Bythotrephes brevimanus, Lilljeborg, 1901
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4550.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:4A6046CC-B1AE-4CFC-8468-5B9FA733EF1D |
|
DOI |
https://doi.org/10.5281/zenodo.5944236 |
|
persistent identifier |
https://treatment.plazi.org/id/A54D87B2-FFE1-C322-FF23-FC5B4507A6BE |
|
treatment provided by |
Plazi |
|
scientific name |
Bythotrephes brevimanus |
| status |
|
Hybrid form Bythotrephes brevimanus x Bythotrephes cederströmii
( Figs. 1 View FIGURE 1 A–6G)
The hybrid specimens are excluded from the scope of International code of zoological nomenclature (ICZN 1.3.3).
Material examined. Sweden. Västergötland: 1) sample (MEUU, N 636 b with specimens from sample N 2689) labeled “ B. c. connectens minor, Sw, Vg, Mullsjön, 14.7.1882, Lillj.”, 12 ad; 2) a slide (MEUU, N 636 c) with specimens from sample N 2317 labeled “ Bythotrephes cederströmii , ♀, Sw, Vg, Hjo, Mullsjön, 14/7 82, Lillj.”, 3 ad.
Finland. Lake Saimaa ,1891, ZMMU, coll. N.V. Slyunin, 22 ad, 2 males, 5 juv.
Russia. River Volga reservoirs: 1) Ivan’kovskoe Reservoir, 9.8.2017, 13 ad, coll. V.I. Lazareva; 2) Rybinskoe Reservoir, 30.7.2008, 178 ad, 1 gam, 4 males , coll. V.I. Lazareva; 3) Uglicheskoe Reservoir , summer 2005, 13 ad, coll. V.I. Lazareva, 23.8.2014, 20 ad, coll. R.S. Sabitova; 4) Gor’kovskoe Reservoir, 3– 5.9.2005, coll. V.I. Lazareva, 17 ad, 3 gam, 8.2008, coll. V.I. Lazareva, 121 ad, 2 gam, 3 males ; 5) Cheboksarskoe Reservoir , August 2008, coll. V.I. Lazareva, 3 juv, 2 males ; summer 2018, coll. D. Gavrilko, numerous ad and juv; 6) Saratovskoe Reservoir , summer 2016, coll. Yu. Malinina, 26 ad, 2 juv; 7) Volgogradskoe Reservoir, summer 2010, coll. Yu. Malinina, 16 ad, 2 gam, 2 males, 3 juv. Permskaya Region : Verhnekamskoe Reservoir on the River Kama, 25.8.2016, coll. V.I. Lazareva, 28 ad. Orenburgskaya Region : Iriklinskoe Reservoir on the River Ural, coll. Yu. Malinina: without date, 17 ad, 2.6.2010, 3 ad, 1 juv, summer 2015, 43 ad, 4 gam, summer 2016, 75 ad, summer 2018, numerous ad and juv . Vologodskaya Region ; coll. E.V. Labunicheva: 1) Vytegorskoe Reservoir , 5.7.2013, 19 ad; 2) Belousovskoe Reservoir, 8.7.2013, 20 ad; 3) River Sheksna, June 2012, 6 ad. 1 juv. 18.9.2013, 2 ad. The Gulf of Finland near Zelenogorsk town : 7.7.2006, coll. L.F. Litvinchuk, 21 ad. Lake Onega : 1898, two total mounts, ZIN, coll. A. Linko, 3 ad. Arkhangelskaya Region : Lake Kumichevo (64°57.736 N; 42° 95.609 E), without date, coll. E.I. Bekker, many ad; 18.7.2018, coll. N.G. Bayanov, numerous ad and juv. Tyumenskaya Region ( West Siberia ): Lake Dolgiy Sor , 3.8.2011, coll. O.A. Aleshina, some ad. Novosibirskaya Province (West Siberia): Lake Chany, 14.8.2004, coll. E.I. Zuykova, 36 ad, 1 juv. GoogleMaps Burjatiya (south of East Siberia): Lake Gusinoe (51°16.608 N; 106°27.693 E), 26.7.2013, coll. N.G. Sheveleva, 26 ad. GoogleMaps Yakutia ( East Siberia ): 1) Lake Saysary near Yakutsk City (coll. V.A. Sokolova), July 2003, 7 ad, 6 juv; July 2004, 20 ad, 6 juv; 2) Lake Homustah, August 2008, coll. I.G. Sobakina, 2 ad.
Kazakhstan (eastern part): Lake Zaysan , 19.6.1906, coll. A. Sidel’nikov, ( ZIN 111–06 View Materials ), 8 ad, 1 juv
Data on body and body parts measurements of specimens of some populations are presented in Table 1.
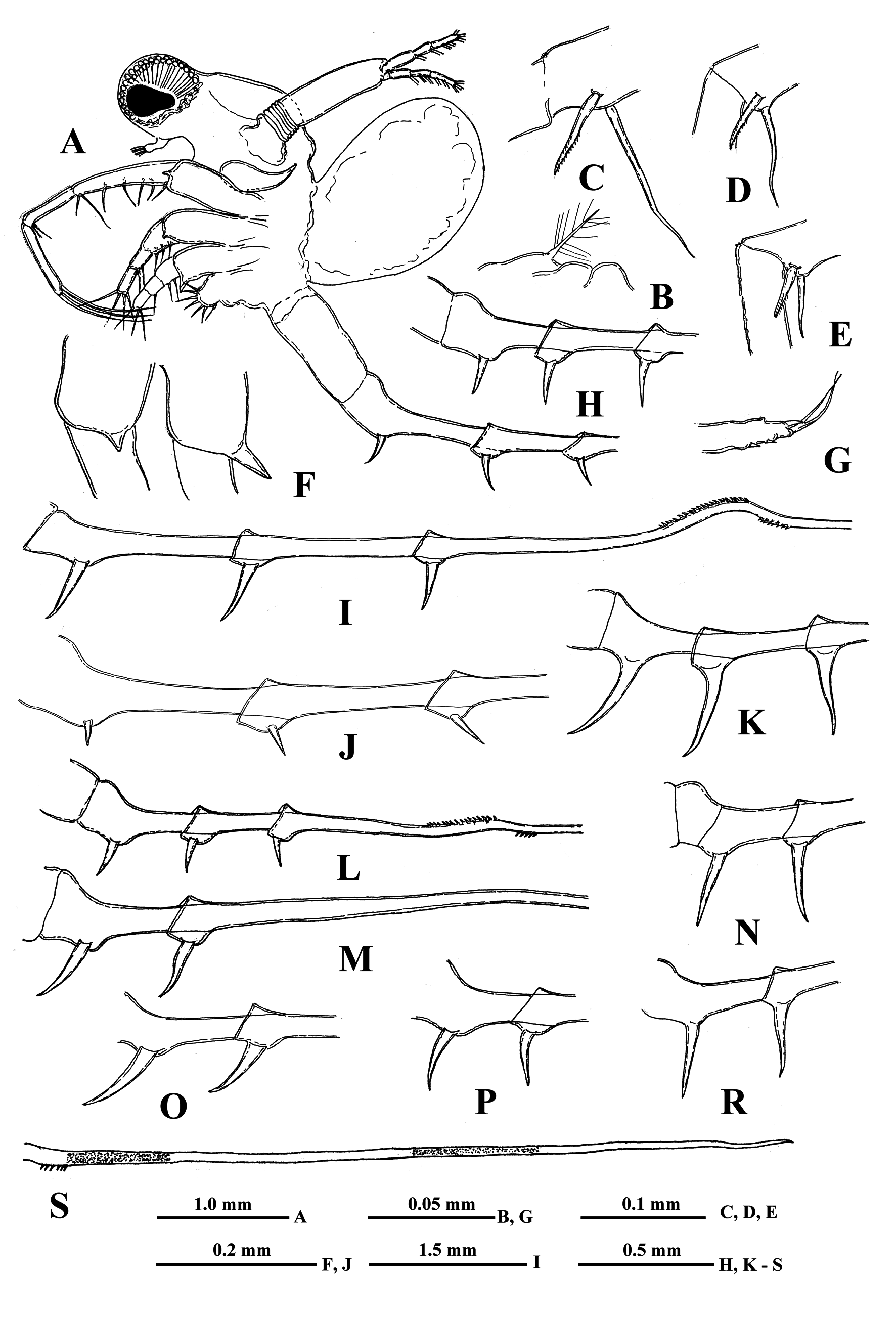
Description. Parthenogenetic female. General body appearance and segmentation. General body appearance and structure as in parental Bythotrephes species. Body elongated and divided into four parts: head, thorax,
abdomen, and postabdomen with long caudal process ( Fig. 1A View FIGURE 1 ). Its longitudinal axis is conspicuously incurved when head is located at almost right angle to the thorax. Also highly movable abdomen can be either in a straight line with the thorax or at different angles to it. Head large with rounded anterior part filled by the enormously developed compound eye and bearing small antennules ventrally. Posterior part of head bearing long swimming antennae and mouth parts consisting of mandibles, maxillules (mx I), and upper lip (labrum). Thorax with strongly developed muscular ventral side bearing four pairs of thoracic limbs of different sizes directed antero-ventrally. Dorsally, thorax bears a sack-like carapace transformed into a brood pouch, sometimes reaching large size. Abdomen (metasome) is elongated, cylindrical, inconspicuously three-segmented (see Korovchinsky 2015) and flexible; connected with a small postabdomen bearing ventrally a pair of claws, and posteriorly a long caudal process with one-two pairs of claws proximally. Body length of females (without caudal process) reaches 3.4 mm while the length of caudal process may exceed the body length by 1.4–4.0 times (on average, about two times).
Head very large (on average about 40.0 % of body length) and subdivided into two parts: rounded anterior part mostly filled by large compound eye and posterior part bearing dorsally a large saddle-shaped neck-organ, swimming antennae and mouth parts. Large pigment spot occupies about one-third or at most half of the eye’s volume. Ocellus (naupliar eye) is absent.
Antennules. Small and situated on the ventral side of the anterior head part beneath the eye. They are bulbous and sit on the joint basis slightly split anteriorly. Terminally they bear five regular aesthetascs in two groups of two and three, and one shorter and thinner aesthetasc-like structure, situated in a group with two regular aesthetascs.
Swimming antennae. Comparatively long, with elongated cylindrical basipodite ( Fig. 1A View FIGURE 1 ) which has a dorsal thin feathered seta on its folded proximal part ( Fig. 1B View FIGURE 1 ). Of the two antennal branches, the lower three-segmented one (endopodite), sitting on the apical basipodital prominence, is slightly longer than the upper branch. The upper branch is four-segmented and lower branch is three-segmented. Small proximalmost segment of upper branch lacks setae, while other segments possess two-segmented swimming setae of more or less similar size except distalmost of them which are shorter. All setae bilaterally armed with rows of uniform thin setules. General formula of antennal setae: 0–1–2–5/1–1–5 (see Korovchinsky (2015) for more details).
Mouth parts. They are represented by upper lip (labrum), mandibles, and maxillules (maxilla I) which are similar to those of the parental Bythotrephes species (see e.g., Korovchinsky 2018).
Carapace. This resembles a bag-like structure, strongly modified into a closed brood pouch ( Fig. 1A View FIGURE 1 ) widely connected in its base with the dorsal surface of the thorax. It may be often well developed and massive, being filled by large embryos.
Thoracic limbs. Four pairs of strongly chitinized, stenopodous limbs are densely situated along the muscular ventral side of thorax and directed antero-ventrally ( Fig. 1A View FIGURE 1 ). All of them have complex and variously setaceous armament along their inner side. Limbs of the three anterior pairs are five-segmented, and those of the last fourth pair are three-segmented. Protopodites of all of them, covered by comparatively softer cuticle, are inconspicuously delimited into two parts (segments), coxa and basis, while the endopodites of limbs of the three anterior pairs are composed of three well developed segments whereas those ones of the fourth pair are unsegmented.
First pair of limbs (tl I) especially long and strong, their length rarely almost equal to the body length (up to 99.2 % of its length) but usually shorter (minimally 58.0 %, on average 62.9–79.4 % of body length). General structure and armament of the limbs as in parental species. The first segment of the endopodite is long and bears 5– 7 anterior lateral setae. Distally, this segment bears a shorter anterior seta and long posterior seta ( Fig. 1C View FIGURE 1 ). The second segment of the endopodite is conspicuously shorter and bears only two apical setae similar to those on the end of previous segment but usually shorter ( Figs. 1D, 1E View FIGURE 1 ). The terminal third segment of the endopodite varies in length, being mostly shorter than the proximal first endopodital segment (minimally 60.0 %, on average 72.5–93.1 % of the latter one but sometimes it may be longer, even exceeding the length of the first segment (up to 125.0 %) and always bearing apically four long roughly spinulated setae, two of them terminally and two subterminally.
Second pair of limbs (tl II) considerably shorter. The first basal segment of their endopodite bearing a row of 5–7 rather long anterior lateral setae; also often there is 1, rarely 2, posterior lateral seta of the same type on this segment. Second segment of the endopodite is short with only two setae, while its distal segment bears four setae, two terminal and two subterminal ones.
Third pair of limbs (tl III) generally similar to the previous ones, differing in some details. The external outgrowth of their protopodite is conspicuously larger ( Fig. 1F View FIGURE 1 ) and lateral anterior and posterior setae (if present) of first segment of endopodite are fewer (3–6 and 1, respectively).
Fourth pair of limbs (tl IV) considerably reduced; their protopodite bearing slightly spinulated seta sited on a short cylindrical base. The only segment of the endopodite has two rows of comparatively short spine-like setae. The external row (group) always consists of two setae, and the internal row of 5–7 setae, differing in their appearance and armament.
Abdomen (metasome) ( Fig. 1A View FIGURE 1 ) is often deformed in examined specimens. It is inconspicuously delimited in two segments, short proximal and long distal one, often having a prominent fold more or less in the middle dorsal side.
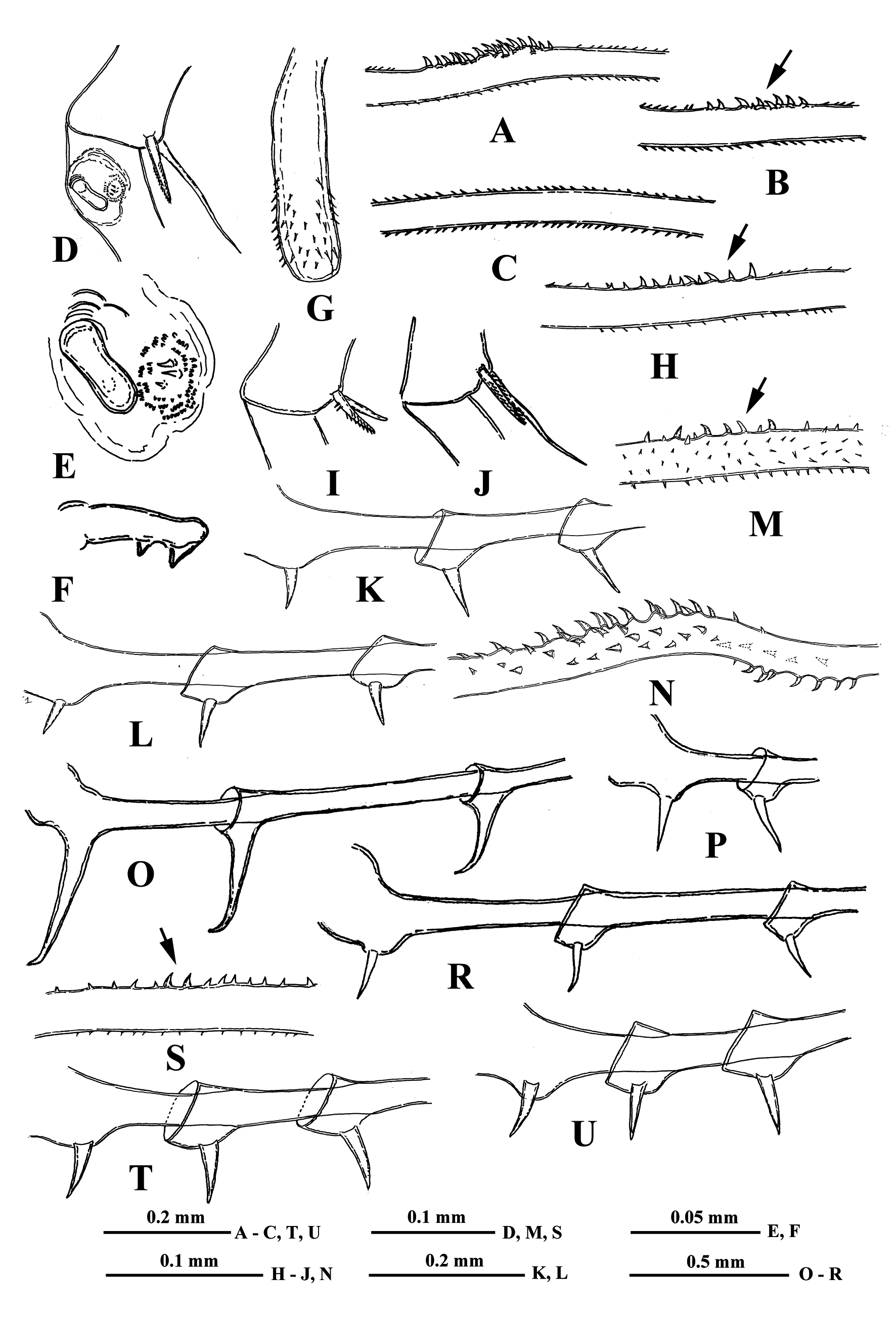
“ Postabdomen” actually consists of two parts: the last small abdominal segment and the postabdomen per se (see Korovchinsky (2015)), which is comparatively small, with the anal opening situated between the postabdominal claws. These claws are of very different size (2.7–36.0 %, on average 6.4–17.1 % of body length) and shape ( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 ).
Caudal process is directly connected with the postabdomen and proceeds as a very long, proximally thicker spine-like structure, variable in length (138.0–405.0 %, on average 173.0–296.0 % of body length), thus exceeding the body length by 1.4–4.0 times. Generally, the caudal process is strongly chitinized and its surface covered by numerous minute spinulae. Besides, it often possesses two either bright or pale colored yellow-orange sclerotized areas ( Fig. 1S View FIGURE 1 ). Caudal process may be straight, sometimes slightly bent but often with more or less developed bend bearing small curved denticles (e.g., Figs. 1I, 1L View FIGURE 1 ). Basally, the caudal process bears one or two pairs of claws similar or not similar to those of the postabdomen but usually smaller (e.g., proximal claws reach 3.1–26.2 % of body length), and apically two minute setae arising from a common base ( Fig. 1G View FIGURE 1 ). Pairs of claws sit either close or quite distant to each other (e.g., distance between postabdominal claws and proximal claws of caudal process (interclaw distance) constitutes 6.7–56.8 %, on average 14.8–36.5 % of body length). Between the latter, the thickness of the structure may be different: 2.3–7.9 %, on average 4.3–6.1 % of body length. Borders separating old molted integuments of caudal process with claws normally are quite conspicuous.
Gamogenetic females differ from parthenogenetic ones only in presence of two-four large yellow-brownish resting eggs (0.39–0.48 mm in diameter) in their brood pouches ( Fig. 3A View FIGURE 3 ).
Males have comparatively shorter first pair (tl I) of thoracic limbs (66.0–76.0 % of body length) as well as each segment of them, especially the distal one (50.0–70.0 % of length of proximal endopodital segment), which is slightly swollen proximally and bears on its inner side a small strongly chitinized clasping hook with two inner denticles, and a field of tiny prominences situated under it ( Figs. 2D, 2E, 2F View FIGURE 2 ). The copulatory appendages are small (9.0–10.0 % of body length) and armed terminally with numerous minute spinulae ( Fig. 2G View FIGURE 2 ). Caudal process always bearing one pair of claws.
Size. Parthenogenetic females body length, 1.34-3.39 mm. Gamogenetic females body length, 1,71-2,06 mm. Males body length, 1.20-2.08 mm.
Intra- and interpopulation variability of females. According to data of Table 1, the smallest adult individuals were observed in Uglicheskoe Reservoir whereas the largest ones were in the West Siberian Lake Chany. The intermediate character of features of hybrids is expressed also in small size of individuals having the “ B. cederströmii ” appearance (normally, the body size of B. cederströmii is rather large, see Korovchinsky (2015, 2018)) which were obtained from water bodies of Vologodskaya Region (Central European Russia).
All the measured parameters shown in Table 1 demonstrate the increased morphological variability of hybrid individuals in comparison with the parental species which is especially characteristic of shape and size of postabdominal and caudal process’ claws as well as of interclaw distance (the highest values of CV = 50.2–55.6 which were observed in populations from Rybinskoe, Uglicheskoe reservoirs and Siberian lakes Chany and Gusinoe). The high variability of length of distal segment of tl I, caudal process, and interclaw thickness (the highest values of CV = 20.3–38.8 were detected in specimens from the same water bodies as well as in those from Gor’kovskoe Reservoir) was observed as well.
In most hybrid populations that were studied, five morphotypes have been distinguished ( Tab. 2). Generally, all of them were dominated by a form “ B. cederströmii ” (on average, 26 %) and especially by the intermediate one without a bend on caudal process (on average, 42 % of all specimens!). The specimens of the latter form were observed in all populations, sometimes in a large number (up to 50–77 % of all studied specimens in Gor’kovskoe, Volgogradskoe, Iriklinskoe, Verhnekamskoe reservoirs and in Chany and Gusinoe lakes). Two other intermediate forms were less frequent (on average, 13 %). The one with a well-developed denticulated bend dominated only in Rybinskoe reservoir (31 %), whereas the second one with weakly developed bend has never shown dominance. The form “ B. cederströmii ” dominated in Uglicheskoe, Belousovskoe, Vytegorskoe reservoirs and in Yakutian Lake Saysary (48–70 %) and was absent only in one population from Volgogradskoe Reservoir which might be a consequence of a small number of individuals. On the other hand, the form “ B. brevimanus ” was the least numerous (on average, 6 %), being absent in five populations while in others its proportion was low (4–12 %). This variability may be of a seasonal nature and populations should be studied over a full annual cycle (see further).
Remarks. The hybrid populations are characterized by increased morphological variability and usually include both specimens typical of the parental species B. cederströmii and B. brevimanus and specimens of different intermediate morphotypes. However, Litvinchuk (2002, 2007) demonstrated that there was no concordance between morphological and genetic traits of hybrid specimens which may have the parental appearance. Therefore, it is not possible to discriminate morphologically among hybrid and parental specimens which can co-occur. For this reason, all specimens of such high variable populations should conditionally be considered hybrids.
The specimens from Mullsjön (Västergötland, Sweden) labeled by Lilljeborg variably, “ B.cederstrōmii robustus minor ”, “ B. cederströmii var. connectens & forma minor” or “ B. c. connectens minor “, earlier synonymized with B. cederströmii ( Korovchinsky 2018) now should be attributed to the hybrid form B. brevimanus x B. cederströmii as a consequence of high variability of their caudal process and claws.
Because of high morphological variability of hybrid populations and populations of parental species, B. cederströmii and B. brevimanus (for the latter ones see Korovchinsky 2018), numerous specimens should be compared for accurate taxonomic determination. Single hybrid specimens can be correctly identified only if they possess both caudal process bearing either denticulated bend or just its traces (enlarged denticles on its place) and postabdominal and caudal claws that differ from those typical for B. cederströmii (in the latter species, they are usually large with apical end curved forward).
Differential diagnosis. Generally, the specimens of the hybrid form B. brevimanus x B. cederströmii are most similar to those of B. cederströmii because they often possess a long caudal process with denticulated bend and large claws. Moreover, the variability of caudal processes of hybrids and in some populations of the latter species may be similar in which the denticulated bend can be either well or weakly developed, marked only by a group or single large denticles or its traces can be absent at all. In such situation, the only reliable difference between the hybrid form and B. cederströmii is the presence of claws of very variable shape in the former, both in one individual and in different individuals (either large, medium or small, curved or straight, directed forward, down or backwards, sitting more or less closely or distantly to each other) ( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 ) and more uniform claws, curved apically forward in the latter species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |