Nuntianus Miranda, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4822.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:E3B5713F-ADE8-4075-9ABF-8F6DE9D3A88E |
|
DOI |
https://doi.org/10.5281/zenodo.4449985 |
|
persistent identifier |
https://treatment.plazi.org/id/BDB84EA7-970F-439A-AF85-E5E0B2AE9BC7 |
|
taxon LSID |
lsid:zoobank.org:act:BDB84EA7-970F-439A-AF85-E5E0B2AE9BC7 |
|
treatment provided by |
Plazi |
|
scientific name |
Nuntianus Miranda |
| status |
gen. nov. |
Genus Nuntianus Miranda View in CoL View at ENA gen. nov.
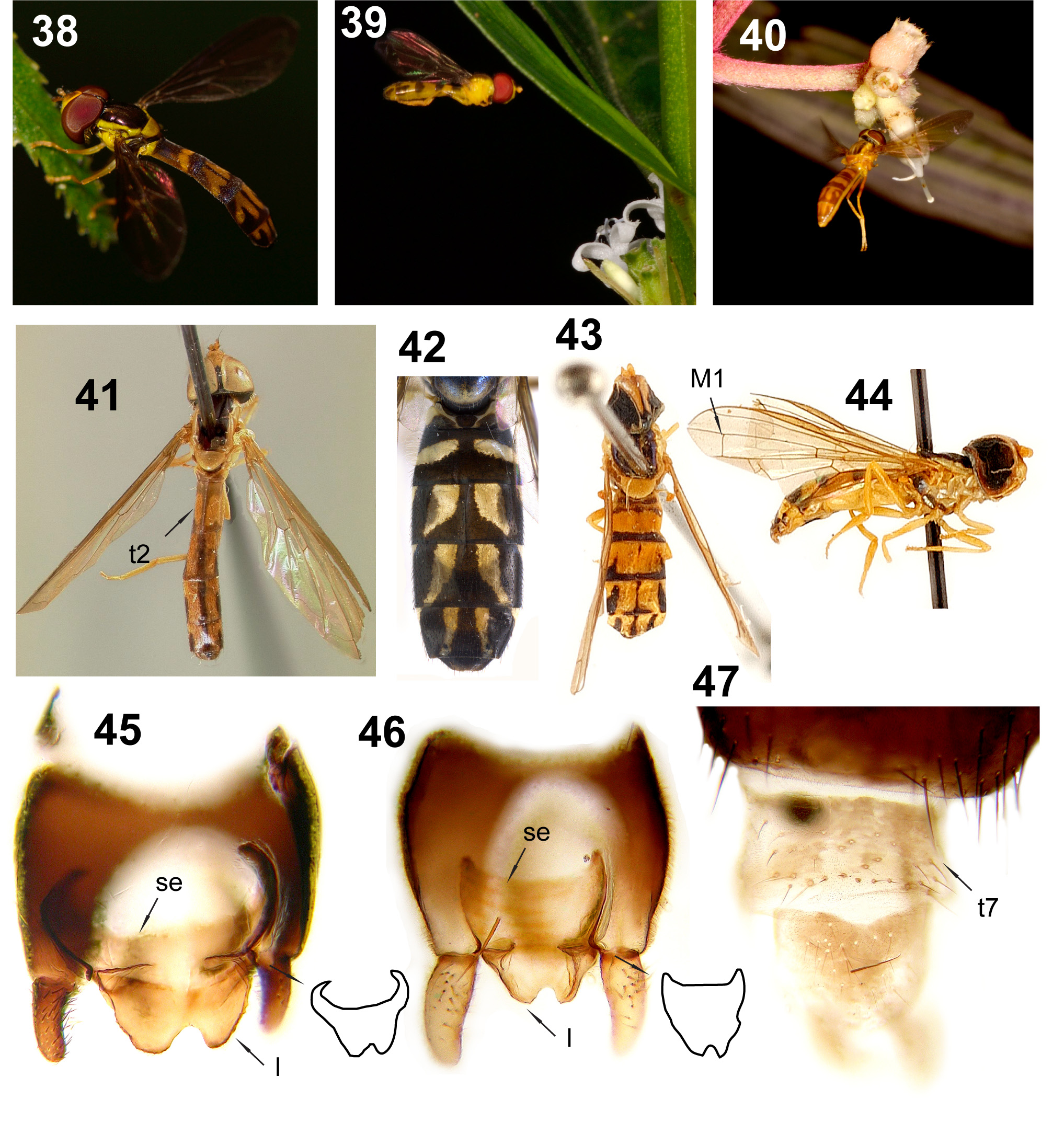
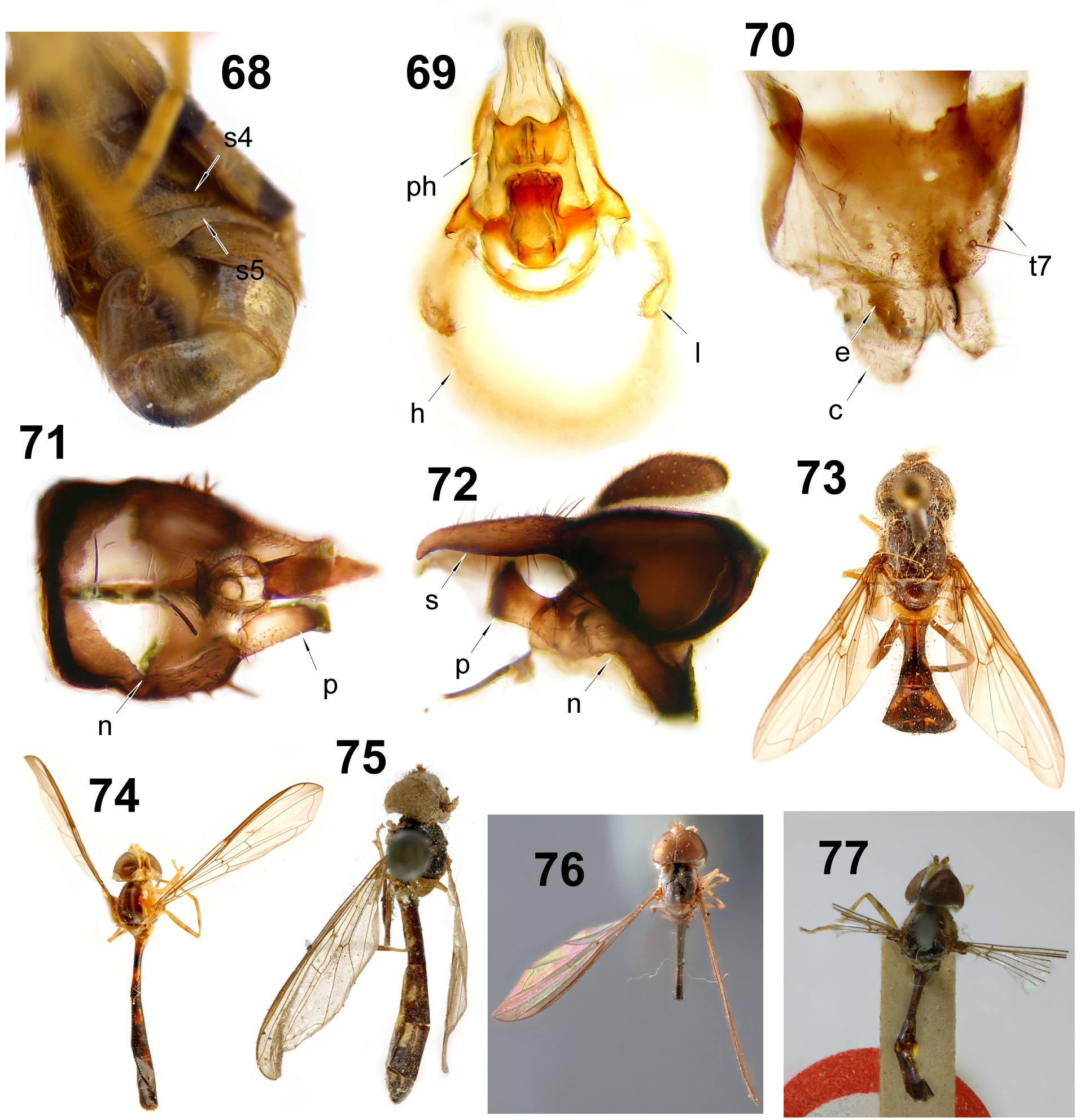
Figs 23–47 View FIGURES 23–37 View FIGURES 38–47 , 75 View FIGURES 68–77. 68 , 86–87 View FIGURES 78–88. 78 , 96–97 View FIGURES 89–102. 89
rn:lsid:zoobank.org:act: BDB84EA7-970F-439A-AF85-E5E0B2AE9BC7
Type species: Baccha lepida Macquart, 1842
Baccha lepidus species group, in part. Hull (1949a)
Ocyptamus lepidus species group. Thompson (1981); Miranda et al. (2016); Mengual et al. (2018)
Description. Head. Face very narrow to narrow (between 1/4 and less than a 1/3 of head width) and usually pale; tubercle usually weak and medially positioned, slightly concave dorsally and ventrally ( Fig. 87 View FIGURES 78–88. 78 ). Antennal insertions confluent. Frons very narrow (~1/4 of head width) to normal (~ 1/3 of head width), white microtrichia usually homogeneously distributed but sparse on a median longitudinal area and absent dorsal to lunule. Female ocellar triangle adjacent to or ~1 ocellus-width from lateral eye margin ( Fig. 86 View FIGURES 78–88. 78 ). Vertex either homogeneously covered by dull white microtrichia, or with sparse white microtrichia, or without microtrichia and shiny. Dorsal occiput with one row of pile ( Figs 86, 87 View FIGURES 78–88. 78 ). Thorax. Scutum usually pale laterally from postpronotum to post-alar callus, and usually without distinct anterior row of pile ( Figs 23–37 View FIGURES 23–37 ). Scutellum usually entirely pale. Anterior anepisternum pilose (except bare on N. croceus and the species group N. hyalipennis ). Katatergite usually with short microtrichia that gives the sclerite a ‘velvet’ appearance. Metaepisternum pilose or bare. Metasternum bare. Upper calypter margin bare or with pile shorter than pile on the ventral calypter margin. Metafemur with normal pile. Wing. Alula usually narrow (as wide as c cell). Wing either hyaline or entirely light yellow to light brown. Vein M1 sometimes straight ( Fig. 44 View FIGURES 38–47 ). Abdomen. Abdomen and terga colour pattern variable. Terminalia. Female tergum 7 only *lightly sclerotized ( Fig. 47 View FIGURES 38–47 , except in the N. hyalipennis species group); tergum 8 variable. Male surstylus usually with homogeneously distributed setulae or setae; *subepandrial sclerite ‘crescent’ shaped ( Figs 45 and 46 View FIGURES 38–47 ) and usually with pair of apical projecting lobes between bases of surstyli; hypandrium usually oval and quite robust apically, with ventral pilosity sub-apically or apically; postgonite with sub-apical acute dorsal extremity and with either a rounded or acute ventral extremity; basiphallus teardropshaped, distiphallus membranous with dorsal sclerotized triangular region ( Figs 92, 96, 97 View FIGURES 89–102. 89 ).
Included species (63): N. abata ( Curran, 1938) comb. nov. [2, 4, type lost], N. aeolus ( Hull, 1943a) comb. nov. [1b], N. anona ( Hull, 1943e) comb. nov. [1b], N. arabella ( Hull, 1947a) comb. nov. [2], N. banksi ( Hull, 1941a) comb. nov. [1b], N. cecrops ( Hull, 1958) comb. nov. [1a], N. chapadensis ( Curran, 1930a) comb. nov. [1b], N. confusus ( Goot, 1964) comb. nov. [4, type lost], N. crocatus ( Austen, 1893) comb. nov. [1b], N. croceus ( Austen, 1893) comb. nov. [2], N. cubanus ( Hull, 1943a) comb. nov. [1b], N. cultratus ( Austen, 1893) comb. nov. [1a (synonym, Baccha currani Hull ), 2, 3, 4], N. cymbellina ( Hull, 1944) comb. nov. [1b], N. debasa ( Curran, 1941) comb. nov. [1b], N. delicatissimus ( Hull, 1943b) comb. nov. [1b], N. dryope ( Hull, 1958) comb. nov. [1a], N. fervidus ( Austen, 1893) comb. nov. [2], N. filii ( Doesburg, 1966) comb. nov. [1b, 4], N. flavens ( Austen, 1893) comb. nov. [1b], N. geijskesi ( Doesburg, 1966) comb. nov. [1b, 4], N. gilvus ( Austen, 1893) comb. nov. [2], N. halcyone ( Hull, 1949b) comb. nov. [1a], N. hippolite ( Hull, 1957) comb. nov. [1a], N. hyalipennis ( Curran, 1930b) comb. nov. [1b], N. inornatus ( Walker, 1836) comb. nov. [1b], N. io ( Hull, 1944) comb. nov. [1b], N. iona ( Curran, 1941) comb. nov. [1b], N. lepidus ( Macquart, 1842) comb. nov. [1b, 4], N. lucretia ( Hull, 1949c) comb. nov. [1a], N. luctuosus ( Bigot, 1884) comb. nov. [1a (synonym, Baccha papilio Hull ), 4, type lost], N. micropyga ( Curran, 1941) comb. nov. [1b], N. minimus ( Hull, 1943b) comb. nov. [1b], N. murinus ( Curran, 1930a) comb. nov. [1b], N. myiophagus (Thompson in Mengual et al., 2018) comb. nov. [1a], N. neoparvicornis ( Telford, 1973) comb. nov. [2, 4], N. neptunus ( Hull, 1943d) comb. nov. [1b], N. neuralis ( Curran, 1934) comb. nov. [1b], N. niobe ( Hull, 1943c) comb. nov. [1b], N. nora ( Curran, 1941) comb. nov. [1b], N. obliquus ( Curran, 1941) comb. nov. [1b], N. octomaculatus (Thompson in Thompson et al., 1976) comb. nov. [1a], N. oriel ( Hull, 1942a) comb. nov. [1b], N. panamensis ( Curran, 1930c) comb. nov. [1b], N. peri ( Hull, 1943a) comb. nov. [1b], N. philippianus ( Enderlein, 1938) comb. nov. [2, type lost?], N. prenes ( Curran, 1930a) comb. nov. [1b], N. prudens ( Curran, 1934) comb. nov. [1b], N. pullus ( Sack, 1921) comb. nov. [1a (synonym, Baccha sepia Hull ), 1b (synonym, Baccha danaida Hull and Baccha violacea Hull )], N. punctifrons ( Williston, 1891) comb. nov. [1b], N. pyxia ( Hull, 1943a) comb. nov. [1b], N. saffrona ( Hull, 1943c) comb. nov. [1b], N. spatulatus ( Giglio-Tos, 1892) comb. nov. [2], N. vanessa ( Hull, 1949a) comb. nov. [1a], N. variegatus ( Macquart, 1842) comb. nov. [1b], N. verona ( Curran, 1941) comb. nov. [1b], N. victoria ( Hull, 1941b) comb. nov. [1a], N. vierecki ( Curran, 1930a) comb. nov. [1b], N. xanthopterus ( Wiedemann, 1830) comb. nov. [2], N. xantippe ( Hull, 1949a) comb. nov. [1a], N. zenillia ( Curran, 1941) comb. nov. [1b], N. zita ( Curran, 1941) comb. nov. [1b], N. zobeide ( Hull, 1943e) comb. nov. [1b], N. zoroaster ( Hull, 1943a) comb. nov. [1b].
Etymology. The name is a reference to the Latin word for ‘messenger’ or ‘message’ since there are still more information/messages to be discovered inside the genus. The name is to be treated as masculine.
Comments. This is the former Ocyptamus lepidus species group. Most common species can be recognized by the abdominal pattern of dark apical extensions (one medial and a sub-lateral pair) into a mostly pale background ( Figs 23 View FIGURES 23–37 and 43 View FIGURES 38–47 ); this pattern is what earlier authors called the yellow ‘inverted V-shaped’ markings ( Figs 30 and 32 View FIGURES 23–37 ). Other species have abdominal patterns that seem to be variations of the common one ( Figs 24, 27, 28, 33, 34 View FIGURES 23–37 and 42 View FIGURES 38–47 ). Species of Nuntianus can be readily distinguished from the superficially similar Hybobathus Enderlein, 1938 by the absence of the contrasting ocellar triangle present in the latter (see Fig. 78 View FIGURES 78–88. 78 in Mengual et al. 2018). Besides the abdominal pattern, the genus can also be quickly separated from other taxa by the often entirely light yellow to brown wings. Despite the variability in superficial color characters, the monophyly of Nuntianus is strongly supported in the combined molecular analysis of Miranda et al. (2016). One of the possible unique synapomorphies, so far not present in any other lineage, for the genus lies on the condition of the female tergum 7: most closely related lineages have a distinct tergum 7, but in Nuntianus it is almost wholly membranous with weakly sclerotized basal areas ( Fig. 47 View FIGURES 38–47 ).
Larval predatory habits seem quite diverse in this genus. Besides the predation of aphids as seen in N. cubanus (but see below) ( Mengual et al. 2018), there are records of N. luctuosus as aquatic predators in bromeliads ( Rotheray et al. 2000) and records of N. myiophagus as a predator of adult insects ( Ureña & Hanson 2010).
Nuntianus variegatus ( Fig. 75 View FIGURES 68–77. 68 ) was previously allocated in the Ocyptamus melanorrhinus species group ( Mengual et al. 2018), but type images indicate that it belongs to Nuntianus due to its similarity to N. zita .
Nuntianus is the largest of the taxa recently removed from the old ‘ Ocyptamus’ assemblage, and includes subgroups yet to be properly defined. One such group is the N. hyalipennis species group ( N. hyalipennis , N. neuralis , N. obliquus and N. panamensis ), once thought to be related to Calostigma Shannon, 1927 ( Thompson 1981) but strongly supported as part of Nuntianus by molecular characters ( Miranda et al. 2016). These small flies differ from most congeners in having a straight, or nearly so, vein M1 [see comments in Miranda et al. (2016: 172)]; the N. hyalipennis species group can be further distinguished from Calostigma by the scutum with 3 long and white microtrichose stripes (the lateral pair wider than the median stripe) (similar to Fig. 27 View FIGURES 23–37 ), yellow scutellum ( Fig. 43 View FIGURES 38–47 ), brownish yellow and almost completely microtrichose wings, and the abdominal terga mainly pale and with apical dark stripes ( Fig. 43 View FIGURES 38–47 ).
Other possible subgroups of Nuntianus comprise the species with wide parallel-sided ( Fig. 23 View FIGURES 23–37 ) to oval ( Fig. 25 View FIGURES 23–37 ) abdomens ( N. cultratus , N. fervidus , N. geijskesi , N. gilvus , N. iona , N. lepidus , N. luctuosus , N. myiophagus , N. neoparvicornis , N. neptunus , N. peri , N. pullus , and N. prudens ), species with petiolate ( Fig. 30 View FIGURES 23–37 ) abdomens ( N. abata , N. aeolus , N. anona , N. arabella , N. banksi , N. cecrops , N. chapadensis , N. crocatus , N. croceus , N. cubanus , N. debasa , N. filii , N. flavens , N. halcyone , N. hippolite , N. io, N. lucretia , N. murinus , N. niobe , N.octomaculatus , N.oriel , N. prenes , N. punctifrons , N. pyxia , N. saffrona , N. spatulatus , N. vanessa , N. variegatus , N. verona , N. victoria , N. vierecki , N. zita , and N. zobeide ), and species with relatively slender ( Fig. 37 View FIGURES 23–37 ), rather than petiolate, abdomens ( N. confusus , N. cymbellina , N. delicatissimus , N. dryope , N. micropyga , N. minimus , N. nora , N. zenillia , and N. zoroaster ). A few species are of uncertain grouping due to insufficient information on the shape of the abdomen, because the literature is inadequate, the type is not available for examination, or type is greatly damaged ( N. inornatus , N. philippianus , N. xantippe , and N. xanthopterus ). Nuntianus lepidus was chosen as the type species for the genus based on the long history of the ‘ lepidus species group’.
Nuntianus cubanus ( Fig. 41 View FIGURES 38–47 ), the only Nuntianus species that occurs in the Nearctic region (Florida, USA), seems to be distinct from the rest of the genus.A neighbour-joining analysis using the COI gene (analysis not shown here) places specimens of this species in a separate cluster far from the remaining Nuntianus . Abdominal tergum 2 of N. cubanus has a more extensive pale pattern than in congeners, but the pattern is similar to the common pattern found on tergum 4 ( Fig. 23 View FIGURES 23–37 ) of other Nuntianus species. We refrain from any taxonomical action at this moment, but this species in particular should be considered carefully in future analyses and revisions.
There was a misidentification of a specimen of Ocyptamus prenes ( Curran, 1930a) (INPA-DIP0000246) in the works of Miranda (2017b) and Mengual et al. (2018). The specimen is actually a representative of N. cecrops (which still makes it a new record for the state of Amazonas but also makes it a new record for Brazil as well).
Ocyptamus isthmus Thompson in Thompson et al., 1976 was a replacement name (International Code of Zoological Nomenclature - ICZN art. 57.3.1) for Callostigma panamensis Curran, 1930c (March 26th 1930). The replacement name was needed when this species and Baccha panamensis Curran, 1930a [February 28th 1930 = Pelecinobaccha transatlantica ( Schiner, 1868) , synonymized in Mengual et al. (2018)] were both placed in Ocyptamus ( Thompson et al. 1976) . This emendation is no longer justified so we reinstate (ICZN art. 59.4) Calostigma panamensis Curran, 1930c , now Nuntianus panamensis , and synonymize O. isthmus under it (ICZN art. 61.3.4 and 72.7).
The Ocyptamus morphospecies of Reemer (2010) seem to fall into the following taxa: Ocyptamus SUR-01 in Nuntianus (mainly due to the presence of the alula), O. SUR-04 in Nuntianus or the V. attenuata species group, O. SUR-05 and O. SUR- 10 in Nuntianus , O. ( Calostigma ) SUR-06b is part of the N. hyalipennis species group (mainly due to abdominal shape and pattern).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Nuntianus Miranda
| Miranda, Gil Felipe Gonçalves, Skevington, Jeffrey H. & Marshall, Stephen A. 2020 |
Ocyptamus lepidus
| Miranda & Skevington & Marshall 2020 |