Spirostomum teres, Claparede & Lachmann, 1858
|
publication ID |
https://doi.org/ 10.4467/16890027AP.20.002.12158 |
|
persistent identifier |
https://treatment.plazi.org/id/AF2EDF67-7706-FFF1-FCEC-ACA296904DA6 |
|
treatment provided by |
Felipe |
|
scientific name |
Spirostomum teres |
| status |
|
Spirostomum teres View in CoL annual cycle and stratification
During last decade, the interest of the protozoologists has turned to the so called rare biosphere including species able to survive in extreme conditions, e.g. anaerobic ones, that are present in the ecosystem but outside their specialised niche and/or following special change in environmental conditions appeared below common method detection limits ( Weisse 2014). However, with some exceptions, the studies in medium to large stratified lakes avoided to quantify protists in anoxic hypolimnia with microaerophilic / anaerobic ciliates while the studies on small ponds and lakes, in particular meromictic and eutrophic ones were abundant (compare Madoni 1990; James et al. 1995; Ha- das and Berman 1998; Peštová et al. 2008; Bautista-Reyes and Macek 2012; Sánchez-Medina et al 2016 vs. Finlay 1981; Bark 1985; Psenner and Schlott-Idl 1985; Neidl 1989; Madoni 1991; Guhl et al. 1994; Finlay and Esteban 2009; Oikonomou et al. 2014, 2015; Tirjaková et al. 2016). It explained why we are lacking data to be compared on the ciliate of interest from an oligomesotrophic anoxic environment.
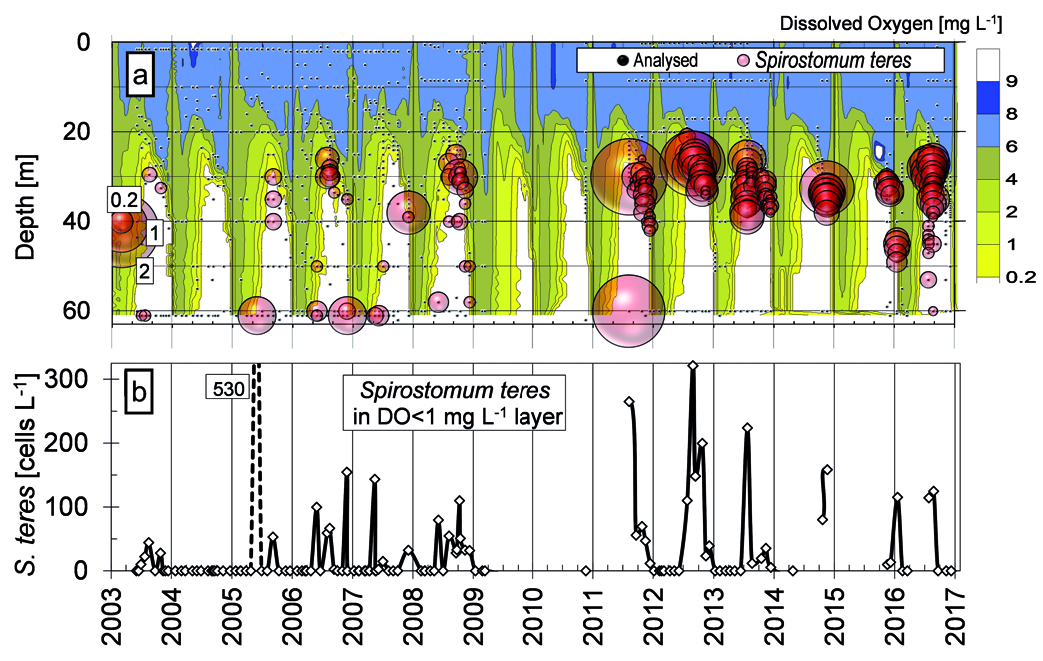
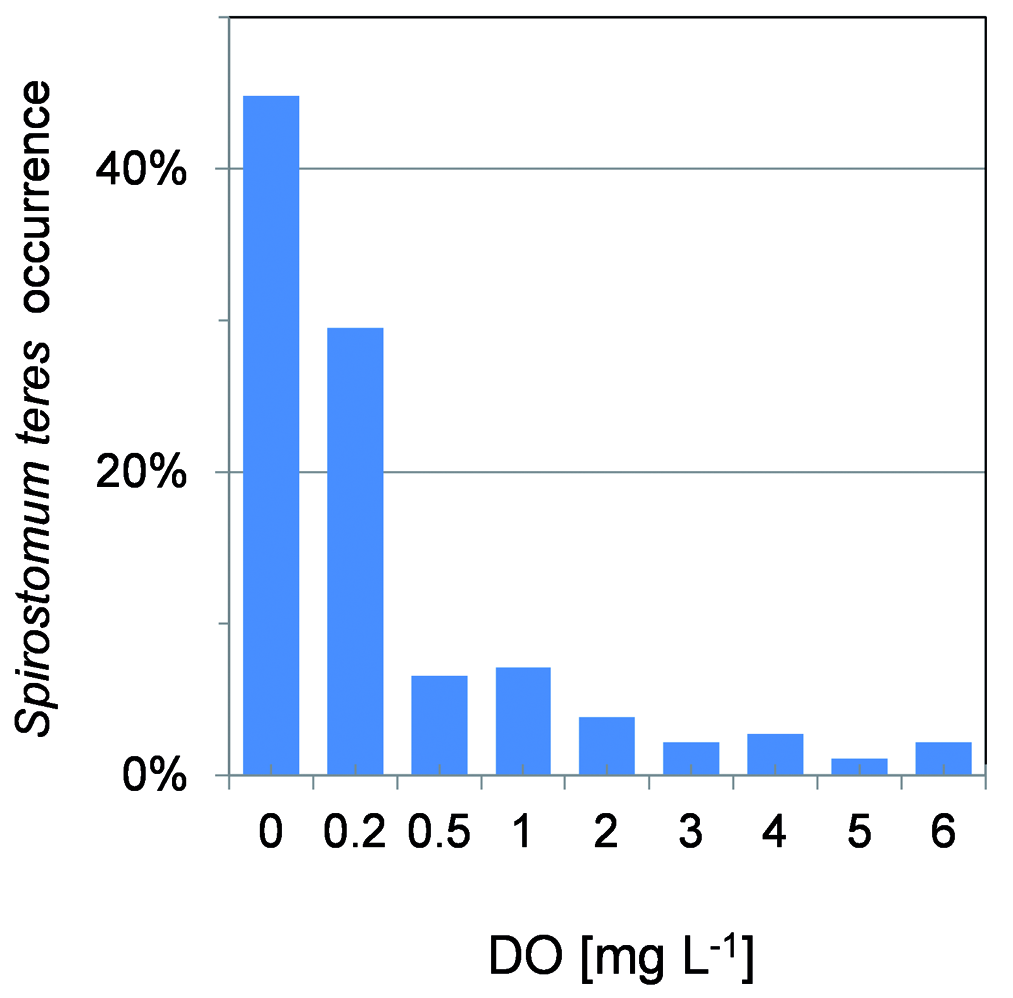
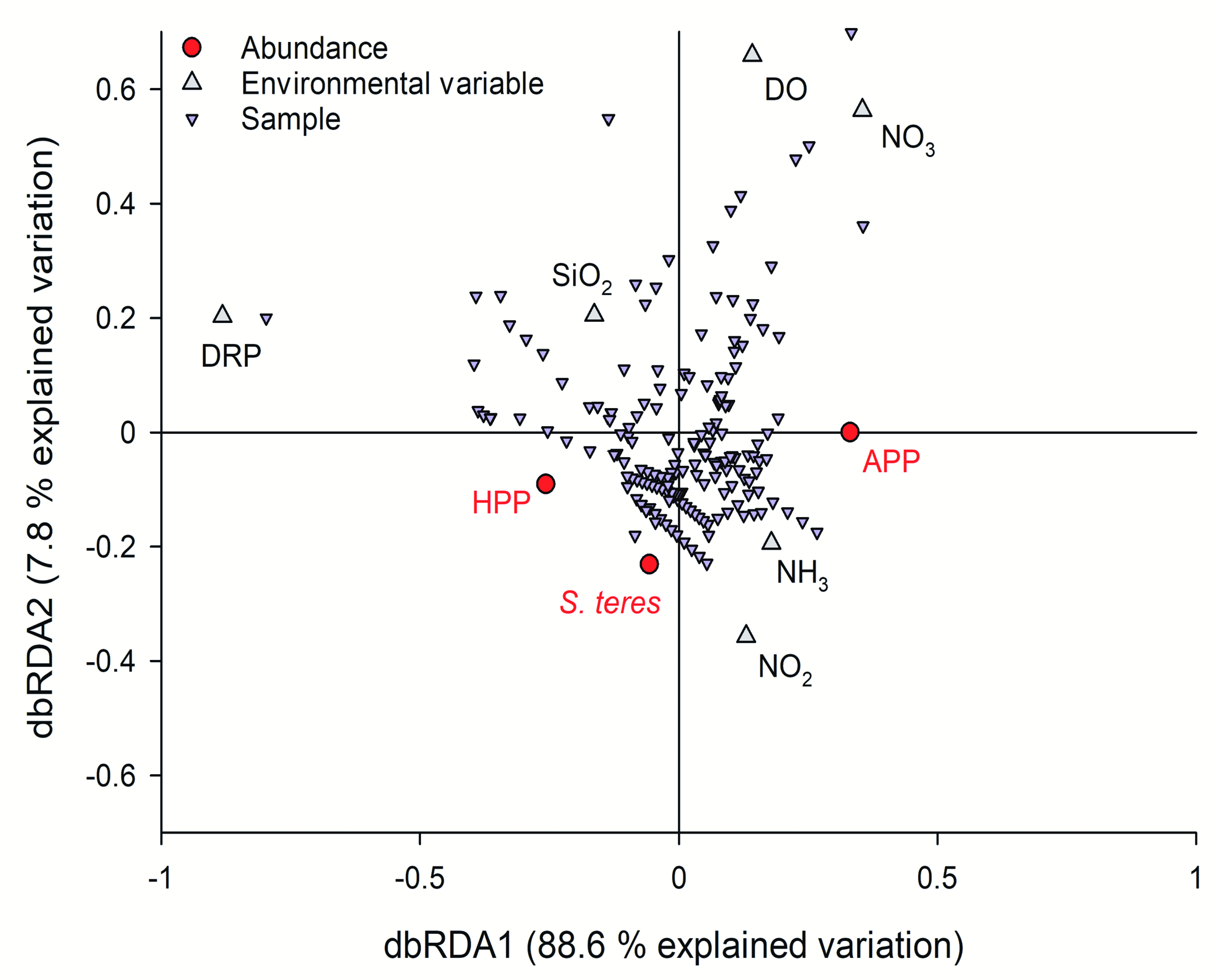
Migration of naturally benthic organisms was pro- posed and proven as a source of microaerophilic ciliates, including S. teres in an anoxic hypolimnion and oxycline in small water bodies ( Bark 1981, 1985; Finlay 1981; Madoni 1991; Finlay et al. 1996; Finlay and Este- ban 2009). Definitively, S. teres distribution in the water column layer of Lake Alchichica was tightly connected with oxygen stratification ( Figs. 3–5 View Fig View Fig View Fig and 11–12 View Fig View Fig ), and also the bottom population has been observed regularly until 2009 (isolated observation occurred also in 2011 and 2016). Apparently, during last years the redox potential in the above bottom layer has been dropping (the robust data covering the study are not available) and the conditions have become unsupportable for the ciliate. But the studies of above mentioned authors were based on annual migration by less than 10 m within the water column or below the duckweed surface cover ( Madoni 1991) comparing to 20 to 40 m distance to the bottom of 60 m in lake Alchichica. One must accept also the dial migration of S. teres within the water column that was proposed to be as much as 5 (10) m ( Bark 1981) and well documented in a eutrophic gravel pit lake ( Rossberg and Wickham 2008). In the latter one, the mean depth of population varied up 1 m comparing day and night, even though taking into account changes in the width of the layer colonised by S. teres , real migration was wider. However, it should not explain bottom- and oxycline-population communication in our deep lake. It seems that the oxycline / anoxic hypolimnion Alchichica population was proliferating separately and/or, hypothetically, it was related to littoral or less deep parts of the lake. In the hypolimnion of Lake Alchichica, the temperature was increasing gradually to 15.3° C during the last two decades that has reached almost the optimum temperature for S. teres growth ( Laybourn and Finlay 1976, Finlay 1977) giving, theoretically, genera- tion time of <100 h. Under such conditions, the res- piration would increase as well. Statistical significance can hardly be obtained for any limnological variable to be linked as explicative for S. teres distribution and abundance due to the fact that thermal stratification, the position of the oxycline, and the deep chlorophyll maximum ( DCM) were tightly co-correlated in Lake Alchi- chica. In this regard, a dbRDA ordination analysis was the best approach in order to determine to what extend environmental variables can explain the differences in the abundance of S. teres, APP and HPP in the abun- dance matrix ( Fig. 16 View Fig ). The dbRDA ordination shows that S. teres was related to microaerophilic and anoxic phosphorus-rich environments. On the other hand, S. teres is supposed to be microaerophilic, i.e. oxygen respiring upon low DO concentrations while it is able to slow down oxygen consumption with decreasing oxygen tension – so called oxygen conformer ( Finlay 1977). However, our data demonstrated the occurrence, even with higher abundance, of S. teres in anoxic conditions, as it has been proven in many studies (e.g., Fin- lay 1981, Psenner and Schlott-Idl 1985, Madoni 1991, Sánchez-Medina et al. 2016).
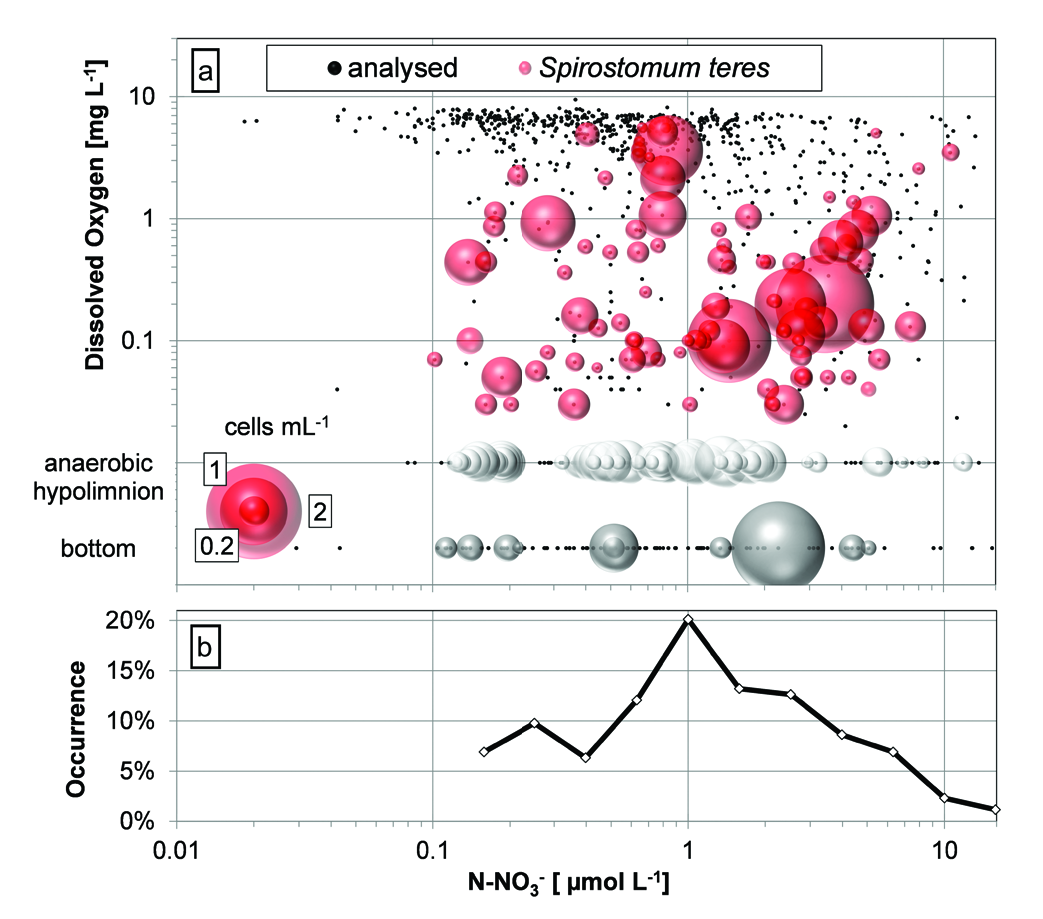
Until now, the nitrate respiration, i.e. nitrite produc- tion was proven for Loxodes spp. ( Finlay 1985) and oth- er protozoa ( Hadas et al. 1992). In the case of S. teres, Psenner and Schlott-Idl (1985) observed apparent relation of the ciliate peaking and nitrate drop coinciding with nitrite peak (not quantified) in lake Piburgersee. In the same lake, Neidl (1989) tried to correlate nitrite data with S. teres occurrence but as he had analysed only nitrates, he applied nitrite profiles taken during preceding year. In lake Alchichica, many samplings of nutrients were not sufficiently numerous throughout the water column to describe local nitrite peaks, cleared by a linear interpolation of lacking stratification data (except, e.g. data in Fig. 4 View Fig ) but it is apparent that the hypolimnion S. teres maximums coincided with higher nitrate concentrations ( Fig. 12 View Fig ). It was confirmed in the dbRDA ordination analysis showing that the abundance of S. teres along with HPP is associated with higher ni- trite ( NO 2 –) and ammonium ( NH 3) concentrations. The relationship would not be observed directly, since the activity of nitrogen cycle-bacteria was proven in such layers ( Pajares et al. 2017). Very recently, a transcriptome of proteins involved in rhodoquinol-dependent fu- marate reduction to respire in the oxygen-depleted habitats were found in a mixotrophic zoochlorella bearing S. semivirescens ( Hines et al. 2018) . Even though the S. teres occurrence shows preferred PAR between 0.04 and 0.6%, with slight preference for 0.2 to 0.6% interval, the statistical tests may not be applied. Such values might have a considerable measurement error during cloudy days when a low absolute PAR was registered. Moreover, the occurrence of S. teres was also considerable in the near-zero illumination /dark hypolimnion and bottom showing even higher abundances.
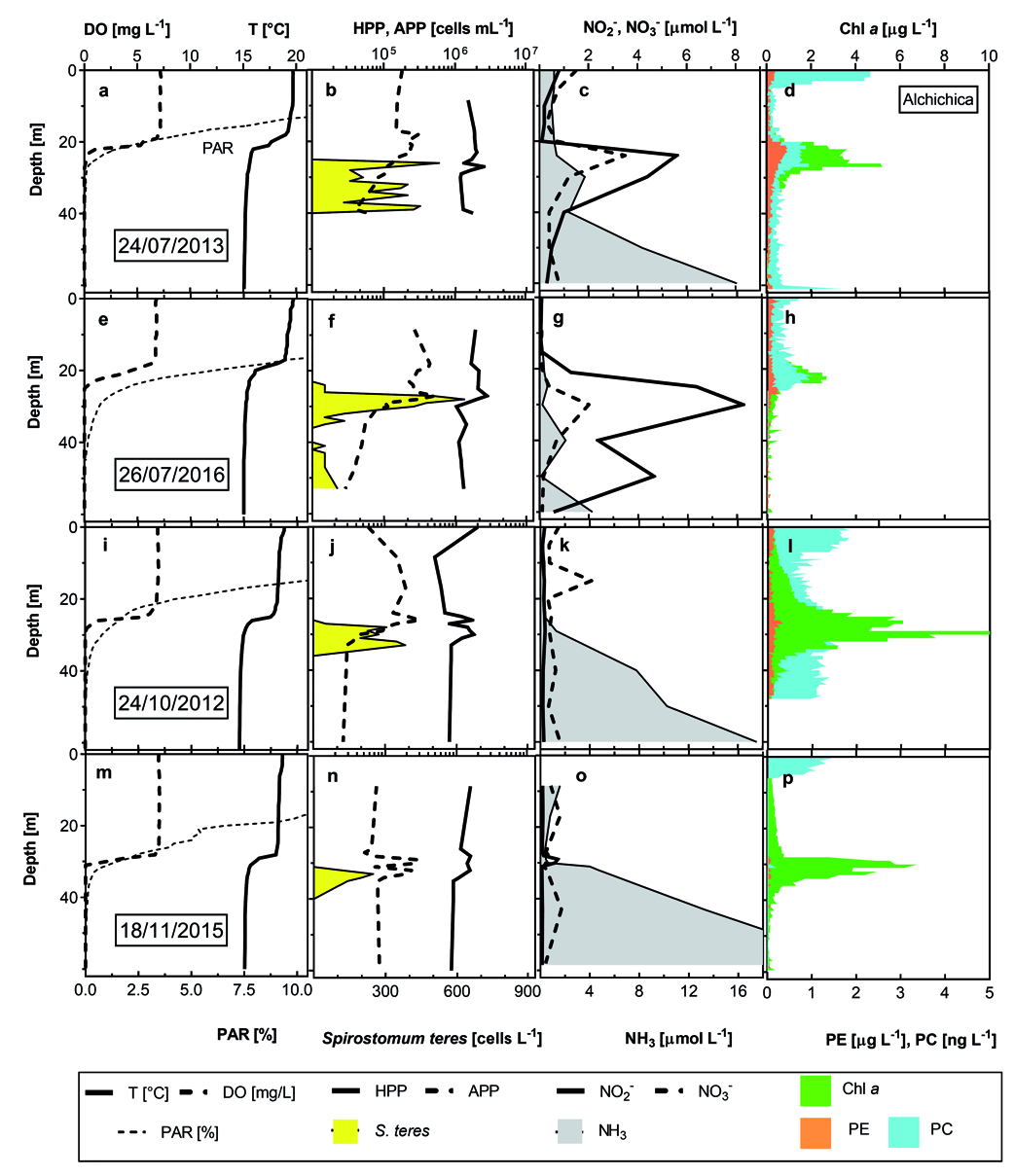
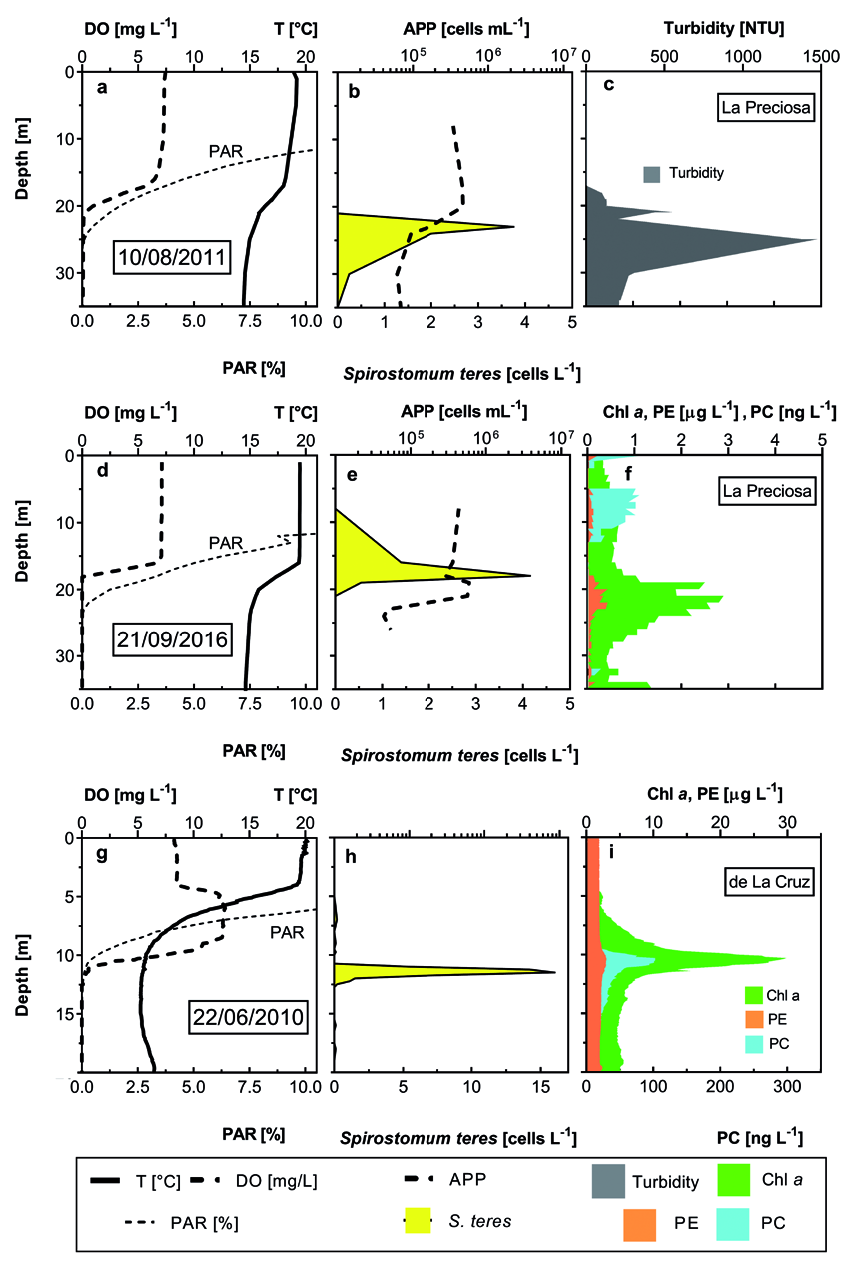
Spirostomum sp. occurrence in the environment was apparently related to a deep chlorophyll maximum. Rossberg and Wickham (2008) observed the typical Spirostomum sp. layer just above the DCM but in that case, it was form by Chromatium View in CoL -like purple sulphur bacteria. In Lake Alchichica, DCM was composed, generally, by large diatoms Cyclotella alchichicana , accompanied by a minute C. choctawhatcheeana , chlorophytes Monoraphidium minutum and, frequently, by a local peak of picocyanobacteria. However, the in situ registered fluorescence did not reveal cyanobacteria peaking: “phycoerythrin” stratification resembled that of diatoms; only cyanobacterial colonies/aggregates were apparently registered as “phycocyanin”. Just with- in the DCM, it was possible to observe S. teres View in CoL , e.g., in July when the thermocline was very thick presenting DO and PAR gradient there. DO dropped to zero just around DCM layer coinciding with about 0.1% PAR. Typically, S. teres View in CoL maximums were found 2 to 4 meters below DCM / zero DO but still above the layer with abundant Thiocapsa View in CoL -like purple bacteria, depending on even lower redox potential ( Fig. 4 View Fig ). Due to the lack of chlorophyll data from the dark layers (untill 2012), we were not able to use pigment data in the statistical analysis. Different situation was found in Lake La Preciosa, where S. teres View in CoL occupied the layers just above the DCM but below zero DO ( Fig. 5 View Fig ). In contrary, Tirjaková et al. (2016) found abundant S. teres View in CoL within cyanobacterial blooms with pretty high DO saturation (> 50%); no data on bacteria and picocyanobacteria were measured. Also Beaver and Crisman (1989) stated primarily the Spirostomum spp. surrounding community as “plankton of very productive ponds and lakes”. Bacteria with IR fluorescence (supposingly anoxigenic photosynthetic bacteria in chains of cells with much brighter fluores- cence than that of purple bacteria from Alchichica) apparently did not serve as a feeding source, they were toxic and/or occupied the layers with the environmental variables, which already did not support the ciliate growth. Either in Lake La Cruz ( Spain) or La Preciosa ( Mexico), S. teres View in CoL did not penetrate the maximum of photosynthetic anoxygenic bacteria, observed via their autofluorescence registered with infrared camera.
Even though Spirostomum spp. have been study exhaustively from the point of view of prokaryotic symbionts ( Fokin et al. 2005), there is a lack of infor- mation on their feeding behaviour and data on their feeding rate. Adl et al. (2019) declared the genus as bacterivorous while other classified it as algivorous / omnivorous ( López-Ochoterena 1966, Bick 1958, Finlay 1981), deducing bacterivory ( Psenner and Schlott-Idl 1985) and again, Finlay and Esteban (2009) defined S. teres View in CoL as a mainly bacterivorous, functionally complex upstream filter feeder. On the other hand, Foissner et al. (1992) stated S. teres View in CoL as a bacterivorous, particularly purple- and green-sulphur bacteria feeder, which ingested even minute chrysophytes, diatoms and desmids. We have observed during the study nearly all these organisms inside the cells: Populations from all three lakes ingested in different proportion minute plankton bacteria, purple sulphur bacteria, diatoms C. choctawhatcheeana , green algae Monoraphidium spp. and Oocystis spp. However, the observed prey that was almost dominant for the populations were the picocyanobacteria. It is possible that the authors, which were not using fluorescence micros- copy for the ciliate observations, were not be able to distinguish them either for the ciliate pigmentation or for the colour of cyanobacteria different to blue-green:
2
isolated Alchichica and La Preciosa picocyanobacte- ria were mainly purple to violet ( Callieri et al. 2013) and La Cruz cyanobacteria were observed yellowish on the membrane filters. On the other hand, all cyano- bacteria from the S. teres layers showed both bright chlorophyll a and phycobilins fluorescence while the surface picocyanobacteria were observed almost only through phycobilins. Curiously, according to dbRDA ordination analysis, S. teres and HPP abundances are associated to low oxygen and high DRP concentrations accompanied by nitrite and ammonium while the ordination plot of APP is located in the opposite side with the lowest DRP and higher DO and nitrate con- centrations within the oxic-anoxic gradient ( Fig. 16 View Fig ). Although it was possible to find S. teres in the layers with DO up to 6 mgL –1, we were obliged to follow strictly the recommendations of Massana and Pedrós-Alió (1994) to maintain the feeding experiments upon anoxic conditions. If the BOD bottles were not filled passing minimum three their volumes with the sample coming from the tube of the sampler, S. teres did not show any activity. In Lake La Cruz where small amount of the sample had been obtained via pumping and the washing-out had not been applied, we were not be able to keep the optimum conditions and consequently we had to measure the feeding activity upon laboratory conditions.
Average clearance rates of S. teres ( Table 1) from significantly robust samplings of Mexican lakes (over 100 examined cells per sampling) were found around 2000 nL cell –1 h –1, i.e. in Alchichica, APP uptake rate of 130 to 150 cells cell –1 h –1 but in La Preciosa, up 400 cells cell –1 h –1. The estimated feeding activity in the samples with lower S. teres numbers was also lower. La Cruz population was less active (clearance rate 245 nL cell –1 h –1, i.e. 115 cells cell –1 h –1) but it was in concordance with the lake low temperature (6° C in the maximum S. teres layer) that was followed in the laboratory experiments. According to Finlay (1977), S. teres is nearly inactive upon such conditions.
In absolute values, the feeding rates of S. teres were comparable to efficient plankton ciliates such as Halteria grandinella , which bacteria uptake rate was found 1580 cells cell –1 h –1 but that of APP only 210 cells cell –1 h –1 ( Šimek et al. 1995). However, if the vol- ume specific clearances were calculated according to Fenchel (1986), S. teres did not present an appropriate feeding activity to support its suspension feeding-derived growth upon picocyanobacteria. H. grandinella of a volume of 2.86 × 103 µm 3 and clearance rate of <1200 nL cell –1 h –1 ( Šimek et al. 1995) would have specific clearance of an order of magnitude 105 h –1, which classifyed it as an efficient suspension feeder, while our S. teres of biovolume 2.3 × 106 µm 3 and clearance rate 2000 nL cell –1 h –1 possessed specific clearance only up to 103 h –1. Stentor sp. from the same size category was found to reach a value over 104 h –1. On the other hand, according to our observations, picocyanobacteria remained in the vacuoles along with FLB for a long time (over one hour) without that they were digested, which looked like the ciliate behavioural strategy (for picocynobacteria ingestion/digestion in minute ciliates, see, Dolan and Šimek 1997). Thus, S. teres should use to support the growth another carbon source, e.g. (i) products of photosynthesis of ingested picocyanobacteria, (ii) it could ingest bacteria upon higher feeding rates that APP or (iii) ingestion of larger eukaryotes such as diatoms and chlorophytes is much more important than it had been supposed.
| C |
University of Copenhagen |
| DO |
Société d'Agriculture Sciences et Arts |
| HPP |
University of Helsinki |
| NO |
Tulane University Herbarium |
| NH |
South African National Biodiversity Institute |
| PAR |
Museo de Ciencias Naturales y Antropológicas Prof. Antonio Serrano |
| APP |
Parco Nazionale del Gran Sasso e Monti della Laga - Università di Camerino |
| BOD |
University of Oxford |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Spirostomum teres
| Macek, Miroslav, Medina, Ximena Sánchez, Picazo, Antonio, Peštová, Dana, Reyes, Fernando Bautista, Hernández, Jorge Ricardo Montiel, Alcocer, Javier, Ibarra, Martín Merino & Camacho, Antonio 2020 |
Cyclotella alchichicana
| Olivia, Lugo, Alocer & Cantoral-Uriza 2006 |
C. choctawhatcheeana
| Prasad 1990 |
C. choctawhatcheeana
| Prasad 1990 |
S. teres
| , Psenner and Schlott-Idl 1985 |
S. teres
| , Psenner and Schlott-Idl 1985 |
S. teres
| , Psenner and Schlott-Idl 1985 |
S. teres
| , Psenner and Schlott-Idl 1985 |
S. teres
| , Psenner and Schlott-Idl 1985 |
Spirostomum teres
| , Psenner and Schlott-Idl 1985 |
S. teres
| , Psenner and Schlott-Idl 1985 |
S. teres
| , Psenner and Schlott-Idl 1985 |
S. teres
| , Psenner and Schlott-Idl 1985 |
Thiocapsa
| Winogradsky 1888 |
Chromatium
| Perty 1852 |