara, Kanagawa, will be permanently lodged there and become the property of that museum.
Notes
Labels attached to the neotype are as follows (from top to bottom, a slash indicates a line break): (Nobi, Yatonota (in Japanese “Kanji” characters)) / Yokosuka-shi/ Kanagawa Pref. / VI‒10, 2000 / I. Kawashima leg. (original white label, hand written in black ink); Luciola cruciata / Motschulsky, 1854 / det. I. Kawashima, 2004 (white determination label, printed in black ink); NEOTYPE / Luciola cruciata / Motschulsky, 1854 / = Nipponoluciola cruciata / ( Motschulsky, 1854) / Ballantyne, Kawashima, Jusoh & Suzuki, 2021 (designated) (pink neotype label, printed in black ink).
Other material examined
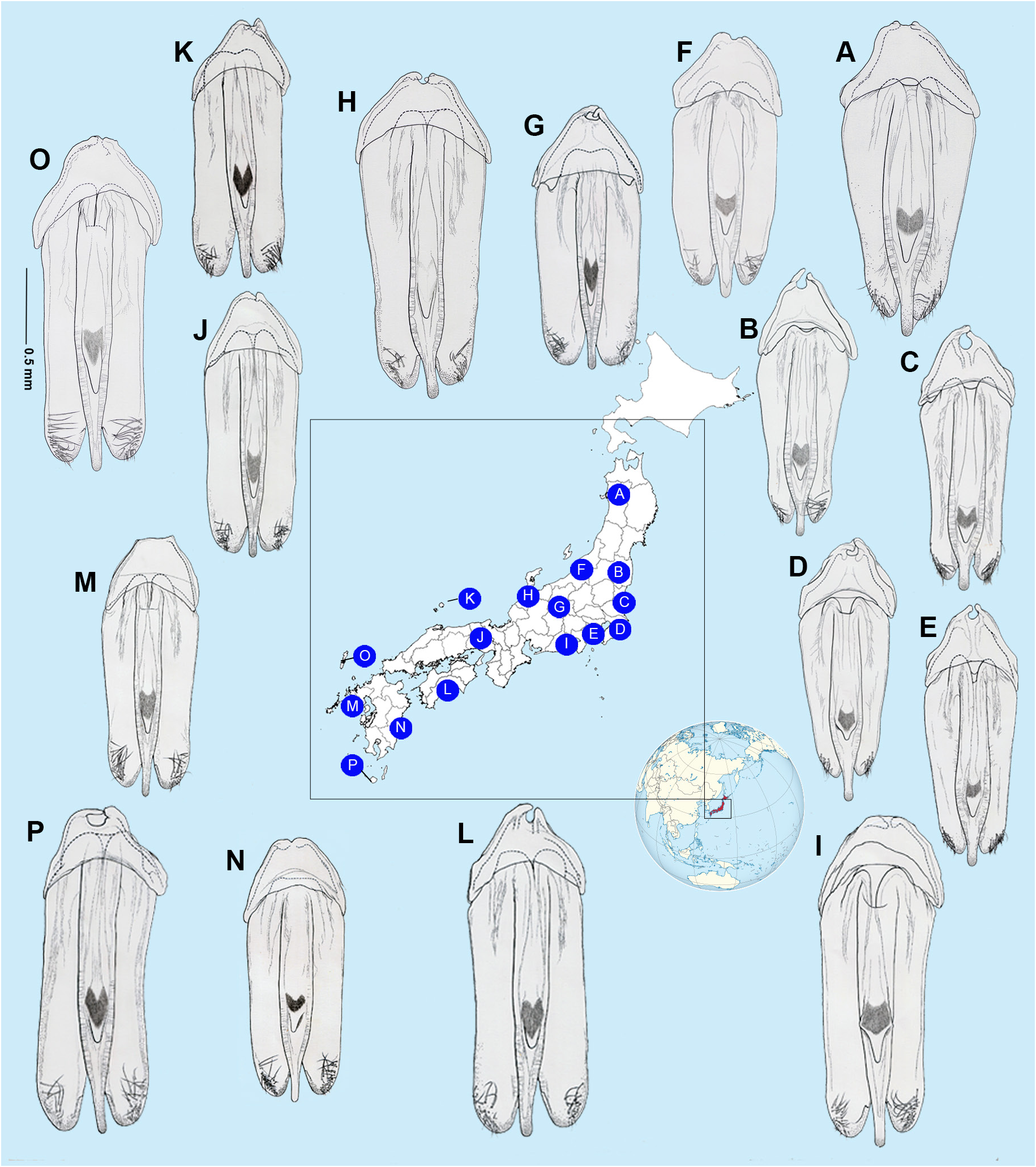
JAPAN ‒ Akita Prefecture • 1 ♂; Higashi, Kawabe-machi ; 39°39′ N, 140°12′ E; 25 Jun. 1994; K. Umetsu leg.; AKPM GoogleMaps • 1 ♂; Sunakobuchi, Kawabe-machi ; 39°44′ N, 140°33′ E; 25 Jun. 1994; K. Umetsu leg.; AKPM GoogleMaps • 1 ♂; Yunosato, Akita-shi ; 39°48′ N, 140°13′ E; Jul. 1995; K. Umetsu leg.; AKPM GoogleMaps • 1 ♂; Noda, Taihei, Akita-shi ; 39°43′ N, 140°20′ E; 25 Jun. 1994; K. Umetsu leg.; AKPM GoogleMaps . • 1 ♂; Mt Ôtaki-yama, Akita-shi ; 39°43′ N, 140°16′ E; 12 Jul. 1988; F. Satô leg.; AKPM GoogleMaps • 1 ♀; Kawabemachi ; 13 Jun. 2002; collector unknown; CIK . ‒ Niigata Prefecture • 1 ♂; Yomogihira, Nagaoka-shi ; 37°36′ N, 138°53′ E; Jul. 1994; N. Harayama leg.; CIK GoogleMaps . ‒ Ishikawa Prefecture • 2 ♂♂; Kamitokuyama, Nomi-shi ; 36°43′ N, 136°54′ E; 3 Jul. 2012; H. Fukutomi leg.; CIK GoogleMaps . ‒ Fukushima Prefecture • 1 ♀; Arakawa ; 37°42′ N, 140°35′ E; Jun. 1993; K. Matsumoto leg.; CIK GoogleMaps • 2 ♂♂; Jikiri-onsen Spa., Nagasaki, Iwaki-shi ; 36°96′ N, 140°93′ E; 3 Jun. 1998; I. Kawashima leg.; CIK . ‒ Ibaraki Prefecture • 2 ♀♀; Ishizuka, Jôhoku-machi , Higashi-ibaraki-gun; 36°47′ N, 140°38′ E; Jun. 1988; K. Nakayama leg.; CIK GoogleMaps • 4 ♂♂; same collection data as for preceding; 30 May 1990; CIK GoogleMaps • 1 ♂; same collection data as for preceding; 1 Jun. 1992; CIK GoogleMaps • 1 ♂; same collection data as for preceding; 20 Jun. 1995; CIK GoogleMaps • 2 ♂♂; same collection data as for preceding; 25 Jun. 1998; CIK GoogleMaps • 2 ♂♂; same collection data as for preceding; 8 Jun. 1999; CIK GoogleMaps • 3 ♂♂; same collection data as for preceding; 10 Jun. 1999; CIK GoogleMaps • 4 ♂♂, 2 ♀♀; same collection data as for preceding; 6 Jun. 2000; CIK GoogleMaps • 1 ♂, 1 ♀; same collection data as for preceding; 6–7 Jun. 2002; CIK GoogleMaps • 1 ♂, 1 ♀; same collection data as for preceding; 3 Jun. 2009; CIK GoogleMaps . ‒ Saitama Prefecture • 2 ♂♂; Shôgun-sawa Riv., Ranzan-machi ; 36°01′ N, 139°33′ E; 5 Jun. 1997; S. Arai leg.; CIK GoogleMaps . ‒ Chiba Prefecture • 13 ♂♂, 1 ♀; Hirasawa-gawa Riv., Ôtaki-machi ; 35°23′ N, 140°22′ E; Jun. 2019; S. Nishihara and I. Kawashima leg.; CIK GoogleMaps • 1 ♂; Tsutsumori, Ôtaki-machi ; 35°22′ N, 140°14′ E; 13 Jun. 2018; I. Kawashima leg.; CIK GoogleMaps . ‒ Tokyo Metropolitan • 10 larvae; Hamura-shi ; 35°45′ N, 139°31′ E; 2001; T. Sano leg.; CIK GoogleMaps . ‒ Kanagawa Prefecture • 3 ♂♂; Kurokawa, Asao-ku, Kawasakishi ; 35°36′ N, 139°45′ E; 9 Jun. 1990; Y. Goto leg.; CIK GoogleMaps • 6 ♂♂; Inukura, Miyamae-ku, Kawasaki-shi ; 35°58′ N, 139°56′ E; 9 Jun. 1991; T. Arikawa leg.; CIK GoogleMaps • 3 ♂♂; Nobi (Aza-Yatonota), Yokosukashi ; 35°21′ N, 139°41′ E; 10 Jun. 2000; I. Kawashima leg.; KPM GoogleMaps • 3 ♂♂; same collection data as for preceding; CIK GoogleMaps • 18 ♂♂; same collection data as for preceding; 11 Jun. 2001; CIK GoogleMaps • 1 ♂; same collection data as for preceding; 26 Jun. 2021; CIK GoogleMaps • 5 ♂♂; Morito-gawa Riv., Sakurayama-Ôyama Pass, Hayamamachi ; 35°28′ N, 139°59′ E; 15 Jun. 2000; I. Kawashima leg.; CIK GoogleMaps . ‒ Shizuoka Prefecture • 1 ♂; Katasumata-gawa Riv., Kanaya, Haibara-gun ; 34°49′ N, 138°13′ E; 15 Jun. 1995; Y. Sato leg.; CIK GoogleMaps • 3 ♂♂; Shizuoka-shi ; 34°58′ N, 138°38′ E; 6 Jun. 1998; Y. Sato leg.; CIK GoogleMaps . ‒ Yamanashi Prefecture • 1 ♂, 1 ♀; Yamanashi ; 35°41′ N, 138°41′ E; 16 Jun. 1978; unknown leg.; (id N. Ohba); ANIC GoogleMaps . ‒ Nagano Prefecture • 2 ♂♂; Sanozaka, Hakuba-mura ; 36°37′ N, 137°50′ E; 13 Jul. 1986; M. Takakuwa leg.; CIK GoogleMaps • 5 ♂♂, 1 ♀; Matsumoto-shi ; 36°23′ N, 137°58′ E; 5 Jul. 1990; Y. Sato leg.; CIK GoogleMaps . ‒ Mie Prefecture • 1 ♂; Osugi ; 34°21′ N, 136°15′ E; 6 Jun. 1932; N. Tamu leg.; (id T. Nakane); ANIC GoogleMaps . ‒ Gifu Prefecture • 4 ♂♂; Gujôhachiman, Gujo-shi ; 35°44′ N, 136°58′ E; 5 Jun. 1988; H. Kuwano leg.; CIK GoogleMaps • 4 ♂♂, 1 ♀; Kitaogi, Tajimi-shi ; 35°33′ N, 137°11′ E; 18 Jun. 1994; H. Suzuki leg.; CIK GoogleMaps . ‒ Hyogo Prefecture • 1 ♂; Kawanishi-shi ; 34°49′ N, 135°41′ E; 1 Jul. 1992; K. Matsuda leg.; CIK GoogleMaps • 3 ♂♂, 3 ♀♀; same locality data as for preceding; 6 Jul. 1996; T. Ochi leg.; CIK GoogleMaps . ‒ Shimane Prefecture, Oki Islands (off W Honshu ) • 1 ♂; Mt Akahage-yama, Chiburi Is.; 36°01′ N, 133°03′ E; 26 Jul. 2003; T. Shimada leg.; CIK GoogleMaps • 7 ♂♂; Araki-gawa Riv., Saigo-chô , Dogo Is.; 36°20′ N, 133°32′ E; 13 Jun. 2003; T. Shimada leg.; CIK GoogleMaps . ‒ Ehime Prefecture • 1 ♂; Nakayama, Omogo-mura, Kami-ukena-gun ( Kumakôgen-chô at present); 33°39′ N, 133°06′ E; 3 Jul. 1999; Y. Goto leg.; CIK GoogleMaps . ‒ Kochi Prefecture • 1 ♂; Amikawa, Tosayamamura ; 33°39′ N, 133°50′ E; 19–20 Jun. 1999; Y. Goto leg.; CIK GoogleMaps • 11 ♂♂; Shimotsui, Shimanto-chô ; 33°29′ N, 132°55′ E; 17 Jun. 2006; I. Kawashima leg.; CIK GoogleMaps . ‒ Fukuoka Prefecture • 1 ♂, 2 ♀♀; Doubaru, Kitakyushu-shi ; 33°46′ N, 130 ° 49′ E; Jun. 1978; M. Nakamura leg.; (id by N. Ohba); ANIC GoogleMaps . ‒ Nagasaki Prefecture • 6 ♂♂, 1 ♀; Tsushima Is. (off N. Kyushu), Kyôzuka, Izuhara-chô ( Tsushimashi at present); 33°46′ N, 129°55′ E; 18 Jun. 1990; Y. Goto leg.; CIK; GoogleMaps 9 ♂♂; Urakami-gawa Riv. , Azebettô-chô, Nagasaki-shi; 32°48′ N, 129°55′ E; 20 May 2018; K. Tanaka leg.; CIK GoogleMaps . ‒ Kumamoto Prefecture • 3 ♂♂, 1 ♀; Yubune, Kyokushi-mura ( Kikuchi-shi at present); 32°55′ N, 130°52′ E; 29 May 1991; Y. Goto leg.; CIK GoogleMaps . ‒ Miyazaki Prefecture • 10 ♂♂; Kita-gawa Riv., Kitagawa-chô ; 32°43′ N, 131°39′ E; 3 Jun. 1993; Y. Goto leg.; CIK GoogleMaps . ‒ Kagoshima Prefecture • 7 ♂♂, 4 ♀♀; Yaku-shima Is. (off S. Kyushu), Miyanoura to Ryûjinsugi-tozanguchi, Yakushima-chô ; 30°37′ N, 130°52′ E; 29 May 2021; A. Ueno leg.; CIK. GoogleMaps – Without locality • 7 ♀♀, pupa; reared by Y. Haneda; ANIC GoogleMaps .
Description
Male ( Figs 1–3 View Fig View Fig View Fig , 5–6 View Fig View Fig , 10–12 View Fig View Fig View Fig )
BODY LENGTH. 10.5–15.8 mm ( Jeng et al. (2003) listed a range of 10.5‒16.5 mm long).
COLOUR ( Figs 1–2 View Fig View Fig , 6 View Fig ). Entirely black except for pale pronotum with underlying pink fat body, and usually with a series of black markings extending narrowly across the anterior margin, continuous with a narrow median band that expands just anterior to midsection into short arms (the median cross) which may be wide or narrow, and continuing to posterior area where there may be a wide expansion across the posterior margin ( Fig. 6 View Fig ). Aged pinned and ethanol preserved specimens may fade and the femoral base may appear brownish. A few specimens have very reduced pronotal colour patterns, and even fewer have no dark pronotal markings at all. IK observed many individual variations in pronotal colour among specimens from the same locality (see Pronotal colouration variations below).
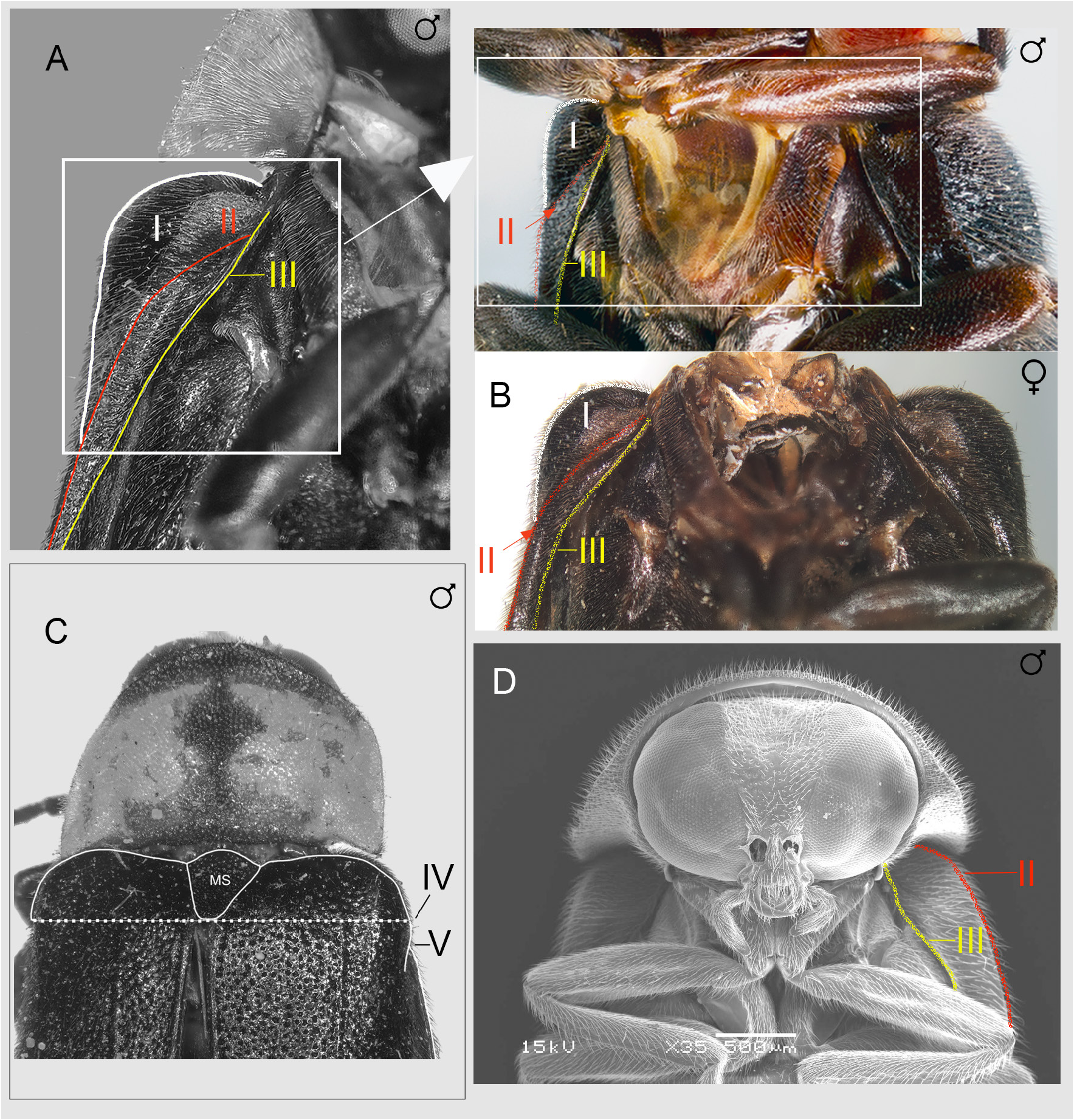
ABDOMEN ( Fig. 2 View Fig ). Jeng et al. (2003: fig.7) illustrated a narrow MPP on V7 with well-defined posterolateral corners which were not prolonged.
AEDEAGAL SHEATH ( Fig. 5 View Fig ). Anterior margin of sheath tergite entire and slightly and broadly emarginated; sternite emarginated strongly on right side. Jeng et al. (2003: fig. 27) depict the same stronger emargination of the sheath sternite on its right side as we do here.
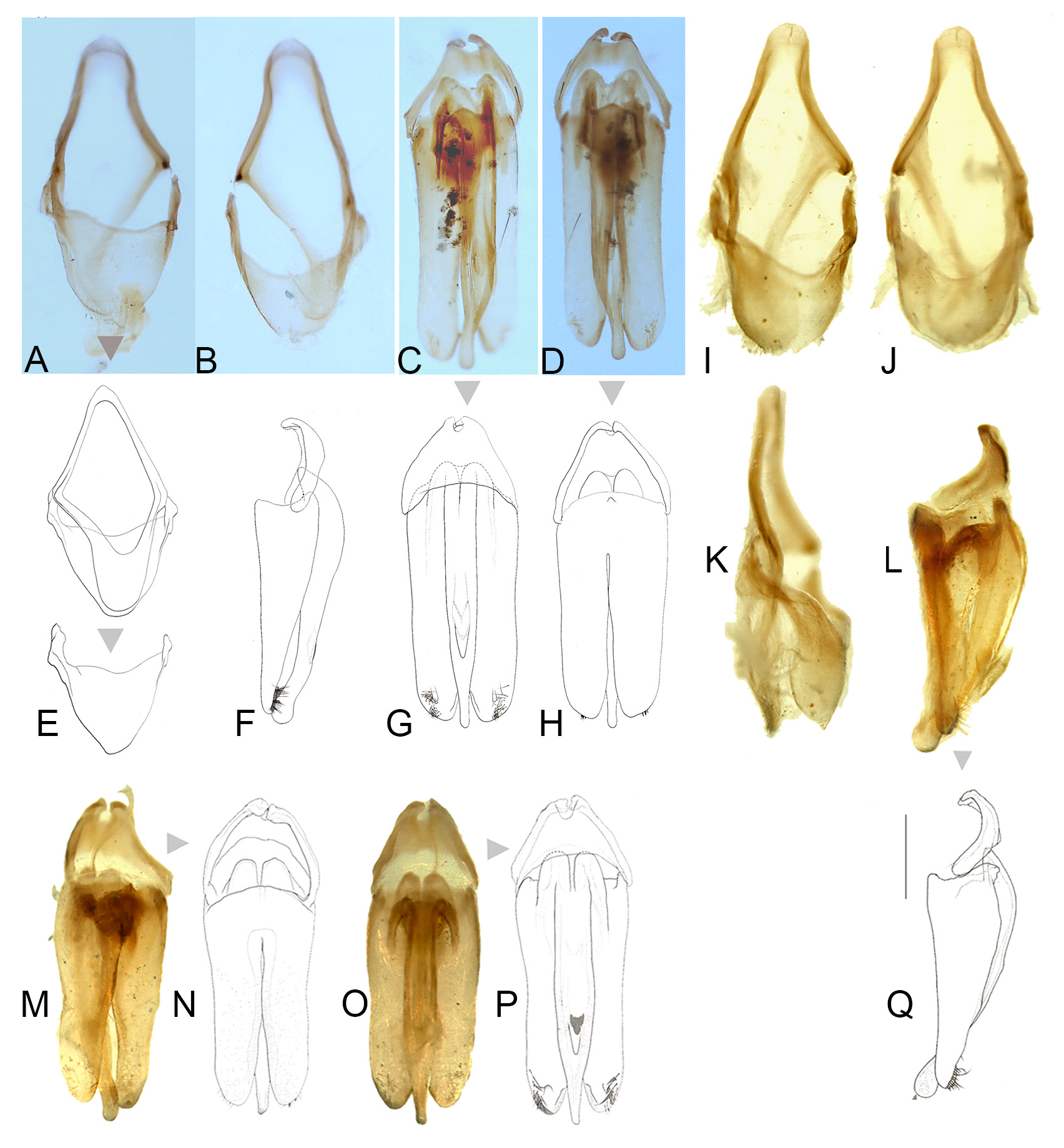
AEDEAGUS ( Figs 5 View Fig , 10–12 View Fig View Fig View Fig ). L/W 3/1; either subparallel-sided or with lateral margins converging slightly; maximum width across LL/maximum width of ML 2.57–3.57; LL separated along the dorsal surface longitudinally by7/₉ of their length; LL apex width considerably wider than width of apex of ML; dorsal base of LL symmetrical, somewhat irregularly rounded; ML symmetrical, expanding slightly along its length to a maximum width around the ejaculatory orifice then narrowing in apical 1/6 or less where it is approximately 2/7‒2/9 the width of the more anterior portion; with rounded apex; BP well sclerotised, not hooded, emargination along anterior margin, may be absent. Jeng et al. (2003: figs 33–34) show an anterior median notch in the BP and the ML narrowing towards the apex.
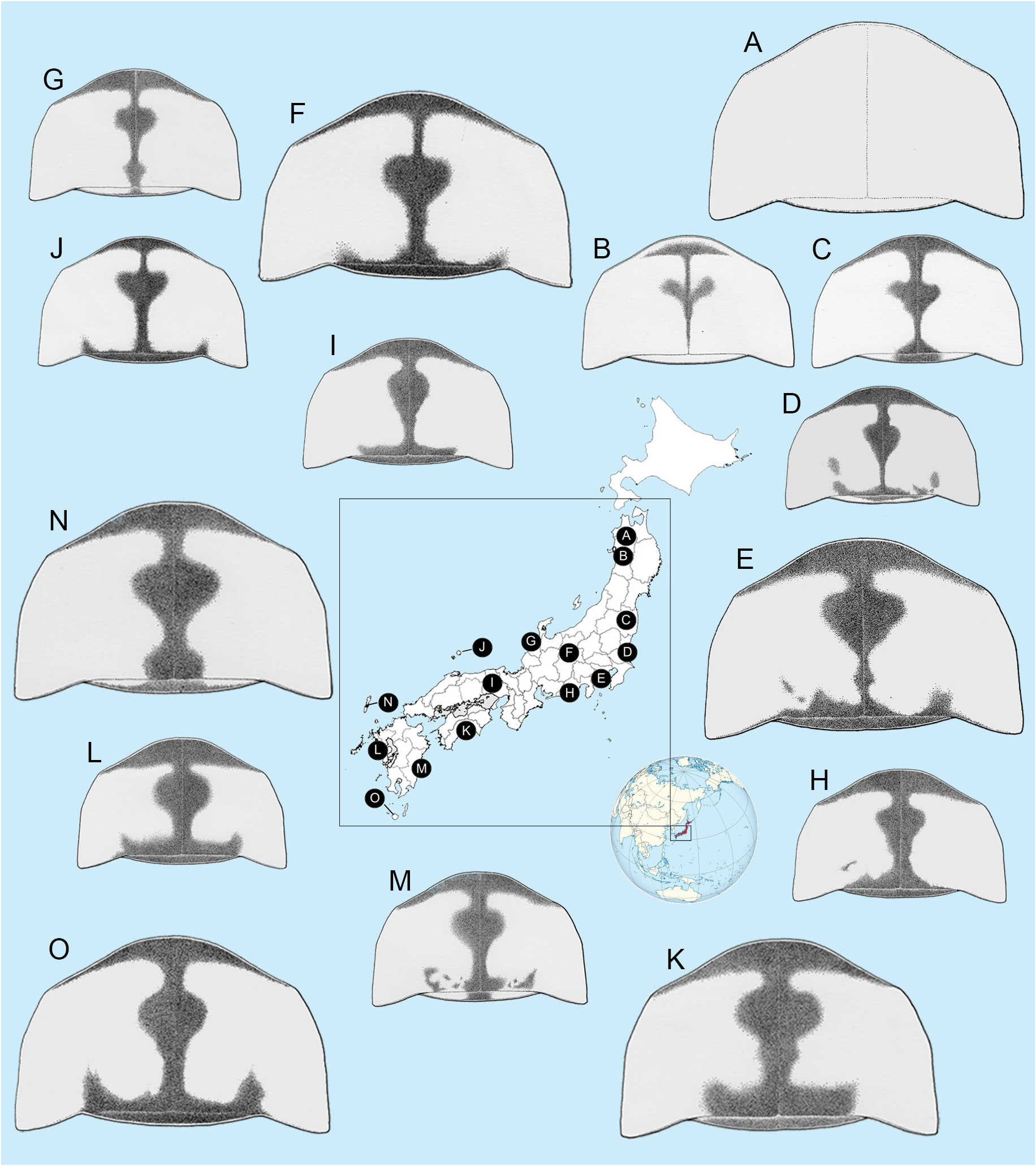
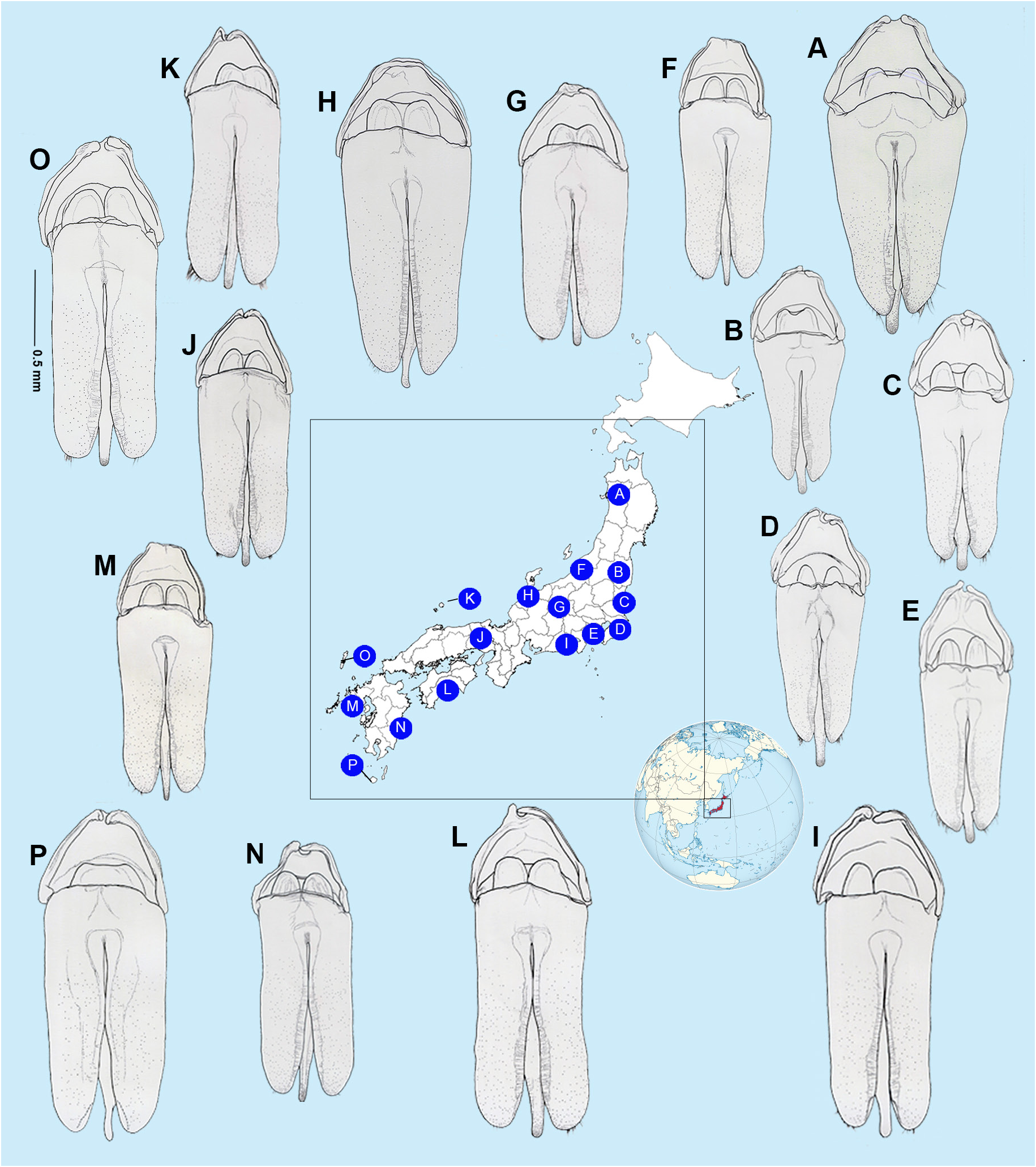
AEDEAGAL PATTERNS ( Figs 10–12 View Fig View Fig View Fig ). For the first time we are able to demonstrate intraspecific variation in the aedeagal patterns of N. cruciata . Aedeagal patterns corresponded with the areas of east Honshu, west Honshu and the island of Kyushu. In E Honshu the lateral margins of the LL converge slightly; the apices of the LL are slightly narrowed; the ML is broader (LL/ML range: 2.57‒2.78), becoming widest around ⅓ of its length from the apex; when viewed from the side, the apex of the ML is not flattened vertically, but becomes thin and stick-like; the emargination of the anterior margin of the BP is more pronounced ( Figs 10‒12 View Fig View Fig View Fig , A‒E). In W Honshu and Kyushu the lateral margins of the LL are subparallel-sided; the apices of the LL are broader and more rounded; the ML is narrower (LL/ML range: 2.66‒3.57); when viewed laterally, the tip of the ML is depressed along both sides and becomes slightly wider vertically in lateral view; the emargination of the anterior margin of the BP is less pronounced ( Figs 10‒12 View Fig View Fig View Fig , F‒P). In populations in E Honshu, from the north to the Pacific coast ( Fig. 12A‒E View Fig ), aedeagus is generally upturned towards the ventral side when viewed from the side. In contrast, populations from Hokuriku to western Honshu, Shikoku and Kyushu ( Fig. 12F‒P View Fig ) are generally straight.
Depictions of the aedeagus from line figures only are sometimes difficult to interpret. The figures in Jeng et al. (2003) are the exception. Assuming McDermott (1962: 24, fig. 20c) is left lateral then despite the absence of a basal piece, the ML can be interpreted as having a thin apex and curving strongly dorsally, similar to what we depict here for the E. Honshu pattern. Ohba’s (2004b: 91, fig. 3) depiction of the aedeagus shows a basal piece with median notch. Takakura’s (1977: fig. 2l) interpretation of the aedeagus may show a dorsal view but does show narrowing of the apex of the ML.
Female ( Fig. 7 View Fig )
BODY LENGTH. 14.0– 18.4 mm ( Jeng et al. (2003) listed a range of 15.0– 18.6 mm long).
COLOUR ( Fig. 7 View Fig ). Coloured as for male except for abdomen; abdominal ventrites black except for white LO in V6 occupying all but a narrow transparent posterior margin; V7 ( Fig. 7D View Fig ) semitransparent with pink fat body granules visible beneath cuticle and clustered around anterior ⅔; median posterior area of V7 is largely devoid of fat bodies and appears black mainly due to the underlying black V8; V8 (observed when removed from intact specimen; Fig. 7I View Fig ) black with lateral areas light brown; dorsal surface of basal tergites (up to T5), and dorsally reflexed margins of V2–V5 black; T6 ( Fig. 7C View Fig ) black with dorsally reflexed margins of V6 white, semitransparent; T7 ( Fig. 7C View Fig ) semitransparent with median dark stripe, dorsally reflexed margins of V6 appearing pink due to underlying fat body granules; T8 black ( Fig. 7C View Fig ).
ABDOMEN ( Fig. 7C–D, I View Fig ). V8: lateral margins converge posteriorly; median posterior margin shallowly and narrowly emarginated; anterior apodeme and narrow anterior margin of V8 well sclerotised, pale coloured, appearing separate to posterior area of V8 because of intervening transparent area ( Jeng et al. 2003: fig. 15, depict the posterior margin of V7 with a broad shallow emargination).
REPRODUCTIVE SYSTEM ( Fig. 7E–H View Fig ). No intact spermatophores observed but spermatophore digesting gland may contain white particulate material which may represent digested spermatophore; median oviduct plate filling half of the median oviduct, anterior margin slightly irregular, lateral margins straight, posterior margin evenly shallowly emarginated. Ovipositor elongate slender ( Fig. 7J View Fig ).
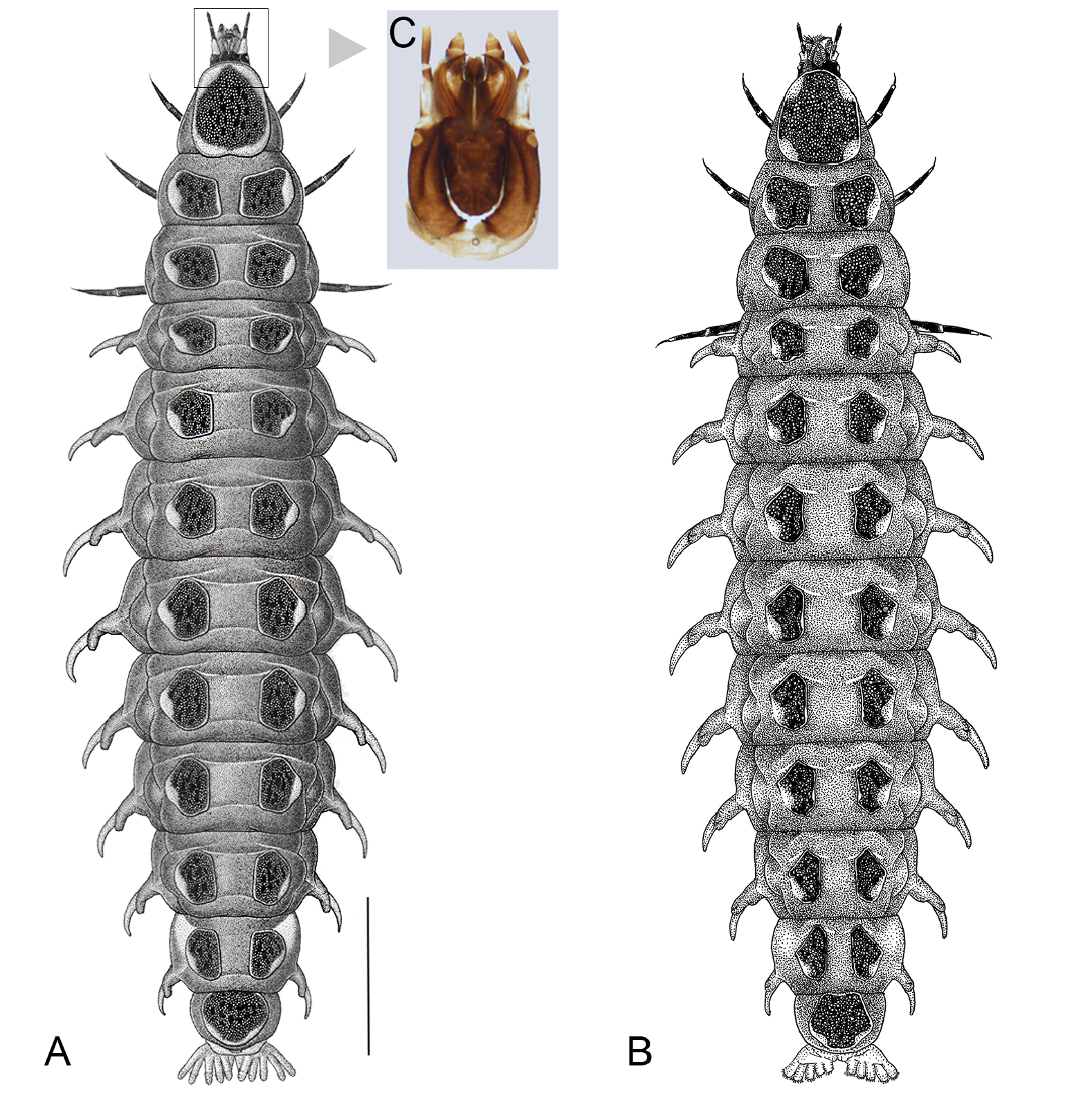
Larva ( Figs 8–9 View Fig View Fig )
Final instar larvae were examined. Approx. body length 25–29 mm; maximum (median) length of protergum 3.2–3.4 mm; maximum width of protergum 2.6–2.9 mm. The following is modified and expanded from Fu et al. (2012b: 8). Living in shallow well oxygenated water, cannot swim. Similar to larvae of Aquatica spp. distinguished from A. hydrophila by the pale marginal markings of the protergum (that of A. hydrophila has no paler marginal markings); from A. lateralis in that the pale markings on both side of protergum are not divided anteriorly and posteriorly, but are more or less continuous, and in that the pale marked areas in lateral margins of each tergite are not covered with minute spines which occur in A. lateralis ; from N. owadai in having remarkably paler ground colour of membranous body and in the tendency of the pale markings on the lateral margins of pronotum to separate anteriorly and posteriorly into four independent ones (in cruciata , the pale markings on the lateral margins of pronotum are more or less continuous anterior and posterior, becoming pale ones along the entire lateral margins). Defensive organs in this species emit a mint-like scent when the larva is disturbed. Organs are transparent when everted, and their colour reflects the amount of haemolymph in them. The protuberances on their surface may have 6 or more irregular apical spines arranged like a crown ( Fu et al. 2009: fig. 4; 2012b: figs 81–82; Hara 1962: figs 2–4; Okada 1928: fig. 1a, pl. viii e). In the pygopodia (caudal legs) there are two rows, dorsal and ventral with each row divided into left and right pairs; each of the two dorsal basal stalks branch into two on each side with a single median strand for a total of ten individual strands; the two ventral stalks branch into two, for a total of 14 individual “legs” ( Kanda 1933: 237; 1935: 57; Hara 1962).
Larvae can climb up the riverbank or canal edges to pupate on rainy nights ( Yuma 1981). Larvae when moulting split their larval cuticle along the sides of the body and not along any ecdysial cleavage line ( Kuribayashi 1979). During the day they hide under stones on the river bottom, but at night they walk around the river bottom, emitting light (IK observations).
Misidentifications
In the absence of specimens, we cannot substantiate all the listings in the table of synonymies above. However all literature, especially that including any pictorial depictions, was reassessed for accuracy of identification. References for which we feel there is the possibility of more than one species are listed as PARTIM. Local knowledge (co-authors IK, HS) of species occurrence permitted us to readdress records in Gorham (1883). He listed L. picticollis from localities in Hokkaido where L. cruciata has not been recorded; together with the adult flight period of July both IK and HS considered this species would have been A. lateralis . Gorham’s reference to L. vitticollis as a larger species, with or without the median pronotal vitta, and a flight period of May suggested this reference is to cruciata ( Fig. 2D View Fig ). While the Olivier collection in MNHN revealed correctly identified specimens ( Fig. 2B–C View Fig ), we consider that in his catalogues spanning 1902‒1910 he still misidentified some specimens. HS & IK considered that Matsumura’s (1918) reference to L. picticollis is most probably to Luciola lateralis . The reference to cruciata (with typological errors, but recognisable) is to cruciata adult but not the larva ( Matsumura 1918: 83, fig. 2–2), which HS & IK considered could be a Pyrocoelia larva. This error is evidenced by the fact that Matsumura (1928) illustrated Pyrocoelia larvae as those of Luciola cruciata vitticollis (= N. cruciata , pl. 7 fig. 14) and L. picticollis (= Aq. lateralis , pl. 7 fig. 16), respectively. McDermott (1966: 108) incorrectly annotated, under a listing of L. lateralis for Kishida (1936), that this reference addressed the introduction of lateralis into Jehol (it was cruciata which was introduced).
Pronotal colour variations ( Fig. 6 View Fig )
Ohba (1988) had described 4 different pronotal colour patterns but did not indicate any geographical distributional bias suggesting intraspecific variation. Variation within populations from the same locality was noted. IK noted a subjective impression that markings tend to become thinner and lighter from SW to NE. He did not find any evidence of the thicker and darker markings in the NE, while these markings were common in the SE. Neither did he observe any individuals in the SE with markings that were either faintly outlined, or completely absent ( Nakane 1987), as he had observed in the NE ( Fig. 6 View Fig ).
Flash communication
Nipponoluciola cruciata males flash synchronously when flying, and recognise the females by their irregular flashes (Ohba 1979, 1984, 2001, 2004a). There is no female response with fixed delay as in other species.
Ohba (1983, 2004a) classified mating behaviour of the Japanese fireflies into six systems. The LC (complex) system observed in N. cruciata is described in several phases: after sunset, the males began to fly and seek females with synchronous or semi-synchronous flashing light; the females emit singlepulsed flashes of light (not synchronised) on grasses; when a male finds the female’s flash of light, the male approaches the female; the male emits flashes with various patterns while approaching and walking around the female; thereafter, they copulate.
Synchronous flashing was first described by Watasé (1902), with 26 flashes per minute (2.3 sec cycle) at 60°F (16°C). Kanda (1935) was the first to notice the difference in the flashing intervals and described synchronous flashing as a 3.7 sec cycle at 16°C and 21°C in Kofu, east area of Honshu, and a 1.8 sec cycle at 18°C in Gifu, west area of Honshu.
Ohba (1984) focussed on synchronous flashing in males swarming while seeking females of the LC system and analysed the flash intervals from video-recorded images of five populations (Yokosuka and Yokohama in east Honshu, Tatsuno in central Honshu, and Kyoto and Toyota in west Honshu). He determined the flash interval was about 2 sec and 4 sec in the west and east area of Honshu, respectively, and the two ecological types, 2 sec type (fast flash type) and 4 sec type (slow flash type) were recognised. The border between the two types was noted in the central area of Honshu. Subsequently, Ohba (1988) through the analysis of 30 populations, confirmed that the border of the two types was the central region of Honshu, and it was speculated that the formation of the Fossa Magna, which divides Honshu into eastern and western areas, may be a factor in the generation of the two types.
Since then, many researchers and amateur researchers observed the synchronous flashing. The relationships between temperature and flash interval, and an intermediate-flash type (3 sec type) were reported. However, the state of observations was not constant. In some cases, flash interval was confused with flash duration, and in other cases, flash interval was measured not in swarming but in solo flight.
Ohba (2001) summarised the geographic variation in morphology and flash patterns in 50 populations through almost all distributional areas of Japan and recognised three flash types: 1) flash interval of about 2 sec and flash duration of about 0.6 sec, 2) flash interval of about 3 sec and flash duration of about 1 sec, 3) flash interval of more than 4 sec and flash duration of about 2 sec. However, it has been observed that some populations have extremely short flash intervals, or that even in western Japan, the flash interval was of the 3 sec type, or that the flash intervals differ locally even in the same population. Iguchi (2010) reported three types (the fast-flash, slow-flash, and intermediate types) from five populations in the Kanto and Chubu regions, but whether the intermediate type is the progeny of a cross between the fast- and slow-flash types is not known. Ohba et al. (2020) reported a quick-flash type in the Goto Islands that is even faster than the fast-flash type, resulting in four types, slow, intermediate, fast and quick.
Genetic analysis
As the flash intervals of males did not change even when N. cruciata from west Japan were transplanted to east Japan and reared in succession, it was speculated that N. cruciata had already differentiated genetically. Therefore, populations of N. cruciata were investigated at allozyme, mt DNA, and genome DNA level.
On the allozyme level, Suzuki et al. (1996a) showed genetic differentiation among east and west Japan based on 17 loci of 13 enzymes in 15 populations. The two genetically differentiated groups corresponded to the synchronous flashing pattern of slow-flash and fast-flash types. The degree of the genetic differentiation was considered as subspecies level based on the comparison of Nei’s genetic distances of other species groups. On the mt DNA level, Suzuki et al. (2002) analysed the COII gene of 62 populations of 494 individuals covering almost all distributional areas and showed three groups, east Honshu, west Honshu-Shikoku, and Kyushu. The east Honshu and west Honshu-Shikoku groups were the most closely related, followed by the Kyushu group. Flash interval of the east Honshu group and the west Honshu-Shikoku and Kyushu groups were slow-flash and fast-flash types, respectively. Furthermore, the three genetic groups divided into six subgroups and the distributional border of the subgroups corresponded to the geological structure of Japanese islands. They proposed a vicariant speciation scenario in which regional differentiation has occurred along with the formation of the Japanese archipelago. On the genome DNA level, Kato et al. (2020) also confirmed the three genetic groups and the vicariant scenario by RAD-Seq analysis, however, relationships among the groups differed from the mitochondrial DNA results in that the west Honshu and Kyushu groups were the most closely related, followed by the east Honshu group. As they could not find any individuals with genome composition between the east Honshu and west Honshu-Kyushu populations, they raised the possibility that mating would not occur between the slow- and fast-flash types.
Flying activity of males
In east Japan, swarming activity decreased after 21:00, but in west Japan, it continued through to midnight, with occasional breaks ( Ohba 1988, 2001). The time difference between Aomori (northern part of Honshu) and Kagoshima (southern part of Kyushu) at sunset is about 15 minutes. Furthermore, flying speed of males in east Japan is slower than that in west Japan ( Ohba 1988).
Spawning behaviour
In west Japan, females fly in a straight line at high speed over the river surface after swarming, and gather on the moss near water one after another. Then females lay their eggs on the moss, and that continues until dawn. On the other hand, in east Japan, females lay eggs alone on the moss without any flight behaviour to search for egg-laying sites ( Kuribayashi 1979; Yuma & Hori 1981; Ohba 1988).
Female recognition by males
In general, flashing is believed to contain information that shows ‘species’, ‘sex’ and ‘position’. However, Kawano (2013) found flashing contained little information other than ‘position’ in this species. He suggested that the factors influencing female recognition by males in the mating behaviour are the position (height), flashing and orientation of females in the long distance stage, while factors other than flashing such as an odorous substance operated during the direct contact stage.
Male approach to artificial flash light
The female responds to the synchronous flashing of the males with single-pulsed flashes of light, which the male detects and he then approaches her. Tamura et al. (2005) examined whether males approach artificial single-pulsed flashes of light at any flash interval, and found that males from east Japan (Aomori and Sendai populations) approached artificial single-pulsed flashes of light with an flash interval of 4 or 5 sec rather than 2 or 3 sec, and males from west Japan (Otsu population) approached artificial light with an flash interval of 2 or 3 sec rather than 4 or 5 sec. Males from the central Japan (Inuyama) showed no particular preference for any flash interval (2, 3, 4, or 5 sec intervals). They proposed pre-mating isolation between the two ecological types.
Factors affecting life cycle and development
Light irradiation affects spawning. In the laboratory using reared specimens, yellow light-emitting diode (LED) irradiation at 0.11 lx inhibited spawning as did LED irradiation of other colours (white, blue and green) at 20 lx ( Miyashita, 2011).
The sex ratio at emergence in this species is male-biased. Last instar larvae were captured (543 and 952 individuals in 2006 and 2007) in the field and emerged in the laboratory at 82.1 and 56.3% emergence rate in 2006 and 2007. Male emergence rate was significantly higher than female, 60.3 and 64.7% in 2006 and 2007 ( Moriya et al. 2009).
Egg size is related to hatching rate and larval growth in this species. Egg size (as weight) decreased with aging of a female, and also seasonally through oviposition period. Heavy and light eggs showed high hatching rates at low (ca 20°C) and high temperature (ca 30°C), respectively. If eggs developed at the favourable temperature, they maximised their larval size and the tolerance of the larvae for starvation ( Yuma 1984). One third of the larvae hatched from the light eggs produced females ( Yuma 1986).
Brightness of the adult habitat at night affects its population density. The borderline for light intensity between the high and low densities (5 individuals per 10 m is boundary) was 0.05‒0.2 lx ( Yuma 2001). Frequent rainfall during rainy and typhoon seasons caused considerable decreases in emergence of adult fireflies, and a decrease in their foraging activity ( Yuma 2007).
Several population characteristics were estimated by Richard and Waloff method. In Kyoto (West Japan), survival rate of adults was 0.76‒0.88 per day, mean life span was 47 days, total number emerging 3200‒3400 ( Yuma & Ono 1985).
Adult males are observed either resting near ground, on trees, or in flight. The proportion of these three behavioural categories was constant through adult life of males, and the season. Seasonal and age-related changes of adult female behaviour were observed. Newly emerged females remained near ground level and copulated there. With increasing age, females changed their resting sites onto broad-leaved trees, and the proportion of females resting on trees increased with the season progression ( Yuma & Hori 1990).
Seasonal variation of body size was observed in both sexes. Daily mean body size of male and female decreased from 12.7 to 12.0 mm and 15.1 to 13.5 respectively, as the season progressed ( Iguchi 2001).
Fireflies intentionally introduced from the Lake Biwa area (West Japan) into Tatsuno area (Central Japan), exhibited a flash interval distinct from populations native to Tatsuno area, but similar to populations native to Lake Biwa area ( Iguchi 2009a, 2009b).
Abundance of both gravel deposit covering the streambed and of freshwater snails significantly affected the firefly population. On the other hand, channel width, flow velocity, dissolved oxygen, and bank height did not always act as environmental factors ( Tomita et al. 2006).
Colour of light organ emissions
Oba et al. (2010) discovered N. cruciata could possess two luciferase isotopes and Oba et al (2013) found these different isotopes of luciferase were responsible for the yellow luminescence in larval and adult light organs, and the dim greenish glow of eggs and whole pupae respectively, in both A. lateralis and N. cruciata .
Occurrence outside Japan?
Specimens of cruciata from Osaka were introduced into Chih-feng (now known as Ulandad, Inner Mongolia), and were seen there for 1‒2 years after introduction, but not since ( Kishida 1936).
Remarks
Nipponoluciola cruciata is distributed throughout mainland Japan with the northern limit of its distribution in Honshu, it never crosses the Braxton line, and is not found in Hokkaido. The southern limit of its distribution is Yakushima Island of Ôsumi Islands, off Kyushu. The genetic differentiation of this species in mainland Japan seems to be influenced by the geological structure, e.g., the Fossa Magna and the Median Tectonic Line. Its habits (e.g., interval period of flashing behaviour and oviposition behaviour) are largely divided between eastern and western Japan, with the boundary near the Fossa Magna. Nipponoluciola owadai is distributed as relict populations on Kume-jima Island of the Okinawa Islands in the central part of Ryukyus, located in the southwestern part of Japan.
Since the recognition of two ecological types differing in flash interval, genetic differentiation has been confirmed, but no morphological characters distinguishing the two types have been reported until now, and they are summarised below.
We attempted unsuccessfully to determine if there is correlation between the extent of the pronotal markings and flash patterns as we have been able to show for certain aedeagal patterns ( Figs 5 View Fig , 10‒12 View Fig View Fig View Fig ). Ohba (2001) depicted 50 examples of males and females showing variability in pronotal pattern but could not distinguish any relationship. It is difficult to determine the extent of the anterior broad pronotal marking in his figures, one of which is surely A. lateralis ( Ohba 2001: fig. 12). In Ohba (2004b) the extent of the median darker marking on the pronotum varied, although females ( Ohba 2004b: figs 10, 16, 48) and males ( Ohba 2004b: fig. 48) have a narrow median pronotal stripe. Ohba’s (2004b) illustrations on page 104 show a gradation depicting a progressive loss of the wide posterior area to pronota having no colour at all, but at no time did he depict the wide anterior marking we show here. Ohba (1988) described four patterns of the pronotum: 1) black cruciate marking thick and clear, 2) cross is thin, 3) cross is traceable, 4) no marking, but there were no corresponding geographic distributional biases suggesting intraspecific variation. Nakane (1987) proposed subspecies L. cruciata towadensis based on two female specimens with no pronotal marking collected at Aomori pref. (northern limit of distribution). Later, Ohba (2001) indicated a geographic distributional separation for the marking patterns; the black cruciate marking is thick and clear in Kyushu (southern limit of distribution), gradually becoming thin to Shikoku, Kinki (central distribution), Kanto, and it is traceable and no marking from Kanto to north east Honshu. However, Kawashima et al. (2003) synonymised the subspecies as interspecific variation. We could not detect any clear cut geographical distributional biases relating to the different marking patterns as we had for aedeagi.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nipponoluciola cruciata ( Motschulsky, 1854 )
| Ballantyne, Lesley, Kawashima, Itsuro, Jusoh, Wan F. A. & Suzuki, Hirobumi 2022 |
Luciola cruciata cruciata
| Geisthardt M. & Sato M. 2007: 232 |
Luciola cruciata towadensis
| Kawashima I. & Suzuki H. & Sato M. 2003: 247 |
Luciola cruciata
| Thapa V. K. 2000: 115 |
Luciola cruciata towadensis
| Nakane T. 1987: 173 |
Luciola cruciata vitticollis
| Matsumura S. 1928: 59 |
Luciola cruciata vitticollis
| Matsumura S. 1928: 59 |
| Okamoto H. 1924: 182 |
| Matsumura S. 1918: 86 |
Luciola Cxuca’a var. vitticollis
| Matsumura S. 1918: 82 |
Luciola cruciata var. vitticollis
| McDermott F. A. 1966: 111 |
| Olivier E. 1910: 42 |
| Olivier E. 1907: 54 |
| Olivier E. 1902: 71 |
| Olivier E. 1902: 188 |
Luciola vitticollis
| Okada Y. K. 1931: 146 |
| Gorham H. S. 1883: 409 |
Luciola picticollis
| Gorham H. S. 1883: 409 |
Luciola vitticollis
| Okada Y. K. 1931: 146 |
| Gorham H. S. 1883: 409 |
Luciola picticollis
| Nakane T. 1983: 111 |
| McDermott F. A. 1966: 111 |
| Okada Y. K. 1931: 130 |
| Matsumura S. 1918: 87 |
| Olivier E. 1910: 45 |
| Olivier E. 1907: 54 |
| Olivier E. 1902: 85 |
| Olivier E. 1902: 189 |
| Lewis G. 1879: 17 |
| Harold E. 1877: 357 |
Luciola picticollis Kiesenwetter, 1874: 262
| Kiesenwetter E. A. H. 1874: 262 |
Luciola cruciata
| Kato D. & Suzuki H. & Tsuruta A. & Maeda J. & Hayashi Y. & Arima K. & Ito Y. & Nagano Y. 2020: 1 |
| Ballantyne L. A. & Lambkin C. L. & Ho J-Z. & Jusoh W. F. A. & Nada B. & Thancharoen A. & Wattanachaiyingcharoen W. & Yiu V. 2019: 163 |
| Jusoh W. F. A. & Ballantyne L. A. & Lambkin C. L. & Hashim N. R. & Wahlberg N. 2018: 14 |
| Ballantyne L. A. & Lambkin C. L. & Luan X. & Boontop Y. & Nak-Eiam S. & Pimpasalee S. & Silalom S. & Thancharoen A. 2016: 204 |
| Ballantyne L. A. & Lambkin C. L. & Boontop Y. & Jusoh W. F. A. 2015: 8 |
| Fu X. H. & Ballantyne L. A. & Lambkin C. L. 2012: 6 |
| Fu X. H. & Ballantyne L. A. & Lambkin C. L. 2012: 14 |
| Oba Y. & Branham M. A. & Fukatsu T. 2011: 771 |
| Fu X. H. & Ballantyne L. A. & Lambkin C. L. 2010: 3 |
| Ballantyne L. A. & Lambkin C. L. 2009: 21 |
| Fu X. H. & Ballantyne L. A. 2009: 243 |
| Fu X. H. & Ballantyne L. A. 2009: 155 |
| Takeda M. & Amano T. & Katoh K. & Higuchi H. 2006: 177 |
| Ohba N. 2004: 228 |
| Suzuki H. & Sato Y. & Ohba N. & Bae J-S. & Jin B-R. & Sohn H-D. & Kim S-E. 2004: 297 |
| Branham M. A. & Wenzel J. W. 2003: 28 |
| Ohba N. 2001: 45 |
| Suzuki H. 2001: 99 |
| Sawada Y. 2000: 93 |
| Suzuki H. 1997: 1 |
| Suzuki H. & Sato Y. & Fujiyama S. & Ohba N. 1996: 191 |
| Suzuki H. & Sato Y. & Fujiyama S. & Ohba N. 1996: 682 |
| Ohba N. & Azuma S. & Nishiyama K. & Goto Y. & Suzuki H. & Sato Y. & Kawashima I. 1994: 13 |
| Sato M. 1989: 352 |
| Sato M. 1985: 121 |
| Ohba N. 1984: 23 |
| Ohba N. 1983: 24 |
| Sato M. 1978: 17 |
| Ohba N. 1978: 25 |
| Sato M. 1974: 133 |
| Bertrand H. P. I. 1973: 107 |
| Bertrand H. P. I. 1972: 599 |
| Minami K. 1961: 21 |
| Nakane T. 1960: 36 |
| Nakane T. & Ohbayashi K. & Nomura S. & Kurosawa Y. 1959: 170 |
| Hasama B. 1944: 155 |
| Hasama B. 1943: 23 |
| Hasama B. 1942: 366 |
| Hasama B. 1942: 378 |
| Kishida K. 1936: 12 |
| Kanda S. 1935: 31 |
| Okada Y. K. 1931: 134 |
| Okada Y. K. 1928: 102 |
| Matsumura S. 1918: 86 |
| Lewis G. 1879: 17 |
| Harold E. 1877: 357 |
| Motschulsky V. 1866: 167 |
Luciola cruciata
| Jeng M. - L. & Lai J. & Yang P. - S. 2003: 541 |
| Kawashima I. & Suzuki H. & Sato M. 2003: 247 |
| Takakura Y. 1977: 7 |
| McDermott F. A. 1966: 102 |
| McDermott F. A. 1962: 24 |
| Olivier E. 1910: 41 |
| Olivier E. 1907: 51 |
| Olivier E. 1902: 77 |
| Olivier E. 1902: 188 |
| Gorham H. S. 1880: 102 |
| Heyden L. 1879: 350 |
| Lacordaire J. T. 1857: 338 |
Luciola cruciata
| Motschulsky V. 1854: 53 |