Xyleborus bispinatus Eichhoff, 1868
|
publication ID |
https://doi.org/10.11646/zootaxa.5174.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:5A05EB20-9355-4265-9712-8169DC7422BD |
|
DOI |
https://doi.org/10.5281/zenodo.6987949 |
|
persistent identifier |
https://treatment.plazi.org/id/A31687CA-FFEB-1D7B-2C99-F90FFD3A0DE1 |
|
treatment provided by |
Plazi |
|
scientific name |
Xyleborus bispinatus Eichhoff |
| status |
|
Xyleborus bispinatus Eichhoff View in CoL
Description. Faccoli et al. (2016) compared the morphology of X. bispinatus with the other European Xyleborus species based on previous descriptions and photos ( Kirkendall & Jordal 2006; Atkinson et al. 2013). All collected specimens were females ranging between 2.8–3.3 mm in size, and characterized by a flat elytral declivity bearing a pair of large tubercles on interstriae 3 that are closer to the declivity base than apex. Additionally, one to three small tubercules can also present on the declivity ( Figure 1 View FIGURE 1 ; B,G).
Xyleborus bispinatus has similar morphological features to other native European Xyleborus ( Figure 1 View FIGURE 1 ). In addition to the characters given above, this species can be differentiated by its more gradually sloped declivity and a pair of large tubercles on declivital interstriae 3 ( Figure 1 G View FIGURE 1 ) compared to the steeper sloped declivities in the native species which lack large tubercles on declivital interstriae 3 ( Figure 1 F View FIGURE 1 , H-J). In accordance with Faccoli et al. (2016), the presence of the large pair of declivital tubercles may lead to misidentification as X. eurygraphus (Ratzeburg) ( Figure 1 View FIGURE 1 , D, I) or X. monographus (Fabricius) ( Figure 1 View FIGURE 1 , A, F) which are superficially similar in appearance. For example, the pronotum of X. eurygraphus is clearly subtruncate ( Figure 1 View FIGURE 1 , D), and is typically larger in size than X. bispinatus (3.4–4.0 mm). The declivital tubercles of X. eurygraphus are less stout than X. bispinatus , and placed near the elytral suture, on interstria 1 ( Figure 1 View FIGURE 1 , B, D). Xyleborus monographus partially overlaps with X. bispinatus in size (3.0– 3.5 mm), but its declivity is armed with two pairs of conical tubercles on interstria 1 that are arranged in irregular square ( Figure 1 View FIGURE 1 , A). Xyleborus bispinatus could also be misidentified as X. dryographus Ratzeburg ( Figure 1 View FIGURE 1 , C, H), although its declivity is armed only with small granules ( Faccoli 2008), and Xyleborinus saxesenii (Ratzeburg) ( Figure 1 View FIGURE 1 , E, J), which has very different, conical scutellum and much smaller size ( 2–2.4 mm).
Distribution. Due to the long-standing synonymy with X. ferrugineus (Fabricius) , the precise distribution of X. bispinatus is still unclear ( Kirkendall & Jordal 2006). Xyleborus bispinatus is native to Neotropical and Neosubtropical regions where it co-occurs with X. ferrugineus . Thus, in accordance with Atkinson (2018), some records of X. ferrugineus may actually refer to X. bispinatus . Xyleborus bispinatus was recorded from the northern part of South America to Mexico and in the Eastern coast of the USA, where it was accidentally introduced ( Faccoli et al. 2016; Gomez et al. 2018). Xyleborus bispinatus was initially found in Europe in 2014 attacking Ficus carica on Sicily ( Italy), and five specimens were captured in 2017 in traps in southern France (Nice) ( Faccoli et al. 2016; Barnouin et al. 2020). Our work enlarges the known European distribution of this alien species to the Eastern Iberian Peninsula.
Host plants. As previously discussed, due to the synonymy with X. ferrugineus , only a few host plants are noted precisely for X. bispinatus . Atkinson et al. (2013), Pérez et al. (2015), Faccoli et al. (2016) and Atkinson (2020) recorded attacks on Eschweilera biflava (Lecythidaceae) , Ficus carica (Moraceae) , Hevea brasiliensis (Euphorbiaceae) , Lonchocarpus macrophyllus (Fabaceae) , Persea americana , Persea palustris (Lauraceae) , Quercus spp. (Fagaceae) , Swietenia macrophylla (Meliaceae) and Wodyetia bifurcata (Aracaceae) .
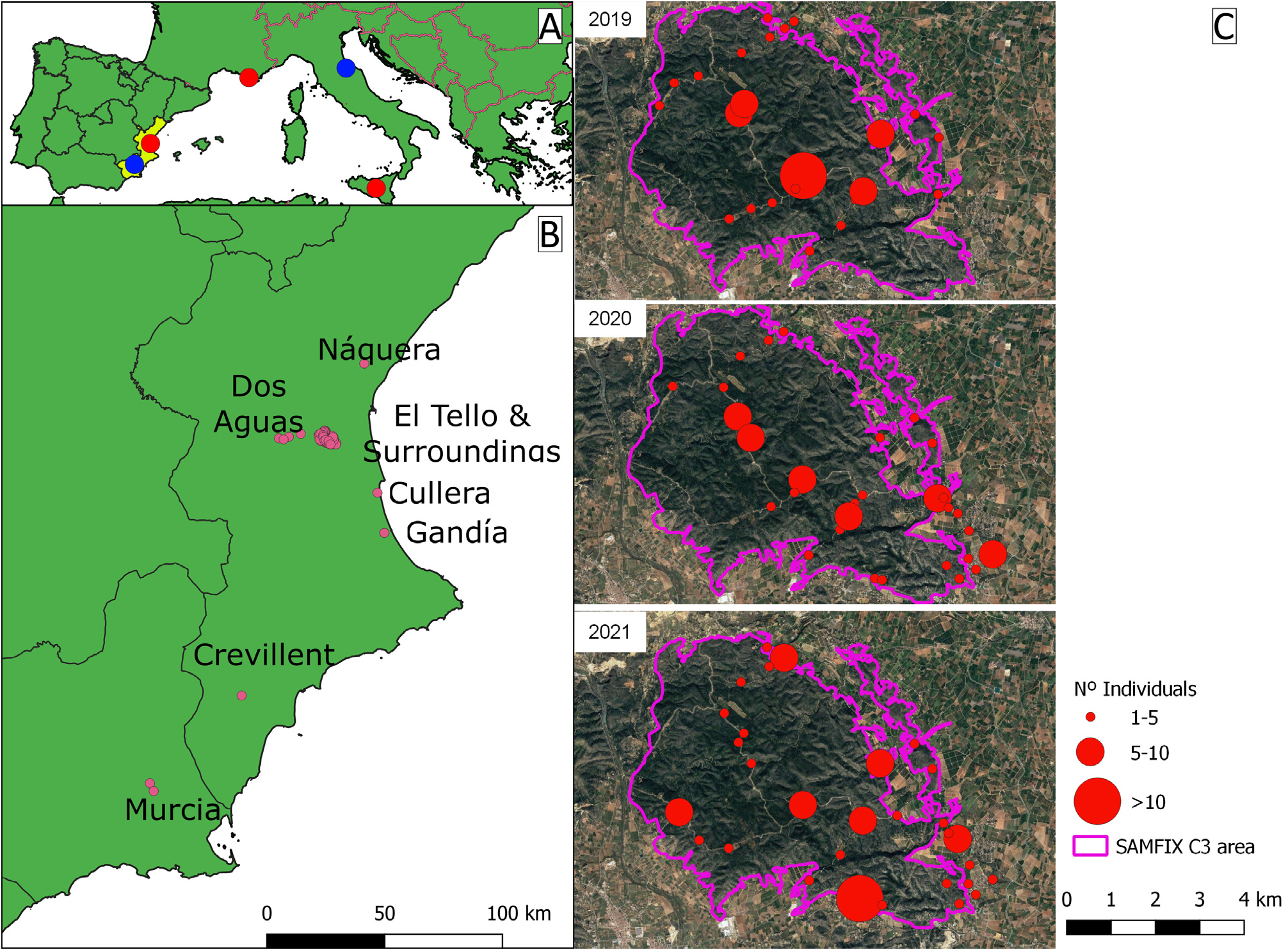
Distribution in the Iberian Peninsula. During two different periods, in 2009 and from 2018 to 2021 ( Table 1 View TABLE 1 ), 410 individuals of X. bispinatus were collected in seven locations of the Eastern Iberian Peninsula. Specimens were collected in traps of four systematic trapping networks: Estaciones de Seguimiento Forestal Permanentte (ESFP), Red de Muestreo Fitosanitario y Forestal en Ecosistema Tipo (MUFFET), trapping network of Life project Saving Mediterranean Forests from Invasions of Xylosandrus beetles and associated pathogenic fungi (SAMFIX), and Red de Alerta Temprana (RAT) and in an experimental trapping set up installed inside a private pomegranate orchard. ESFP and MUFFET are long-term monitoring projects in Mediterranean pine forests ( Gallego 2019; Generalitat Valenciana 2020) which are considered twin trapping networks covering Eastern and Southeastern regions of Spain. They are implemented by the Regional Governments of Murcia and Valencia Regions. Networks are composed of 20 and 15 plots, respectively, provided with data loggers, two band dendrometers and a cross vane trap (Crosstrap, Econex, Spain) baited with a generic lure (Econex, Spain). This lure is composed of a blend of attractants that mimic a stressed tree and are thus attractive to saproxylic beetles ( Faccoli et al. 2020). These specific attractants are made of an ethanol dispenser (ultrahigh release rate of 1.5 g per day at 20 ºC), a dispenser of (–) alpha-pinene (release rate of 30 mg per day at 20) and a dispenser that contains a blend of the conifer bark beetle pheromones ipsdienol, ipsenol, and cis-verbenol ( 300 mg of each component, with 1.5 mg per day of release rate at 20 ºC). Traps were revised monthly.
SAMFIX is a trapping network composed of 62 traps Crosstrap model, divided in C3 (50 traps) and C5 (12 traps) subnetworks ( LIFE SAMFIX 2020), baited only with ethanol and alpha-pinene. The eight traps of C3 subnetwork installed in Náquera were also baited with ethanol and alpha-pinene, plus quercivorol and alpha-copaene dispensers (Synergy, Canada) SAMFIX traps are located in “El Tello and Surroundings” and Náquera, in Valencia province ( Table 1 View TABLE 1 , Figure 2B View FIGURE 2 ). RAT is a web of 16 traps deployed in ports and surrounding forests implemented by the Regional Government of Valencia, traps and lures are the same used for the MUFFET network. Finally, the insects collected in the mentioned pomegranate orchard (Cullera, Valencia, Spain) were unintentionally captured using cross vane traps baited with ethanol and alpha-pinene aimed to capture mainly the species Apate monachus Fabricius (Bostrichidae) in a pomegranate orchard.
The European Xyleborini Fauna. The European Xyleborini fauna is frequently changing due to the establishment of invasive alien species. Currently eight genera- Ambrosiodmus Hopkins , Ambrosiophilus Hulcr & Cognato , Anisandrus Ferrari , Xyleborus Eichhoff , Xyleborinus Ratzeburg , Cyclorhipidion Hagedorn , Xylosandrus Reitter and Heteroborips Reitter are broadly distributed across Europe ( Faccoli 2008; Knížek 2011). Recently two more genera have been recorded, Euwallacea Hopkins and Cnestus Sampson ( Schuler et al. 2021; Colombari et al. 2022) but these have not yet become widespread across the continent. One of these genera, Xylosandrus , is represented in Europe by four alien species: X. morigerus (Blandford) lives only in tropical orchids in greenhouses; X. germanus (Blandford) has been recorded in Europe since at least 1932 and has silently become widespread throughout European forests (review in Galko et al. 2019); X. crassiusculus (Motschulsky) was first collected in 2003 in traps set up in Tuscany, Italy (revision in Gallego et al. 2017), and X. compactus (Eichhoff) which was first detected in Campania and Tuscany ( Italy) in 2010 (Pennacchio et al. 2003; Francardi et al. 2012; Garonna et al. 2012). Moreover, these last three species were reported occupying, in co-presence, the same evergreen Mediterranean maquis environment in Latium ( Italy) ( Contarini et al. 2020). Ambrosiophilus atratus (Eichhoff) , is an invasive species native to Eastern Asia, that was first collected in 2007 and 2008, in ethanol baited traps in Legnaro, in the North-eastern part of Italy ( Faccoli 2008), and in 2017 in Slovenia ( Hauptman et al. 2019).
The native European Xyleborus fauna is comprised of four species: X. dryographus , X. eurygraphus , X. monographus and X. pfeilii (Ratzeburg) ; this latter species was possibly introduced from eastern Asia, prior to the nineteenth century, and was thus considered a native European species until the review of Kirkendall and Faccoli (2010), when X. pfeilii was accepted as alien species. Within Xyleborus some alien species were intercepted or became established in Europe within the last two decades. Xyleborus affinis Eichhoff and X. perforans (Wollaston) , which were reported from Austria, Hungary, and the Azores, respectively ( Knížek 2011), were never established here ( Kirkendall & Faccoli 2010; Barnouin et al. 2020). A single specimen interception of X. ferrugineus has been reported from Malta in 1994 ( Mifsud & Knížek 2009), under the bark of imported logs from Central Africa, where it is not considered established ( Rassati et al. 2015). The most recent alien Xyleborus species to Europe is X. bispinatus Eichhoff , which was reported attacking figs ( Ficus carica ) in South-Eastern Sicily Region in 2014 and 2015 ( Faccoli et al. 2016). Moreover, Barnouin et al. (2020) captured five specimens of X. bispinatus in two localities: Mont Boron and Nice (Southern France) in ethanol traps between May and November 2017. Findings of misidentifications between X. ferrugineus and X. bispinatus , lead some authors to correct some of their recording data. For example, the interception of six specimens of X. ferrugineus in baited traps in Ravenna in 2012 (Nord-eastern Italy) ( Rassati et al. 2015) are now considered as an interception of X. bispinatus ( Faccoli et al. 2016) .
Our data show that X. bispinatus has been present in Southeastern Iberian Peninsula since at least 2009. The punctual collection records in Murcia (2009, ESFP network) and in Crevillent (2018, MUFFET network), and the no further catches in the traps until the present, evidence that this species is not established at the Southeastern.
On the contrary, the continuous collection of specimens in SAMFIX C3 ( Table 1 View TABLE 1 , Figure 1B, C View FIGURE 1 ) since the installation of the trap network during the spring of 2019 may indicate the presence of an established population, even if the host plants have not yet been identified. Since 2019 we collected 96 specimens in 23 traps (in 2019), 69 specimens in 29 traps (in 2020) and 150 specimens in 28 traps (in 2021). The collected specimens of SAMFIX C3 at Náquera ( Table 1 View TABLE 1 , Figure 1B View FIGURE 1 ) between 2020 and 2021 may also mark the establishment of a population. In the RAT trap of Gandia port in the surrounding forest, 24 specimens have been collected in non-consecutive years, 2019 and 2021, which may indicate a possible established population. Lastly, the 25 specimens collected in Cullera in 2021 ( Table 1 View TABLE 1 , Figure 1B View FIGURE 1 ) are insufficient to conclude if the population is established or not, since there has only been one year of sampling .
Inspections of putative host plants were carried out in the areas of El Tello, Náquera, Cullera and Gandía, with a special focus on Ficus carica , Persea americana , Quercus coccifera , and Quercus ilex . No attacks of X. bispinatus on these or other plant species were detected, thus, the host plants in Spain remain unknown.
Possible impacts in Spain. Impacts of X. bispinatus on tree crops or on the invaded ecosystems were revised by EPPO (2020). This document only provides scarce host records of X. bispinatus on P. americana in Argentina in 2017, and an existing concern in USA about its potential ability to be vector of the laurel wilt fungal pathogen Harringtonia lauricola . As previously mentioned, X. bispinatus , together with the alien species Cryphalus discretus (Eichhoff) caused large infestations in fig trees in Sicily ( Faccoli et al. 2016), although these authors consider X. bispinatus as secondary pest. Note that Hypocryphalus scabricollis (Eichhoff) has been synonymized with C. discretus after a recent revision of Cryphalini ( Johnson et al. 2020) .
However, after three years (2019 to 2021) of intense monitoring, no damages on any possible host plant have been detected in “El Tello and Surroundings” area ( Valencia). Consequently, X. bispinatus may breed on plants, or plant parts, that remain undetected (i.e. wild fig trees in dense vegetation of some down ravines), or their breeding activity does not produce easily visible conspicuous damages.
Although EPPO (2020) considers this insect a major concern because of its potential to vector a new potential pathogen fungi in Europe, specifically H. lauricola , although to date X. bispinatus has been a non-confirmed vector of H. lauricola ( Saucedo et al. 2018) . However due to this potential risk, surveillance trapping and searching for host plants should continue for both the stable populations found at least in the center of Valencia province and in the other areas where X. bispinatus has been captured.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |