Sylvicanthon foveiventris ( Schmidt, 1920 )
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.467 |
|
publication LSID |
lsid:zoobank.org:pub:8D27AAB8-B7F2-424C-B1A6-66FEFA66EDFF |
|
DOI |
https://doi.org/10.5281/zenodo.3846327 |
|
persistent identifier |
https://treatment.plazi.org/id/A72C87FB-FFD4-FFF5-0D05-0F590FAA93CA |
|
treatment provided by |
Valdenar |
|
scientific name |
Sylvicanthon foveiventris ( Schmidt, 1920 ) |
| status |
|
Sylvicanthon foveiventris ( Schmidt, 1920) View in CoL
Figs 13A View Fig , 14B View Fig , 15B View Fig , 16D View Fig , 17B View Fig , 20 View Fig , 23–24 View Fig View Fig
Canthon foveiventris Schmidt, 1920: 132–133 View in CoL .
Canthon foveiventris View in CoL – Schmidt 1922: 64, 75. — Balthasar 1939: 188–189. — Martínez 1949 a: 287. — Halffter & Martínez 1977: 63. — Krajcik 2012: 63.
Canthon foveiventre – Blackwelder 1944: 199.
Glaphyrocanthon (Glaphyrocanthon) foveiventris – Pereira & Martínez 1956: 126, 128. — Martínez et al. 1964: 5, 8, 10, 14. — Vulcano & Pereira 1964: 662. — Martínez & Pereira 1967: 53.
Sylvicanthon foveiventre – Vaz-de-Mello & Louzada 1997: 57. — Vaz-de-Mello 2000: 195. — Hernández 2002: 598. — Falqueto et al. 2005: 20. — Hernández & Vaz-de-Mello 2009: 610–611. — Hernández et al. 2011: 7–8, fig. 3.
Sylvicanthon foveiventris View in CoL – Durães et al. 2005: 725. — Almeida & Louzada 2009: 37-39. — Culot et al. 2013: 85, 87.
Etymology
From the Latin words ‘ fovea ’ and ‘ ventris ’, a likely reference to the three pairs of foveae present on the sides of the abdomen of females of this species.
Material examined
Lectotype (here designated)
BRAZIL: ♂, Espírito Santo (“9652 / E92 +”, “24 / 56 ”, “ LECTOTYPE Ƌ / Canthon / foveiventris / Schmidt / des. F. Z. Vaz-de-Mello, 20 13 ”, “ Glaphyrocanthon / foveiventris / (Schm.) / P. Pereira det. 66 ”, “ foveiventr.”, “ Esp. Santo ”) ( NHRS) ( Fig. 23 View Fig Ca, b).
Paralectotypes
BRAZIL: 1 ♀, (“ foveiventris / Schm.”, “9653 / E92 +”, “Espir. / Santo”, “ foveiventr ”, “ foveiventris / A. Schm.”, “ PARALECTOTYPE / ♀ / Canthon / foveiventris / Schmidt / des. F. Z. Vaz-de-Mello, 20 13 ”) ( NHRS) ( Fig. 23 View Fig Cc); 1 ♂ (“ PARALECTOTYPE / ♂ / Canthon / foveiventris / Schmidt / des. F. Z. Vazde-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918 ”, “Esp. Santo”) ( SMTD); 1 ♂, (“ PARALECTOTYPE / ♂ / Canthon / foveiventris / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918 ”, “Esp. Santo”) ( SMTD); 1 ♂, (“ PARALECTOTYPE / ♂ / Canthon / foveiventris / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918 ”, “Esp. Santo”) ( SMTD); 1 ♀, (“ PARALECTOTYPE / ♀ / Canthon / foveiventris / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918 ”, “Esp. Santo”) ( SMTD); 1 ♀, (“Typus”, “Esp. Santo”, “Coll. C. Felsche / Kauf 20, 1918 ”, “ Canthon / foveiventris / A. Schmidt ”, “ PARALECTOTYPE / ♀ / Canthon / foveiventris / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”) ( SMTD) ( Fig. 23 View Fig Cd).
Additional material (140 ♂♂, 144 ♀♀)
BRAZIL: 1 ♀, no further data, B. Schwarzer leg.,ex. coll. Balthasar ( NMPC). – Bahia: 1 ♂, no other data [“homeótipo”] (“F. Ohaus S.”) ( MZSP); 1 ♀ ( NMPC). – Espírito Santo: 1 ♂, 1 ♀, no other data ( BMNH); 5 ♀♀ ( NMPC); 1 ♂ ( ZMHB); 1 ♂, Jean-Theodore Descourtilz (“Descourtils”) leg. ( BMNH); 1 ♂, 1 ♀, Conceição do Castelo, Feb. 1994, Vaz-de-Mello leg. ( MCNZ); 4 ♂♂, 1 ♀, Conceição do Castelo, 20º22′ S, 41º15′ W, Feb. 1994, human faeces, Arnaud, Grossi and Vaz-de-Mello leg. ( CEMT); 2 ♂♂, 3 ♀♀, same collecting data as preceding ( CMNC); 1 ♂, 1 ♀, Domingos Martins, Jan. 2000, C.-L. Andrade leg. ( NMPC); 3 ♂♂, 1 ♀, Domingos Martins, Parque Estadual da Serra Azul, 1500 m, Jan. 2000, Lopes-Andrade and Vaz-de-Mello leg. ( CEMT); 1 ♀, Santa Teresa, Estação Biológica de Santa Lúcia, Trilha Indaia-Açu, 19º58′13″ S, 40º32′06″ W, 779 m, 29 Jan. 2015, pitfall trap baited with human faeces, T. Vargas leg. ( CEMT); 1 ♀, Vargem Alta, Jan. 2000, Louzada and Louzada leg. ( CEMT); 1 ♀, Vargem Alta, 680 m, 15 Sep. 1995, J.N.C. Louzada leg. ( CEMT); 1 ♀, Venda Nova do Imigrante, Lavrinhas, 20º12′29″ S, 41º07′23″ W, Jan. 2013, L.F. Vaz-de-Mello leg. ( CEMT); 1 ♀, Venda Nova do Imigrante, Lavrinhas, 20º12′29″ S, 41º07′23″ W, 850 m, 10–14 Jan. 2011, human faeces, F.Z. Vaz-de- Mello leg. ( CEMT); 1 ♀, Venda Nova do Imigrante, Vila Santa Cruz, 20º20′02″ S, 41º08′18″ W, 800 m, 10–14 Jan. 2011, human faeces, F.Z. Vaz-de-Mello leg. ( CEMT); 1 ♀, Venda Nova do Imigrante, Lavrinhas, 20º18′40″ S, 41º08′16″ W, Dec. 2012, L.F. Vaz-de-Mello leg. ( CEMT). – Minas Gerais: 2 ♀♀, Barão de Cocais, Vale Mineração, 19º57′17″ S, 43º33′51″ W, 860 m, 4 Nov. 2011, human faeces, R.N. Mota leg. ( CEMT); 1 ♂, Conceição dos Ouros, Rio Sapucaí, 19 Feb. 2003, without collector ( CEMT); 1 ♂, 1 ♀, Carrancas, Intituto de Permacultura Cerrado-Pantanal (“Inst. Perm. Cer. Pantanal”), 1217 m., -21.4556′ S, -44.6203′ W, 21 Oct. 2008, pitfall with human faeces, Clever Pinto col. ( CEMT); 5 ♂♂, 4 ♀♀, Diamantina, Campus UFVJM, 17 Dec. 2005, S.L. Assis Junior leg. ( CEMT); 1 ♂, Itamonte, 22º21′ S, 44º48′ W, 1737 m, 12 Oct. 2009, T. Vidaurre et al. leg. ( CEMT); 1 ♂, Lavras, May 1997, J. Louzada leg. ( CEMT); 1 ♀, Lavras, 21º19′02.54″ S, 44º59′25.29″ W, 20 Jan. 2008, M.R. Rocha and D.H.T. Takahashi leg. ( CEMT); 1 ♀, Lavras, Serrinha da Bocaina, 27 Apr. 2012, pig dung, A. Díaz- Rojas leg. ( CEMT); 2 ♀♀, Lima Duarte, Parque Estadual do Ibitipoca, Dec. 1997, Souza et al. leg. ( CEMT); 2 ♂♂, Nova Lima, Parque Estadual da Serra do Rola-Moça, 2005, G. Schiffler leg. ( CEMT); 1 ♀, Prados, 21º04′40″ S, 44º08′06.1″ W, 1090 m, 17 Feb. 2012, pitfall with human faeces, Letícia Vieira et al. leg. ( CEMT); 2 ♂♂, Rio Acima, 20º00′20″ S, 43º41′37″ W, 993 m, 29 Nov. 2007, E. Bordoni leg. ( CEMT); 2 ♀♀, Rio Acima, Vale Mineração, 20º03′27″ S, 43º40′23″ W, 1334 m, 10 Oct. 2010, human faeces, R.N. Mota leg. ( CEMT); 2 ♂♂, 1 ♀, São Gonçalo do Rio Abaixo, Estação Ambiental Peti, 19º53′21″ S, 43º21′43″ W, 1 Dec. 2010, F. França leg. ( CEMT); 1 ♀, São João Evangelista, 18º33′11″ S, 42º53′58″ W, 898 m, 2. Apr. 2011, human faeces, R.N. Mota leg. ( CEMT); 2 ♂♂, 2 ♀♀, Viçosa, Mata do Paraíso, 20º48′18″ S, 42º51′20″ W, 750 m, 3 Feb. 2000, F. Génier leg., trap with dung ( CMNC); 1 ♀, Viçosa, Mata do Paraíso, 20º48′18″ S, 42º51′20″ W, 750 m, 4 Feb. 2000, F. Génier leg., trap with dung ( CMNC). – Rio de Janeiro: 1 ♂, no more data ( ISNB); 1 ♂, 1 ♀, Itatiaia, Jan. 1961, Dirings leg. ( MZSP); 1 ♂, Itatiaia, 700 m, Feb. 1959, W. Zikan leg. ( CEMT); 2 ♀♀, same collection data as preceding ( MNRJ); 1 ♀, Itatiaia, Mar. 1992, C. Godinho Junior leg. ( CEMT); 1 ♀, Nova Friburgo, Jan. 1995, F.Z. Vaz-de-Mello leg. ( CEMT); 4 ♂♂, 4 ♀♀, Nova Friburgo, 22º23′04″ S, 42º33′30″ W, 750 m, 21 Jan. 2000, trap with dung, F. Génier and S. Ide leg. ( CMNC); 4 ♂♂, 3 ♀♀, Nova Friburgo, 22º23′04″ S, 42º33′30″ W, 750 m, 23 Jan. 2000, trap with dung, F. Génier and S. Ide leg. ( CMNC); 1 ♀, Nova Friburgo, Macaé de Cima, Jan. 2006, B. Miller leg. ( AMBC); 1 ♂, 1 ♀, Nova Friburgo, Macaé de Cima, 1500 m, Mar. 2000, Lopes-Andrade, Gumier and Vaz-de-Mello leg. ( CEMT); 1 ♀, Nova Friburgo, Muri (“Mury”), 22º21′49″ S, 42º33′07″ W, 1150 m, 22 Jan. 2010, trap with dung, F. Génier and S. Ide leg. ( CMNC); 2 ♀♀, Parque Nacional da Serra dos Órgãos (PARNASO), 850 m, Dec. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PARNASO, 950 m, Dec. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♀, PARNASO, 1030 m, Jan. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♀, PARNASO, 1080 m, Jan. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PARNASO, 1130 m, Jan. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PARNASO, 1150 m, Dec. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PARNASO, 1230 m, Jan. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PARNASO, 1230 m, 28–30 Jan. 2014, pitfall trap, Cristina Araújo and Raissa Andrade leg. ( CEMT); 1 ♂, PARNASO, 1280 m, Jan. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 3 ♂♂, PARNASO, 1330 m, Jan. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♀, PARNASO, 1400 m, Dec. 2014, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, 1 ♀, Parque Nacional do Itatiaia ( PNI), 700 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 2 ♂♂, 1 ♀, PNI, 750 m, 22–24 Oct. 2010, pitfall with human faeces, Wallace Beiroz and Mario Cupello leg. ( MNRJ); 1 ♂, PNI, Casa do Pesquisador, 750 m [sic], 11–13 Nov. 2011, pitfall, Mario Cupello leg. ( MNRJ); 1 ♂, PNI, 750 m, Jan. 2012, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PNI, 800 m, Dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♀, PNI, Casa do Pesquisador, 810 m, 22–25 Feb. 2013, pitfall with human faeces, Mario Cupello leg. ( CEMT); 6 ♂♂, 1 ♀, same sollecting data as for preceding ( MNRJ); 1 ♀, PNI, Casa do Pesquisador, 850 m, 03–06 Oct. 2013, A. Carelli and J. P. Botero leg. ( MNRJ); 1 ♂, PNI, 900 m, dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 2 ♀♀, PNI, 900 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 16 ♂♂, 7 ♀♀, PNI, 1000 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♀, PNI, 1050 m, Dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 3 ♂♂, 6 ♀♀, PNI, 1050 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PNI, 1050 m, Aug. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PNI, 1100 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. CLEI); 1 ♂, PNI, 1200 m, Dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 2 ♂♂, 2 ♀♀, PNI, 1250 m, Dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 2 ♂♂, 1 ♀, PNI, 1250 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 5 ♂♂, 6 ♀♀, PNI, 1300 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, 1 ♀, PNI, 1350 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 2 ♂♂, PNI, 1400 m, Dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, 1 ♀, PNI, 1400 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, 1 ♀, PNI, 1450 m, Dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 2 ♂♂, PNI, 1450 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, 2 ♀♀, PNI, 1500 m, Dec. 2011, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♀, PNI, 1550 m, Aug. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, PNI, 1600 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 2 ♀♀, PNI, 1750 m, Jan. 2012, pitfall with human faeces, Cristina Araújo and Raissa Drufrayer leg. ( CLEI); 1 ♂, Rio de Janeiro, Grajau, Nov. 1965, H. S. Lopes leg. ( CEMT). – São Paulo: 1 ♀, Mogi das Cruzes, Parque das Neblinas, 23º47′28″ S, 46º11′48″ W, 810 m, Nov. 2015, pitfall trap baited with human faeces, R. V. Nunes leg. ( CEMT); 2 ♀♀, Parque Estadual da Serra do Mar, Núcleo Santa Virgínia, Sede Itamambuca, 23º19′30″ S, 45º04′58″ W, 18 Jan. 2012, pitfall trap baited with human faeces, E. Bovy leg. ( CEMT); 7 ♂♂, 12 ♀♀, Parque Estadual da Serra do Mar, Núcleo Virgínia, Sede Vargem Grande, 23º26′15″ S, 45º14′16″ W, 17 Jan. 2012, human faeces, E. Bovy leg. ( CEMT); 18 ♂♂, 25 ♀♀, Parque Estadual da Serra do Mar, Núcleo Santa Virgínia, Sede Itamambuca, 23º19′27″ S, 45º05′08″ W, 18 Jan. 2012, human faeces, E. Bovy leg. ( CEMT); 2 ♂♂, Parque Estadual da Serra do Mar, Núcleo Santa Virgínia, Sede Vargem Grande, 23º26′35″ S, 45º14′21″ W, 17 Jan. 2012, human faeces, Marion Boutefeu leg. ( CEMT); 1 ♂, Salesópolis, Estação Biológica de Boracéia, Jan. 2006, M. Uehara leg. ( CEMT); 1 ♂, Salesópolis, Estação Biológica de Boracéia, Feb. 2006, M. Uehara leg. ( AMBC); 1 ♂, São Luiz do Paraitinga, Parque Estadual da Serra do Mar, Núcleo Santa Virgínia, Mar. 2005, M. Uehara leg. ( CEMT); 2 ♂♂, 1 ♀, São Miguel Arcanjo, Parque Estadual Carlos Botelho, 24º03′43″ S, 47º58′45″ W, 812 m, 29 Jan. 2012, human faeces, E. Bovy leg. ( CEMT); 2 ♀♀, Serra do Japi, 23º14′ S, 46º56′ W, 1050 m, 1998, pitfall with dung, M. I. M. Hernández leg. ( CEMT).
Redescription
COLOURATION. Dorsum lustrous and shiny. Head, pronotum, pygidium and ventrite VI dark purple. Elytra dark green or dark blue. Meso- and metafemora reddish-brown. Venter with purplish reflections.
HEAD. Tegument shiny and covered by dense micropunctation and very fine alveolar microsculpture; sometimes, microsculpture lacking at the posterior region of frons. Clypeus with two apical teeth obtuse and contiguous at base; with a single transverse row of very short setae covering the base of both teeth. Genae with a weak tooth immediately behind clypeal-genal juncture. Posterior edge of head unmargined between eyes (some few specimens with traces of a fine line between eyes).
THORAX. Pronotum with shiny tegument; alveolar microsculpture restrict to a very narrow strip above lateral margins; rest of tegument smooth, with dense micropunctation, almost as dense as on head. Posterior edge with a fine transverse line at middle (extending just beyond the second elytral stria) Hypomeral cavity with tegument with some long yellowish setae at centre; external margin with a minute tubercle. Metaventrite with some few setae close to metacoxae on the sides, and entirely glabrous at centre; anterior region with tegument with strong rivose microsculpture; centre and posterior region with dense micropunctation.
LEGS. Protibiae very narrow and without expansion on its internal margin; at their apical seventh, with two tiny, acute teeth of unequal length. Mesofemora margined anteriorly only at its basal half or third; tegument with sparse, almost imperceptible micropunctation. Metafemora margined only anteriorly; with strong coarse elongate punctation at base and with sparse micropunctation at the rest of tegument ( Fig. 13A View Fig ). Metatarsomeres II and V subequal in length and longer than the others; metatarsomere IV shorter than the others.
ELYTRA. With only eight visible striae: first four to five striae strongly marked, finely carinulate and widened at base; sixth to eighth striae very effaced and discontinuous; seventh stria absent at humerus; all striae lack their carinulae at the apex of elytra, being either marked only by microsculpture or completely indistinct; humeral carina absent. Tegument of interstriae shiny, without microsculpture and with shallow, but evident micropunctation.
ABDOMEN. Ventrite VI smooth at centre and with diffuse microsculpture on the sides ( Fig. 14B View Fig ). Pygidium slightly convex in both sexes and with shiny tegument, without microsculpture and with evident micropunctation.
AEDEAGUS. Parameres almost as long as phallobase and symmetrical, with both external faces flat. In lateral view, simple, without any ventral notch or keel and with truncate apex ( Fig. 17B View Fig ).
SEXUAL DIMORPHISM. Males: protibial spur wide and bifid, with external projection long, straight, and fine, and internal projection shorter, bent, and widened ( Fig. 15B View Fig ). Pygidium very long (length between 1.1 and 1.4 mm). Ventrite VI strongly narrowed at middle; ventrite V without medial expansion on its posterior edge. Females: protibial spur fine and spiniform. Abdomen with three pairs of transverse foveae located in the suture between ventrites I–II, II–III and III–IV, respectively; foveae not margined by row of long setae ( Figs 14B View Fig , 16D View Fig ). Pygidium shorter (between 0.9 and 1.1 mm). Ventrite VI wide at middle, only slightly narrowed by medial expansion on the posterior edge of ventrite V ( Fig. 14B View Fig ).
Measurements
Males (N = 16). TL: AV: 7 ± 0.53; MX: 7.9; MN: 6.2. EW: AV: 5.2 ± 0.29; MX: 5.8; MN: 4.7. PL: AV: 2.4 ± 0.13; MX: 2.6; MN: 2.2. PW: AV: 4.4 ± 0.21; MX: 4.8; MN: 4.1. PgL: AV: 1.2 ± 0.07; MX: 1.4; MN: 1.1. PgW: AV: 2.2 ± 0.12; MX: 2.4; MN: 2.
Females (N = 21). TL: AV: 6.3 ± 0.38; MX: 7.5; MN: 6.36. EW: ME: 5.3 ± 0.39; MX: 6; MN: 4.5. PL: AV: 2.4 ± 0.16; MX: 2.6; MN: 2.1. PW: AV: 4.5 ± 0.3; MX: 5; MN: 3.9. PgL: AV: 1 ± 0.09; MX: 1.1; MN: 0.9. PgW: ME: 2.3 ± 0.12; MX: 2.5; MN: 2.
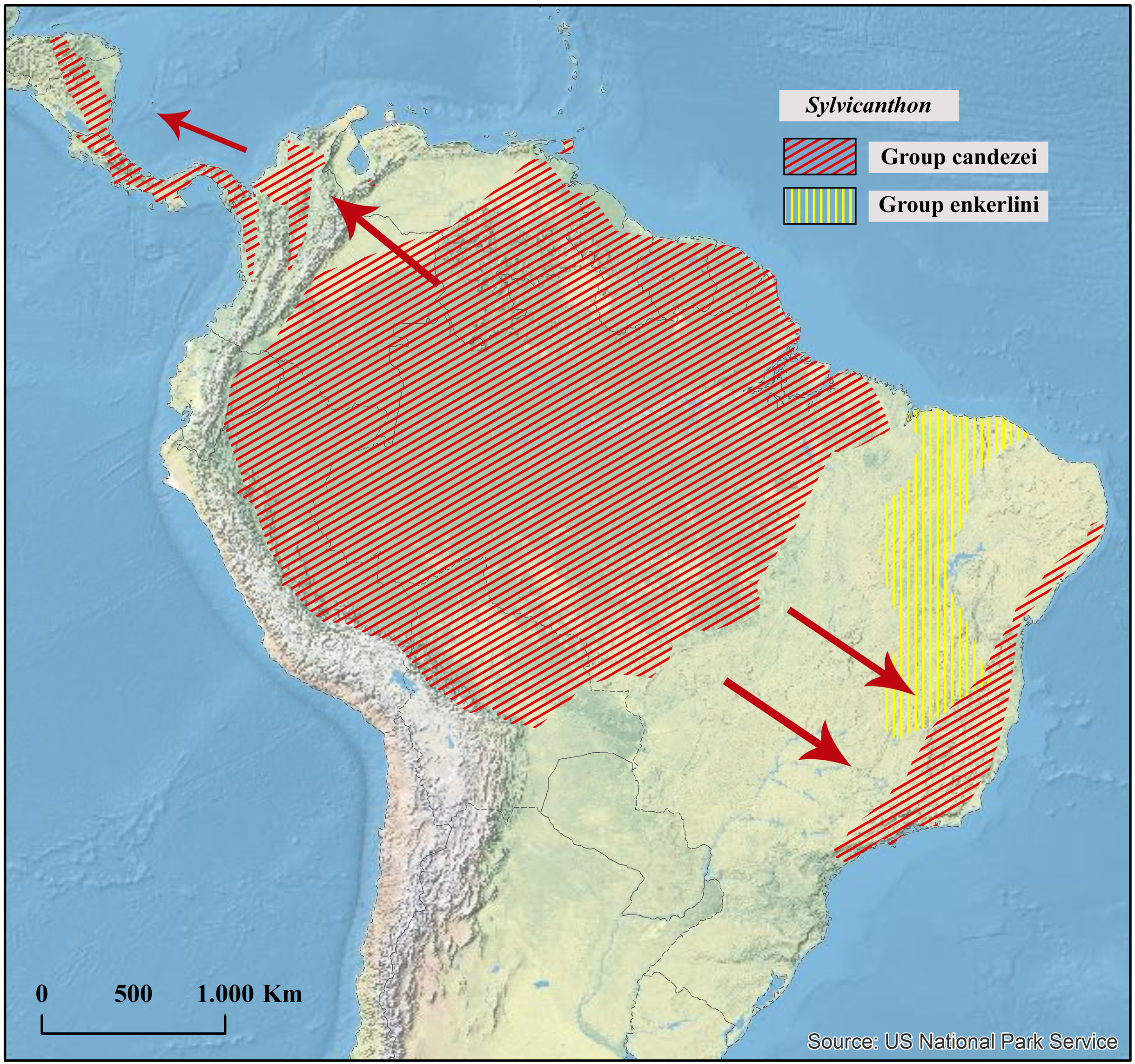
Geographical distribution
Atlantic Forest above 600 m in the Brazilian southeast.
Ecoregions
Bahia Interior Forests, Bahia Coastal Forest, Alto Paraná Atlantic Forest, Serra do Mar Coastal Forests.
Collecting sites ( Fig. 24 View Fig )
BRAZIL. Bahia (?). Minas Gerais: Barão de Cocais, Belo Horizonte (Parque Estadual da Serra do Rola-Moça), Carrancas (Chapada das Perdizes), Conceição dos Ouros, Diamantina, Itamonte, Lavras , Lima Duarte (Parque Estadual do Ibitipoca), Nova Lima (Parque Estadual da Serra do Rola-Moça), Prados, Rio Acima, São João Evangelista, Viçosa. Espírito Santo: Conceição do Castelo, Domingos Martins (Parque Estadual da Pedra Azul), Santa Teresa (Estação Biológica Santa Lúcia), Vargem Alta, Venda Nova do Imigrante. Rio de Janeiro: Itatiaia (Parque Nacional do Itatiaia), Nova Friburgo, Parque Nacional da Serra dos Órgãos, Rio de Janeiro. São Paulo: Mogi das Cruzes (Parque das Neblinas), Parque Estadual da Serra do Mar (Núcleo Santa Virgínia), Salesópolis (Estação Biológica de Boracéia), São Luiz do Paraitinga, São Miguel Arcanjo (Parque Estadual Carlos Botelho), Serra do Japi.
Intraspecific variation and taxonomic discussion
Apart from the colouration of the teneral specimens, little intraspecific variation was observed in S. foveiventris . Although the majority of the specimens do not show any trace of a margin between the eyes, a few individuals have a very short, tenuous line at the centre of the posterior edge of the head. Another noticeable variation refers to the density of coarse punctation at the base of metafemora: at one extreme, this punctation is deep and dense, being easily observed ( Fig. 13A View Fig ). At the other extreme, there are a few short points – in the male from Itamonte (Minas Gerais), especially, these points are almost absent, only weakly marked on the left metafemur. Between those extremes, a complete gradual variation exists. Finally, variation is also seen in the elytral striae, with some specimens showing all the first five striae well marked and carelunate, while others have only the first four striae in that way, whereas the fifth stria is as effaced and discontinuous as the more external striae.
Being a member of the candezei subgroup, S. foveiventris is closely related to S. candezei and S. genieri sp. nov. It differs from both species by the dorsal colouration pattern ( Fig. 23A View Fig ), the presence of a coarse punctation at the base of the metafemora ( Fig. 13A View Fig ), the presence of three pairs of abdominal foveae in females ( Figs 14B View Fig , 16D View Fig ), the shape of the parameres ( Fig. 17B View Fig ), and the distribution ( Fig. 24 View Fig ). From S. candezei , in particular, S. foveiventris is distinct also in the shape of the posterior edge of ventrite V ( Fig. 14B View Fig ) and from S. genieri sp. nov. in the microsculpture of the surface of the pronotum, elytra and pygidium. Table 3 summarizes the differences between these three species.
Since they are found in sympatry in at least some localities in Espírito Santo, specimens of S. obscurus have been misidentified as S. foveiventris in several collections. Nonetheless, it is possible to readily separate the two species by the number of protibial teeth (two in S. foveinvetris , Fig. 11J View Fig , and three in S. obscurus , Fig. 11D View Fig ), shape of the internal margin of the protibiae (straight in S. foveiventris and strongly expanded in the apical half in S. obscurus ), by the coarse punctation at the base of the metafemora (present in S. foveiventris , Fig. 13A View Fig , and absent in S. obscures , Fig. 13B View Fig ), pilosity on the female abdominal foveae (glabrous in S. foveiventris , Figs 14B View Fig , 16D View Fig , and with a row of long setae on the anterior margin in S. obscurus , Fig. 16C View Fig ), among other features. Besides, the distribution of both species does not entirely overlap: S. obscurus occurs throughout the Atlantic Forest from Alagoas to Espírito Santo ( Fig. 41 View Fig ), while S. foveiventris is present in the mountain ranges of southeastern Brazil (Minas Gerais, Espírito Santo, Rio de Janeiro and São Paulo) ( Fig. 24 View Fig ); sympatry was observed only in the municipalities of Santa Teresa and Venda Nova do Imigrante, in Espírito Santo state.
Comments
In a very vague way, without specifying either an exact locality or the source for the new record, Martínez et al. (1964) cited S. foveiventris as being present in Paraguay. Although we have studied the specimens deposited in the Martínez collection (now at the CMNC) and in the MACN, institution where Martínez worked, we could not find any specimens of S. foveiventris from that country or from Brazilian localities bordering Paraguay. Thus, we consider the Paraguayan record for S. foveiventris erroneous and possibly fruit of a misidentification of specimens of Canthon cobosi (e.g., Pereira & Martínez (1960) considered C. cobosi close to S. foveiventris ). In reality, based on the material gathered for this work, S. foveiventris seems to be a species restricted to the mountain ranges of southeastern Brazil; the only other records outside that region are two specimens labelled “ Bahia ” deposited in the MZSP and NMCP, which we consider a doubtful record.
Natural history
In the same way as for the other species in the genus, S. foveiventris seems to be strictly coprophagous, having been attracted to human faeces ( Vaz-de-Mello & Louzada 1997; Hernández 2002; Falqueto et al. 2005; Almeida & Louzada 2009; Hernández & Vaz-de-Mello 2009; Hernández et al. 2011; MC, personal observation; and information from specimen labels) and to a mixture of maned wolf ( Chrysocyon brachyurus (Illiger, 1815)) , capuchin monkey ( Sapajus apella (Linnaeus, 1758)) and coati ( Nasua nasua (Linnaeus, 1766)) dung ( Duraes et al. 2005). Other baits offered apart from dung, such as cow spleen ( Vaz-de-Mello & Louzada 1997; Falqueto et al. 2005; Almeida & Louzada 2009) and rotten bananas and fungi ( Falqueto et al. 2005), did not attract individuals of S. foveiventris . The time of foraging activity is also the one expected for the genus, i.e., nocturnal. In Serra do Japi (São Paulo), Hernández (2002) collected eight specimens at night, four at sunset, and only one at sunrise, having found no specimens during the day.
Sylvicanthon foveiventris seems to be a seasonal species, with adults active mainly during the rainiest and hottest season of the year, which, in the Brazilian southeast, occurs during spring and summer. This is corroborated both by label data (one specimen recorded for September, five for October, 29 for December, 110 for January and 14 for February, and only two specimens in August, in the second half of the winter) and by the results obtained by Hernández & Vaz-de-Mello (2009) during a year of monthly collecting in Serra do Japi. The species was reported exclusively in altitudes between 680 and 1727 m (data compiled from Vaz-de-Mello & Louzada 1997; Duraes et al. 2005; Almeida & Louzada 2009; and specimen labels). Indeed, S. foveiventris is present in several mountain ranges in southeastern Brazil, such as Serra Capixaba, Serra da Mantiqueira, Serra dos Órgãos and Serra do Mar, areas covered predominantly by ombrophilous and dense or semidecidual Atlantic Forest.
The strategy of arboreal foraging, a notable behavioural characteristic of S. foveiventris , was reported for this species by Vaz-de-Mello & Louzada (1997). In forest fragments in Viçosa (Minas Gerais), those authors hoisted some traps baited with human faeces 10 m from the ground and 22 specimens of S. foveiventris were collected as a result. As discussed by Vaz-de-Mello & Louzada (1997) (and, before them, by Howden & Young 1981), arboreal mammal and bird dung can usually attach to leaves and branches high in the trees. Consequently, dung beetles that developed a special capacity of threedimensional foraging (i.e., the ability of searching for food in several different strata in the forest, in contrast to searching only horizontally above the ground) had access to an alternative food source to the dung deposited in the forest floor, which is heavily disputed by a rich guild of coprophagous arthropods. For Vaz-de-Mello & Louzada (1997), perching on leaves, a behaviour displayed by many tropical dung beetles, was a preadaptation to the evolution of arboreal foraging – indeed, one of the specimens of S. foveiventris collected in the Parque Nacional do Itatiaia (Rio de Janeiro) was caught perching on a leaf on the understory (Juan Pablo Botero, 2014, personal communication to MC).
| NHRS |
Swedish Museum of Natural History, Entomology Collections |
| NMPC |
National Museum Prague |
| MZSP |
Sao Paulo, Museu de Zoologia da Universidade de Sao Paulo |
| MCNZ |
Porto Alegre, Museu de Ciencias Naturais da Fundacao Zoo-Botanica do Rio Grande do Sul |
| S |
Department of Botany, Swedish Museum of Natural History |
| W |
Naturhistorisches Museum Wien |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
| F |
Field Museum of Natural History, Botany Department |
| B |
Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universitaet |
| A |
Harvard University - Arnold Arboretum |
| J |
University of the Witwatersrand |
| P |
Museum National d' Histoire Naturelle, Paris (MNHN) - Vascular Plants |
| H |
University of Helsinki |
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
| E |
Royal Botanic Garden Edinburgh |
| M |
Botanische Staatssammlung München |
| I |
"Alexandru Ioan Cuza" University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sylvicanthon foveiventris ( Schmidt, 1920 )
| Cupello, Mario & Vaz-De, Fernando Z. 2018 |
Sylvicanthon foveiventris
| Culot L. & Bovy E. & Vaz-de-Mello F. Z. & Guevara R. & Galetti M. 2013: 85 |
| Almeida S. S. P. & Louzada J. N. C. 2009: 37 |
| Duraes R. & Martins W. P. & Vaz-de-Mello F. Z. 2005: 725 |
Sylvicanthon foveiventre
| Hernandez M. I. M. & Monteiro L. R. & Favila M. E. 2011: 7 |
| Hernandez M. I. M. & Vaz-de-Mello F. Z. 2009: 610 |
| Falqueto S. A. & Vaz-de-Mello F. Z. & Schoereder J. H. 2005: 20 |
| Hernandez M. I. M. 2002: 598 |
| Vaz-de-Mello F. Z. 2000: 195 |
| Vaz-de-Mello F. Z. & Louzada J. N. C. 1997: 57 |
Glaphyrocanthon (Glaphyrocanthon) foveiventris
| Martinez A. & Pereira F. S. 1967: 53 |
| Martinez A. & Halffter G. & Halffter V. 1964: 5 |
| Vulcano M. A. & Pereira F. S. 1964: 662 |
| Martinez A. & Pereira F. S. 1956: 126 |
Canthon foveiventre
| Blackwelder R. E. 1944: 199 |
Canthon foveiventris
| Krajcik M. 2012: 63 |
| Halffter G. & Martinez A. 1977: 63 |
| Martinez A. 1949: 287 |
| Balthasar V. 1939: 188 |
| Schmidt A. 1922: 64 |
Canthon foveiventris
| Schmidt A. 1920: 133 |