Buthids, C. L. Koch, 1837
|
publication ID |
https://doi.org/ 10.18590/euscorpius.2016.vol2016.iss220.1 |
|
DOI |
https://doi.org/10.5281/zenodo.7124606 |
|
persistent identifier |
https://treatment.plazi.org/id/B07187DF-A865-FFE6-FCFE-FABE9BFEF994 |
|
treatment provided by |
Felipe |
|
scientific name |
Buthids |
| status |
|
Hemispermatophores of the Buthids View in CoL
We obtained new data on hemispermatophore morphologies of a variety of Old World buthids from Sri Lanka, that have not previously been described. Our findings may be significant in the context of the larger problem of defining monophyletic genera in the family and understanding relationships of major lineages of buthids. A preliminary buthid phylogeny was derived by Fet et. al. (2005) from the cladistic analysis of certain trichobothrial characters. They proposed a major family subdivision based on whether patella trichobothrium d 3 is positioned internal to the dorsomedian carina, which defined the ' Buthus ' group, or external to it, which is the character state for the remaining five 'non- Buthus ' groups. Hemispermatophores of ' Buthus ' group genera have been relatively well described, including for the genera Androctonus , Apistobuthus , Buthacus , Buthiscus , Buthus , Cicileus , Compsobuthus , Femtobuthus , Gint , Hottentotta , Leiurus , Lissothus , Mesobuthus , Microbuthus , Neobuthus , Odontobuthus , Orthochirus , Picobuthus and Vachoniolus ( Kovařík & Lowe, 2012; Kovařík, Lowe et. al., 2013; Levy & Amitai, 1980; Lowe, 2009, 2010a, 2010b, 2010c; Lowe et. al., 2014; Navidpour & Lowe, 2009; Vachon, 1952a, 1952b, 1958; Vachon & Stockmann, 1968). Their capsule region has a stereotypic 4-lobed configuration, in which the sperm hemi-duct is composed of 3 lobes (i.e. external lobe, carinated median lobe, and internal lobe), and a single basal lobe arises dorsally near the base of the median lobe carina. The flagellum is well separated from these lobes. So far, this '3+1' configuration has been found in all Buthus group members, suggesting that it is a synapomorphy for the group.
Hemispermatophores of non- Buthus group genera, including most of the Sri Lankan buthids (except Hottentotta tamulus ), are more heterogeneous. In the majority of genera, the base of the flagellum is broadly fused to a large carinated lobe, and there may be one or more additional distinct lobes on the internal side. A basal lobe is usually present, and its size and shape varies considerably. This configuration has been described in the genera Ananteris , Australobuthus , Butheoloides , Centruroides , Chaneke , Hemilychas , Isometroides , Isometrus , Parabuthus , Rhopalurus , Tityus and Zabius ( Botero-Trujillo & Florez-Daza, 2011; Francke & Stockwell, 198 7; Gysin & Le Coroller, 1968; Koch, 1977; Kovařík, Teruel, et al., 2015; Kovařík, Teruel & Lowe, 2016; Lamoral, 1979; Lenarducci et. al., 2005; Locket, 1990; Lourenço et. al., 2006; Maury, 1969, 1970, 1974; Ojanguren-Affilastro, 2005; Prendini et. al., 2009; Probst, 1972; Stockwell, 1989; Teruel & Armas, 2012). Here we document this lobe configuration also in the non- Buthus group genera, Buthoscorpio , Charmus , Lychas and Reddyanus stat. n.
Following Stockwell (1989), we have proposed that the carinated lobe of buthids is homologous to and derived from the carinated lobe of the chaerilid hemispermatophore ( Kovařík, Teruel & Lowe, 2016). In the ' Buthus ' group, this lobe sits between two other lobes with more external and more internal positions, and hence was termed the 'median lobe' ( Vachon, 1952). Taking the carina as a conserved landmark, we hypothesize that the median lobe is homologous to the carinated lobe in non- Buthus group genera that is fused to the base of the flagellum. We further hypothesize that the fused state is plesiomorphic because it approximates the chaerilid condition in which the carinated lobe is joined continuously to the distal lamina. Some exceptions to the fused configuration are seen in the genera Ananteris , Babycurus , Grosphus and Uroplectes ( Lamoral, 1979; Lowe, 2000; Kovařík, Lowe et. al., 2015, 2 0 16; Ojanguren-Affilastro, 2005;Vachon, 1950, 1969), in which the flagellum appears well separated from the carinated median lobe. However, in contrast to the Buthus group, a non-carinated external lobe is not interpolated between the flagellum and the median lobe. This arrangement could represent a precursor to the Buthus group configuration.
All Sri Lankan representatives of non- Buthus group genera so far examined ( Buthoscorpio , Charmus , Isometrus , Lychas and Reddyanus stat. n.) exhibit a basic 1+1 lobe configuration (fused median lobe + basal lobe), without additional internal lobes developed. This configuration is shared with a number of other non- Buthus group genera that have been reported, i.e. some species of Ananteris ( Botero-Trujillo & Florez-Daza, 2011; Ojanguren-Affilastro, 2005) and Tityus (Kovařík, Teruel, et. al., 2015; Ojanguren-Affilastro, 2005); and Australobuthus , Hemilychas , Isometroides and Isometrus ( Gysin & Le Coroller, 1968; Locket, 1990; Probst, 1972). This may be a plesiomorphic state from which additional internal or external lobes have arisen independently in different lineages. Even under a basic 1+1 configuration, we observe hemispermatophore differences that are diagnostic at the generic level, e.g. enabling us to differentiate Reddyanus from Isometrus by the size of the basal lobe, and the length and shape of the flagellum. Two novel, unique hemispermatophore morphologies that we found are: (i) a highly elongated capsule in Buthoscorpio , with a long narrow median lobe fused to the base of the flagellum, and a round, blunt basal lobe; and (ii) a short capsule in Charmus , with a truncated median lobe, and a bulging, bilobate basal lobe. These findings reveal an unexplored diversity in buthid hemispermatophores, that could provide new characters for analyzing phylogenetic relationships in this large and ancient family.
Cytogenetic Data on Sri Lankan Buthid Scorpions
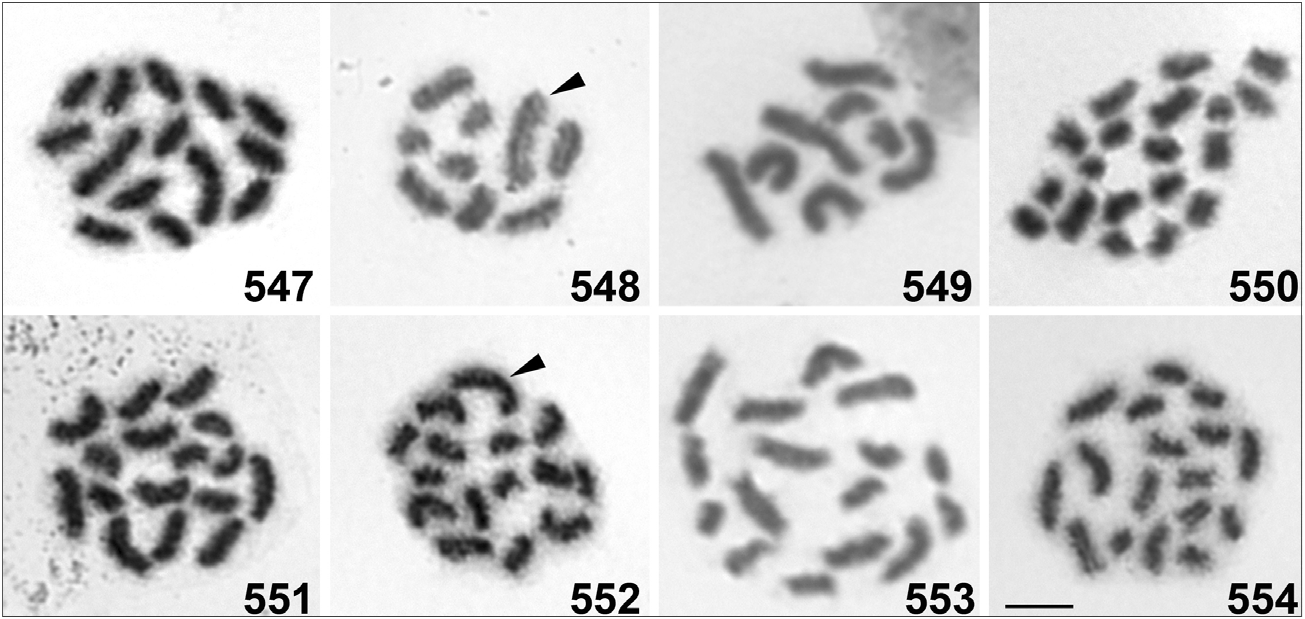
Altogether we analyzed seven buthid species ( Figs. 547–554 View Figures 547–554 , Table 6 View Table 6 ) using standard cytogenetic methods (e.g. Kovařík et al., 2009; Šťáhlavský et al., 2014). The karyotype characteristics of all analyzed species correspond to the cytogenetic attributes typical for the family Buthidae . Chromosomes do not have visible centromere regions which is a typical feature for holocentric chromosomes. This type of organization is only known in the family Buthidae within scorpions (e.g. Mattos et al., 2013). This family is also characterized by low numbers of chromosomes. Half of the cytogenetically investigated species show diploid numbers from 14 to 24 (Schneider et al., 2016). In view of this fact, Charmus laneus (2n=9) ( Fig. 548 View Figures 547–554 ) and Isometrus thwaitesi (2n=8) ( Fig. 549 View Figures 547–554 ) represent exceptions with very low number of chromosomes. Only three known species from the genus Tityus C. L. Koch, 1836 have diploid numbers of chromosomes lower than 10 (see Schneider et al., 2016). This phenomenon has been documented in T. bahiensis (Perty, 1834) and was explained as an effect of intensive fusion of holocentric chromosomes ( Schneider et al., 2009). This type of chromosomal rearrangement may also explain the differences in chromosome size within Buthoscorpio sarasinorum ( Fig. 547 View Figures 547–554 ), Lychas srilankensis ( Fig. 550 View Figures 547–554 ) (both with one extra larger pair of chromosomes) and Isometrus thwaitesi ( Fig. 549 View Figures 547–554 ) (with one extra shorter pair of chromosomes). Moreover, heterozygous chromosomal rearrangements may also explain odd diploid numbers of chromosomes in karyotypes of Isometrus thwaitesi ( Fig. 549 View Figures 547–554 ), Reddyanus loebli ( Fig. 554 View Figures 547–554 ) and one male of Reddyanus basilicus ( Fig. 552 View Figures 547–554 ). In Reddyanus basilicus we found 2n= 16 in a male from locality 15CS. In this case the chromosomes gradually decrease in size ( Fig. 551 View Figures 547–554 , Table 6 View Table 6 ). However, the male of R. basilicus from locality 15CR has 2n=15, including one extra large chromosome ( Fig. 552 View Figures 547–554 , Table 6 View Table 6 ). The intraspecific variability has also been documented in another 8 species from different genera in the family Buthidae (see Schneider et al., 2016). Due to the intraspecific variability and high similarity of basic cytogenetic characteristics (such as number and size of chromosomes) it seems difficult to apply standard cytogenetic techniques to the taxonomy of buthid scorpions. In the future, application of more refined molecular cytogenetic techniques should lead to a better understanding of the organization of genome, which may be the key to detecting specific differences between closely related species.
Key to Scorpions of Sri Lanka
1. Pedipalp patella without ventral trichobothria. .......... Buthidae C. L. Koch, 1837 View in CoL ..……………………......... 3
– Pedipalp patella with three ventral trichobothria. ...... 2
2. Adults 27–45 mm long. Pedipalp femur with 9 trichobothria, of which 4 are dorsal. ……....... Chaerilidae Pocock, 1893 View in CoL , …... Chaerilus ceylonensis Pocock, 1894 View in CoL
– Adults 75–176 mm long. Pedipalp femur with 3 or 4 trichobothria, of which only one is dorsal ..................... Scorpionidae Latreille, 1802 View in CoL ....…………………...... 15
3. Legs III and IV with well developed long tibial spurs ( Figs. 193–195, 198 View Figures 193–200 ) .....……………………................ 4
– Legs III and IV without tibial spurs ( Figs. 196–197, 199 View Figures 193–200 ) .......…………………............................................. 8
4. Telson with a distinct subaculear tooth ( Figs. 407– 408 View Figures 403–429 ). ..........…........ Lychas srilankensis Lourenço, 1997
– Telson without subaculear tooth ( Fig. 421–427 View Figures 403–429 ). ....... 5
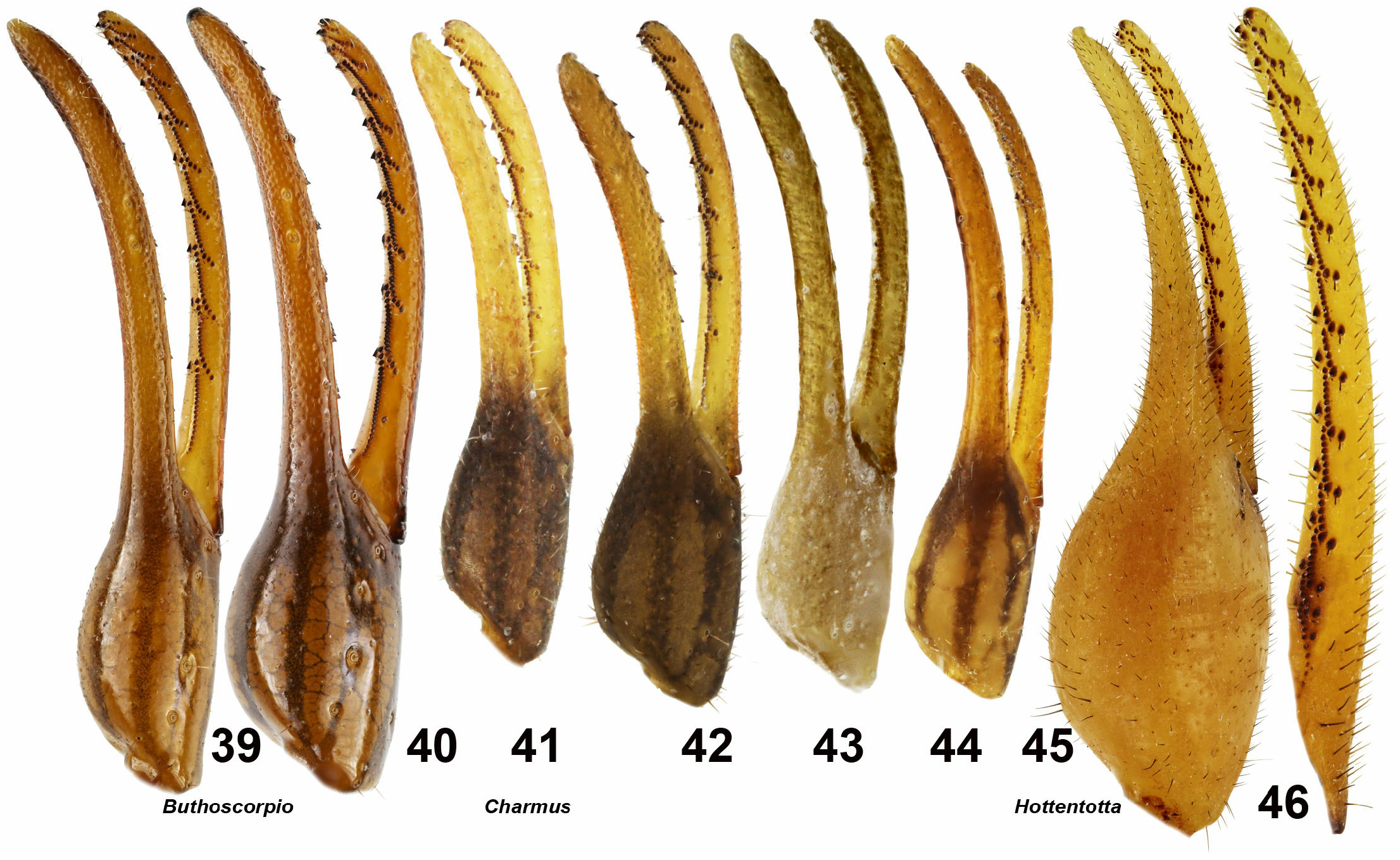
5. Metasomal segments IV–V punctate without developed carinae ( Figs. 24–29 View Figures 24–29 ). Dentate margin of pedipalp chela movable finger with distinct granules divided into 8–11 linear rows, apical rows of 3–6 granules, and 3 terminal granules ( Figs. 39–44 View Figures 39–46 ). ...……......................... 6
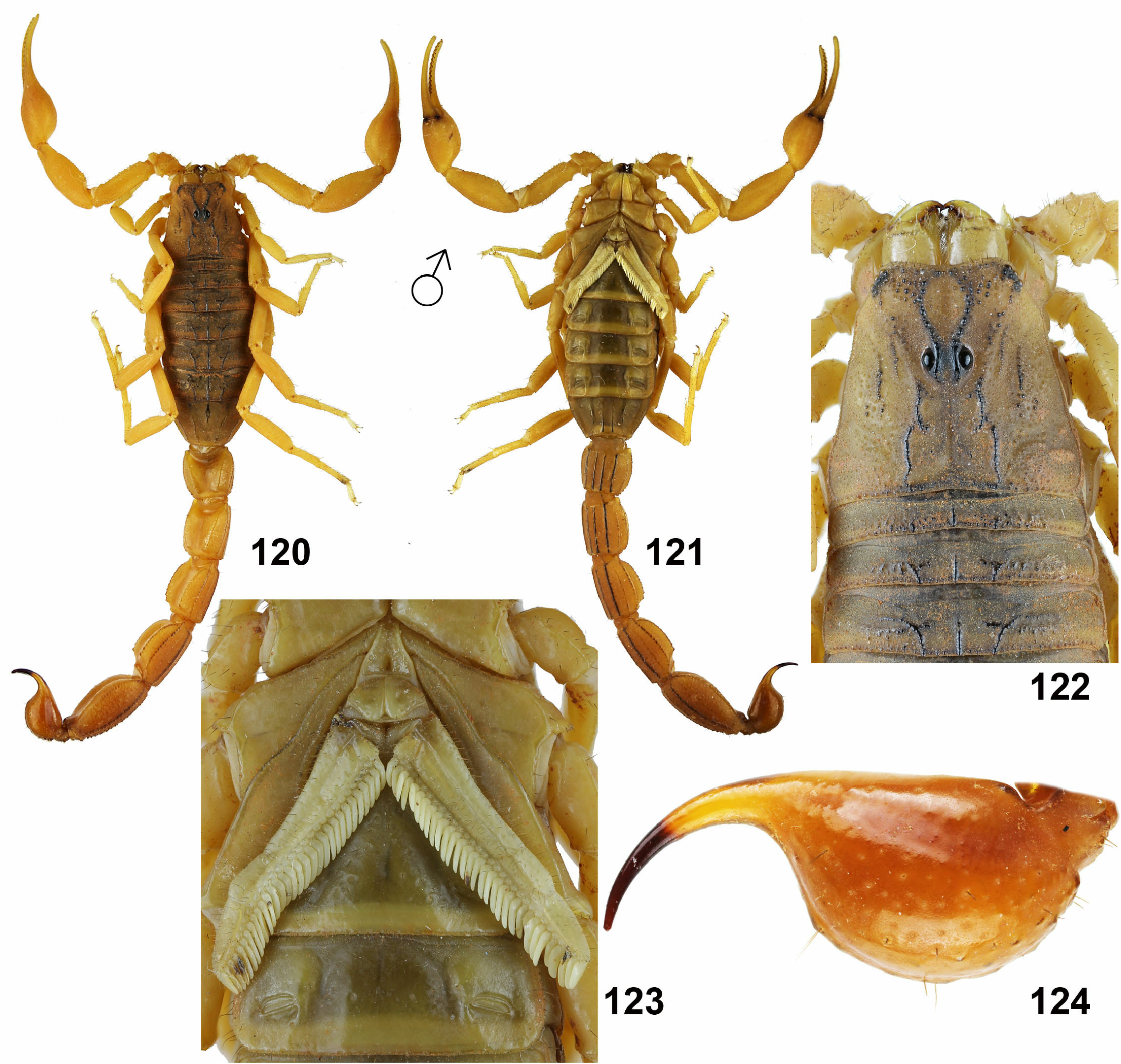
– Metasomal segments IV–V not punctate with well developed carinae ( Figs. 120–121 View Figures 120–124 ). Dentate margin of pedipalp chela movable finger with distinct granules divided into 13–15 linear rows and 5–6 terminal granules ( Fig. 46 View Figures 39–46 ). ..... Hottentotta tamulus ( Fabricius, 1798) View in CoL
6. Adults 25–52 mm long. Pedipalps, metasoma and telson glabrous ( Figs. 24–29 View Figures 24–29 ). ..………….......................... ……...…….. Buthoscorpio sarasinorum ( Karsch, 1892) View in CoL
– Adults 12–25 mm long. Pedipalps, metasoma and telson densely hirsute ( Figs. 71–73 View Figures 71–73 )..……………............ 7
7. Pedipalp patella yellowish with several black spots ( Figs. 118–119 View Figures 107–119 ); metasomal segment V length/ width ratio is 1.28–1.43 in female ( Fig. 83 View Figures 80–84 ); pedipalp chela length/ fixed finger length ratio is 1.69–1.79 in female ( Figs. 42–43 View Figures 39–46 )..……….... Charmus laneus Karsch, 1879 View in CoL
– Pedipalp patella black with several little yellow spots ( Figs. 111, 116 View Figures 107–119 ); metasomal segment length/ width ratio is 1.80 in female ( Fig. 84 View Figures 80–84 ); pedipalp chela length/ fixed finger length ratio is 1.45 in female ( Fig. 44 View Figures 39–46 ). .............. ……………………………..... Charmus saradieli View in CoL sp. n.
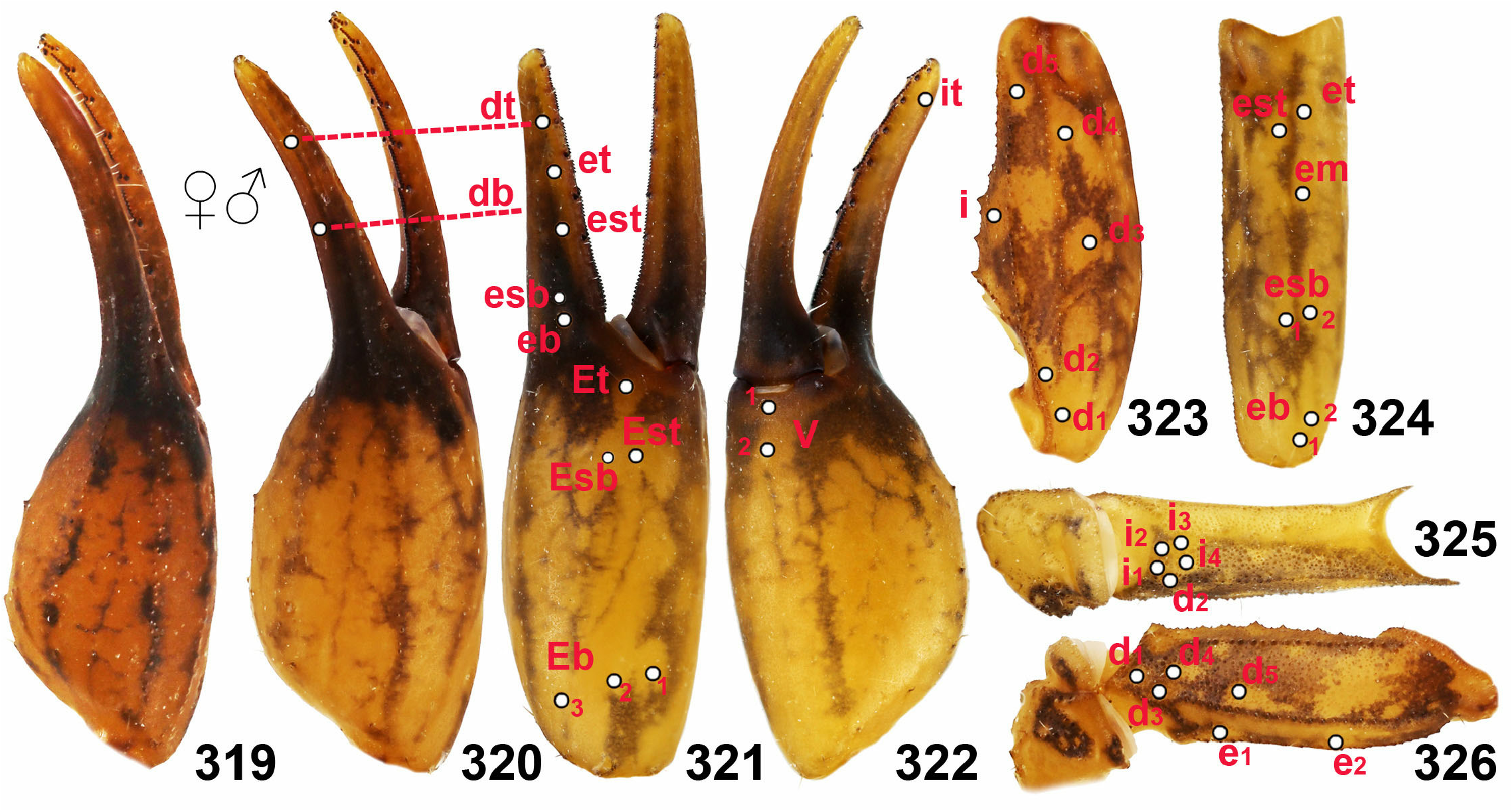
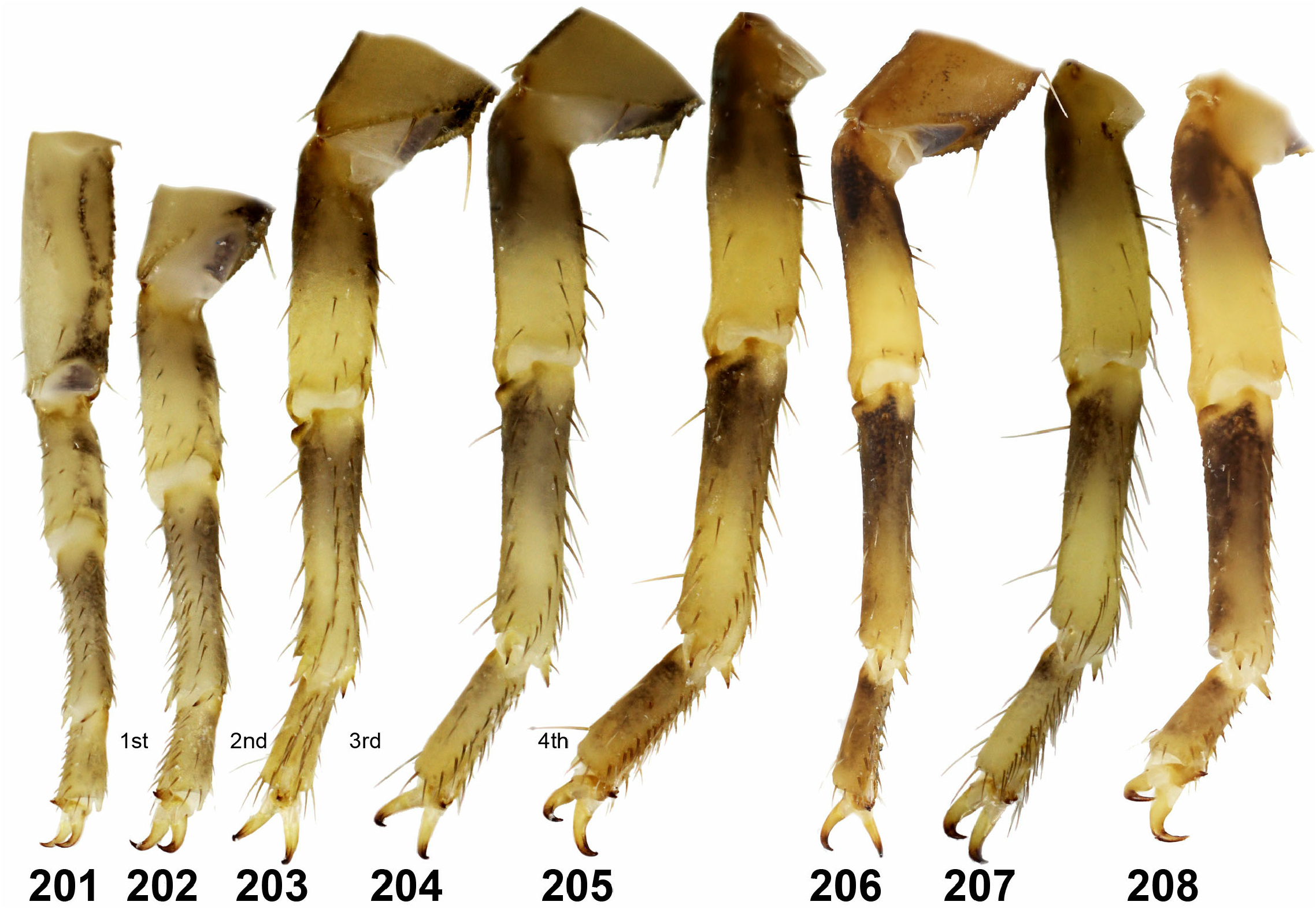
8. Chelal trichobothrium db located between dt and et. Fixed fingers of pedipalps with six rows of granules and six external and internal granules ( Figs. 252–253 View Figures 241–259 ). Tarsomeres II of leg IV with two rows of dense setae ( Figs. 196–197 View Figures 193–200 ). ........ Isometrus Ehrenberg, 1828 View in CoL ….. 9
– Chelal trichobothrium db located between et and est ( Fig. 321 View Figures 319–326 ). Fixed fingers of pedipalps with seven rows of granules and six external and seven internal granules ( Figs. 254–259 View Figures 241–259 ). Tarsomeres II of leg IV with two rows of no more than 20 spiniform setae ( Figs. 199 View Figures 193–200 , 201– 208 View Figures 201–208 ). ............ Reddyanus Vachon, 1972 View in CoL stat. n. ….... 10
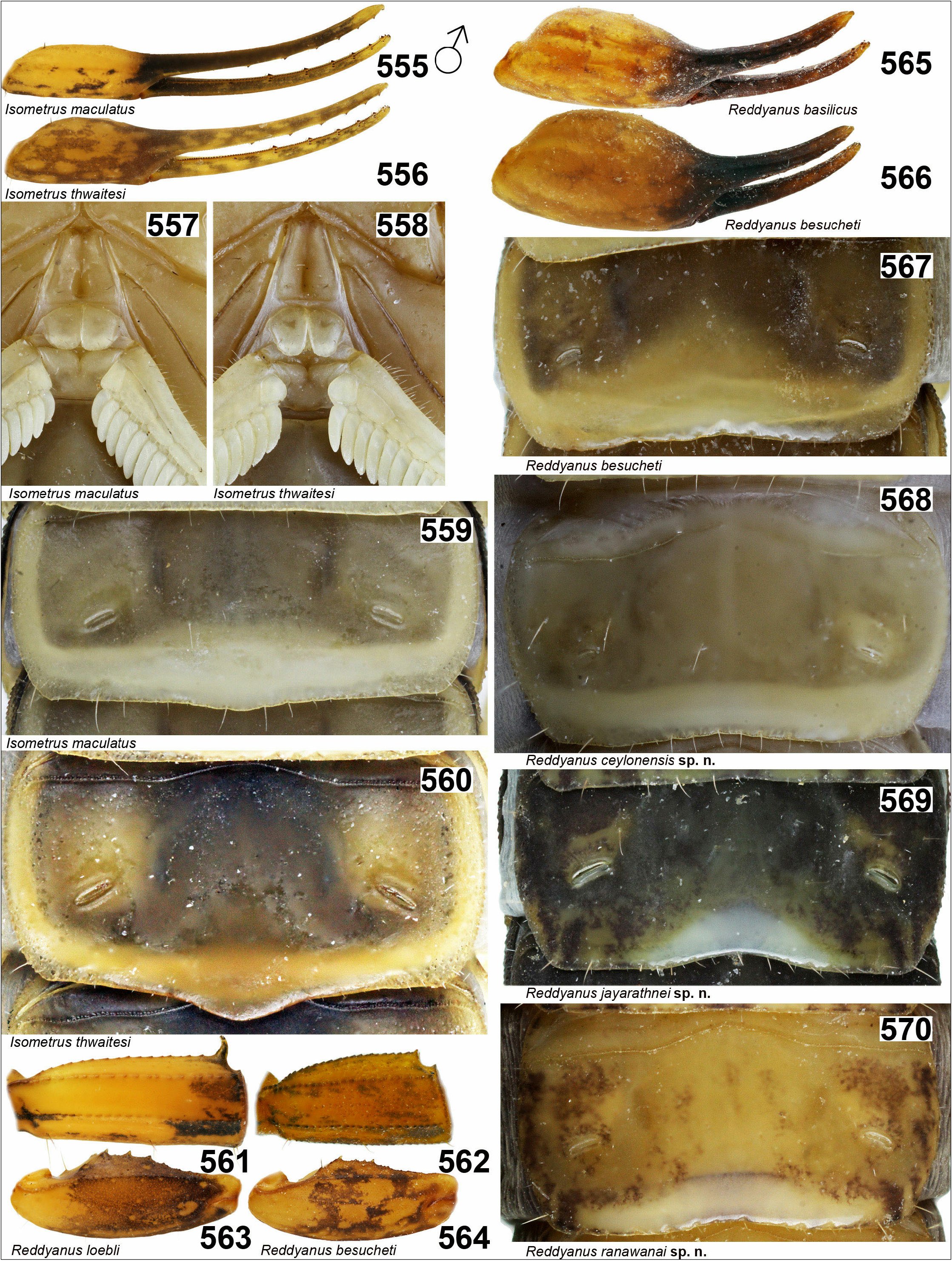
9. First (basal) middle lamella of pectine in both sexes rounded ( Fig. 558 View Figures 555–570 ). Fingers and manus of pedipalp chela the same color, spotted ( Fig. 556 View Figures 555–570 ). Posterior margin of sternite V strongly convex medially ( Fig. 560 View Figures 555–570 ) …….. ….....………................……… I. thwaitesi Pocock, 1897 View in CoL
– First (basal) middle lamella of pectine in both sexes quadrangular ( Fig. 557 View Figures 555–570 ). Manus of pedipalp yellow with several spots, fingers dark ( Fig. 555 View Figures 555–570 ). Posterior margin of sternite V almost straight to very slightly convex medially ( Fig. 559 View Figures 555–570 ). …..... I. maculatus ( De Geer, 1778) View in CoL
10. Terminal tubercle of dorsal carina on second and third metasomal segment of male markedly enlarged ( Fig. 561 View Figures 555–570 ). Pedipalp femur and patella spotted, patella mostly black ( Fig. 563 View Figures 555–570 ), femur mostly yellow. Subaculear tooth spinoid ( Figs. 417–418 View Figures 403–429 ). ..………...... …………………….. R. loebli ( Vachon, 1982) View in CoL comb. n.
– Terminal tubercle of dorsal carina on metasomal segments of male are not enlarged ( Fig. 562 View Figures 555–570 ). Pedipalps with brown spots, identical on femur and patella ( Fig. 564 View Figures 555–570 ). Subaculear tooth wide and rounded ( Figs. 409– 416, 419–420 View Figures 403–429 ). ….............……................................... 11
11. Glabrous zone on posterior part of fifth sternite present medially in male ( Figs. 569–570 View Figures 555–570 ). ………….. 12
– Glabrous zone along posterior margin of fifth sternite absent or indicated ( R. besucheti ) ( Figs. 567–568 View Figures 555–570 ). .... 13
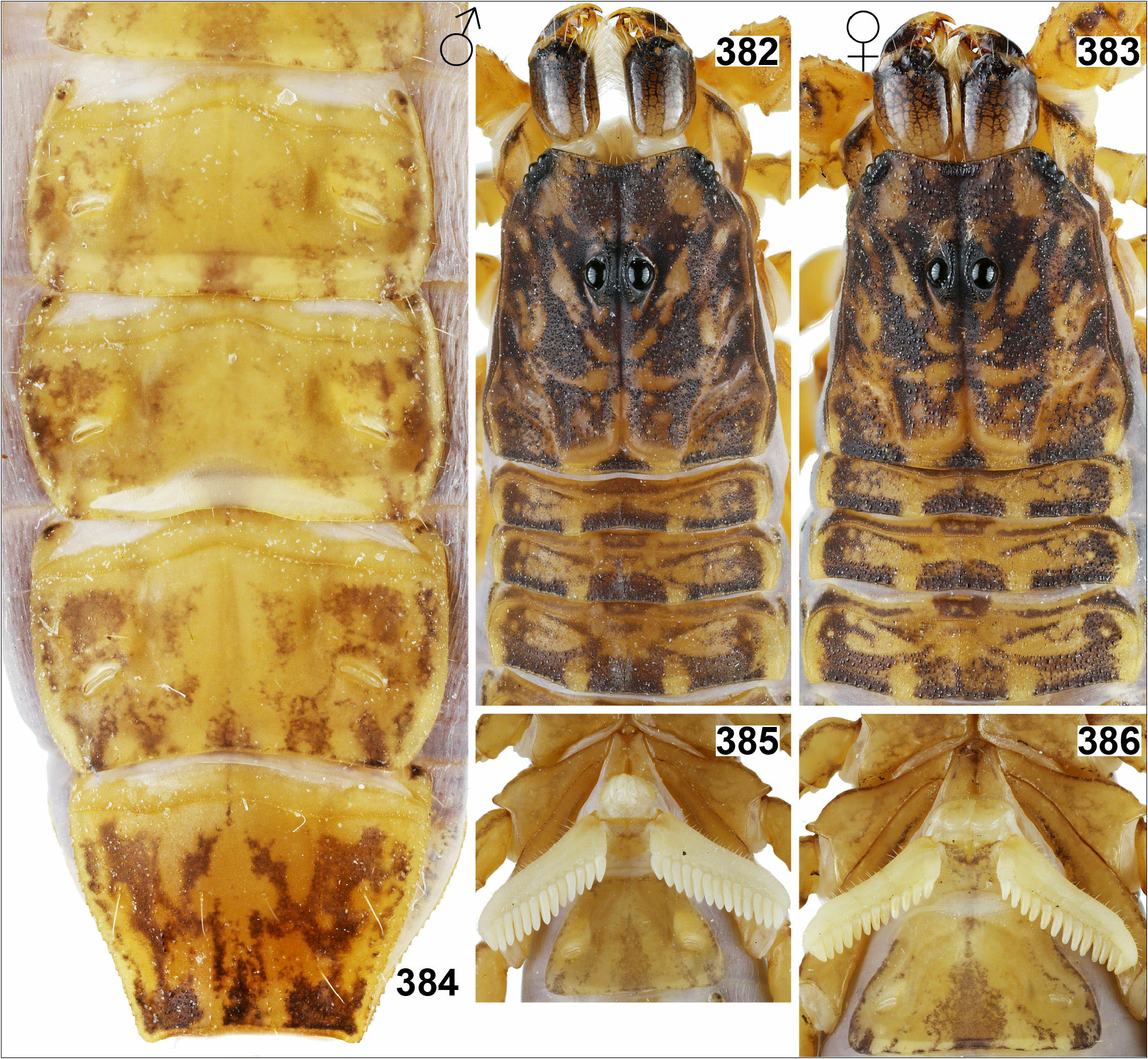
12. Whole mesosoma dark, almost black ( Figs. 338– 339 View Figures 338–348 ). Glabrous zone present in middle part of fifth sternite only ( Fig. 569 View Figures 555–570 ). ....……..... R. jayarathnei View in CoL sp. n.
– Mesosoma lighter, yellowish brown ( Figs. 382–383 View Figures 382–386 ). Glabrous zone stretches almost over whole posterior margin of fifth sternite ( Fig. 570 View Figures 555–570 ).... R. ranawanai View in CoL sp. n.
13. Manus of pedipalp chela wide in male. Ratio pedipalp chela length/width 2.94–3.28 ( Fig. 566 View Figures 555–570 , Table 5 View Table 5 ). Chela wider in male than in female. .......…........... 14
– Manus of pedipalp chela narrow in male. Ratio pedipalp chela length/width 3.41–3.79 ( Fig. 565 View Figures 555–570 , Table 5 View Table 5 ). Chela the same width in both sexes. .................... ………......……… R. basilicus ( Karsch, 1879) View in CoL comb. n.
14. Ratio metasomal segment II length/width 1.56–1.79 in male ( Fig. 211 View Figures 209–224 , Table 5 View Table 5 ). ............................…........... ………...………. R. besucheti ( Vachon, 1982) comb. n.
– Ratio metasomal segment II length/width 1.85–1.97 in male ( Fig. 213 View Figures 209–224 , Table 5 View Table 5 ). ……….... R. ceylonensis View in CoL sp. n.
15. Adults 128–176 mm long. Pectinal teeth number 16– 20. Fifth segment of metasoma longer than pedipalp femur, fourth segment of metasoma about as long as pedipalp femur ..…........ H. swammerdami Simon, 1872
– Adults 75–130 mm long. Pectinal teeth number 10–16. Fifth segment of metasoma about as long as pedipalp femur, fourth segment of metasoma shorter than pedipalp femur. .....…………………………………….... 16
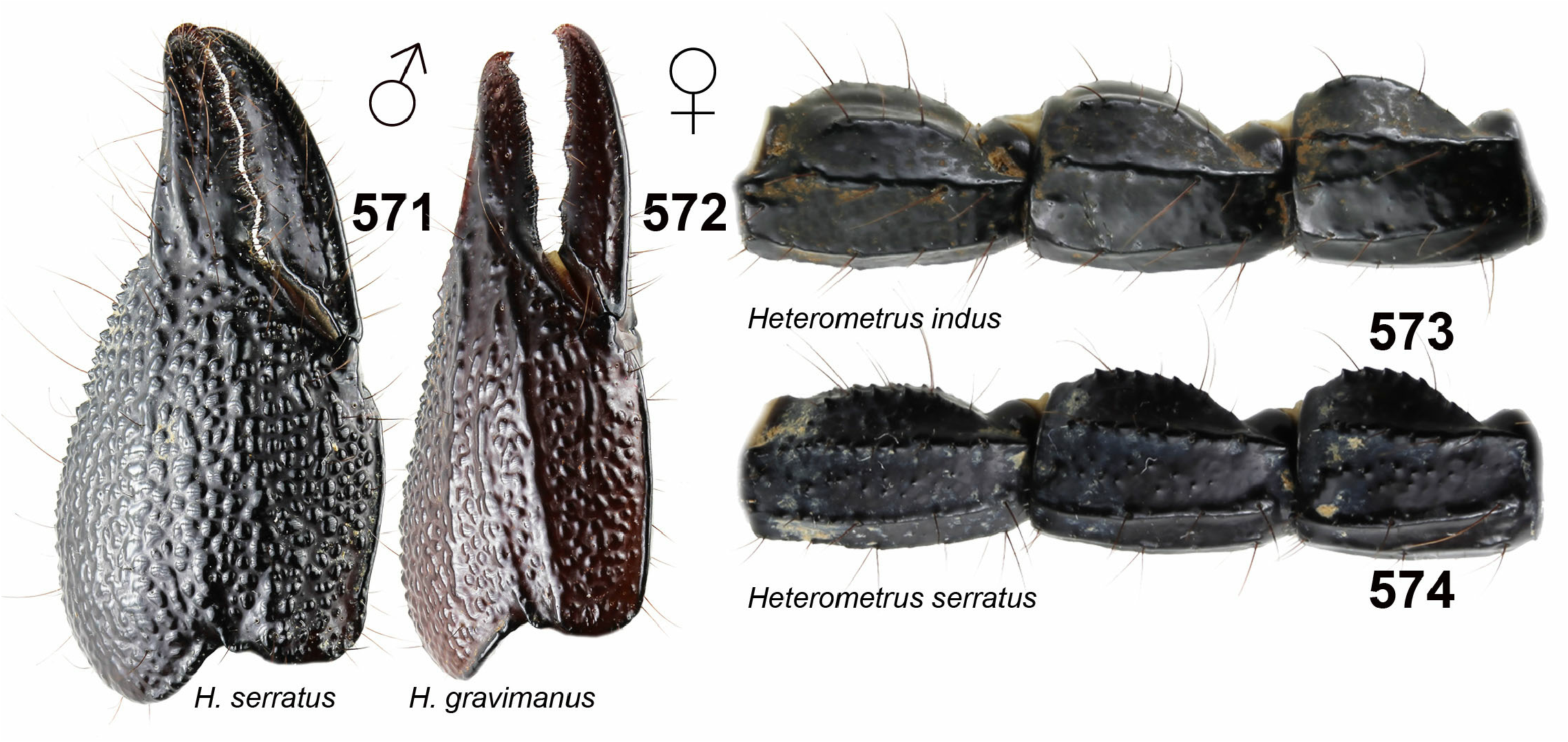
16. Pedipalp chela with carinae on dorsoexternal surface ( Fig. 572 View Figures 571–574 )..………......... H. gravimanus ( Pocock, 1894)
– Pedipalp chela without carinae on dorsoexternal surface ( Fig. 571 View Figures 571–574 ). .......…………..................................... 17
17. Dorsal and dorsolateral carinae of metasomal segments smooth ( Fig. 573 View Figures 571–574 ). ….. H. indus ( De Geer, 1778)
– Dorsal and dorsolateral carinae of metasomal segments granulated ( Fig. 574 View Figures 571–574 ). ..….... H. serratus ( Pocock, 1900)
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |