Smeringopina bioko
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3713.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:C5F0BC11-92C0-4B30-9DB3-200882AC8950 |
|
DOI |
https://doi.org/10.5281/zenodo.6161957 |
|
persistent identifier |
https://treatment.plazi.org/id/B20287ED-FFE1-FF93-B990-C0D1FCB23DED |
|
treatment provided by |
Plazi |
|
scientific name |
Smeringopina bioko |
| status |
|
Smeringopina Kraus, 1957 View in CoL View at ENA
Smeringopina Kraus 1957: 233 ; type species by original designation: Smeringopina beninensis Kraus, 1957 .
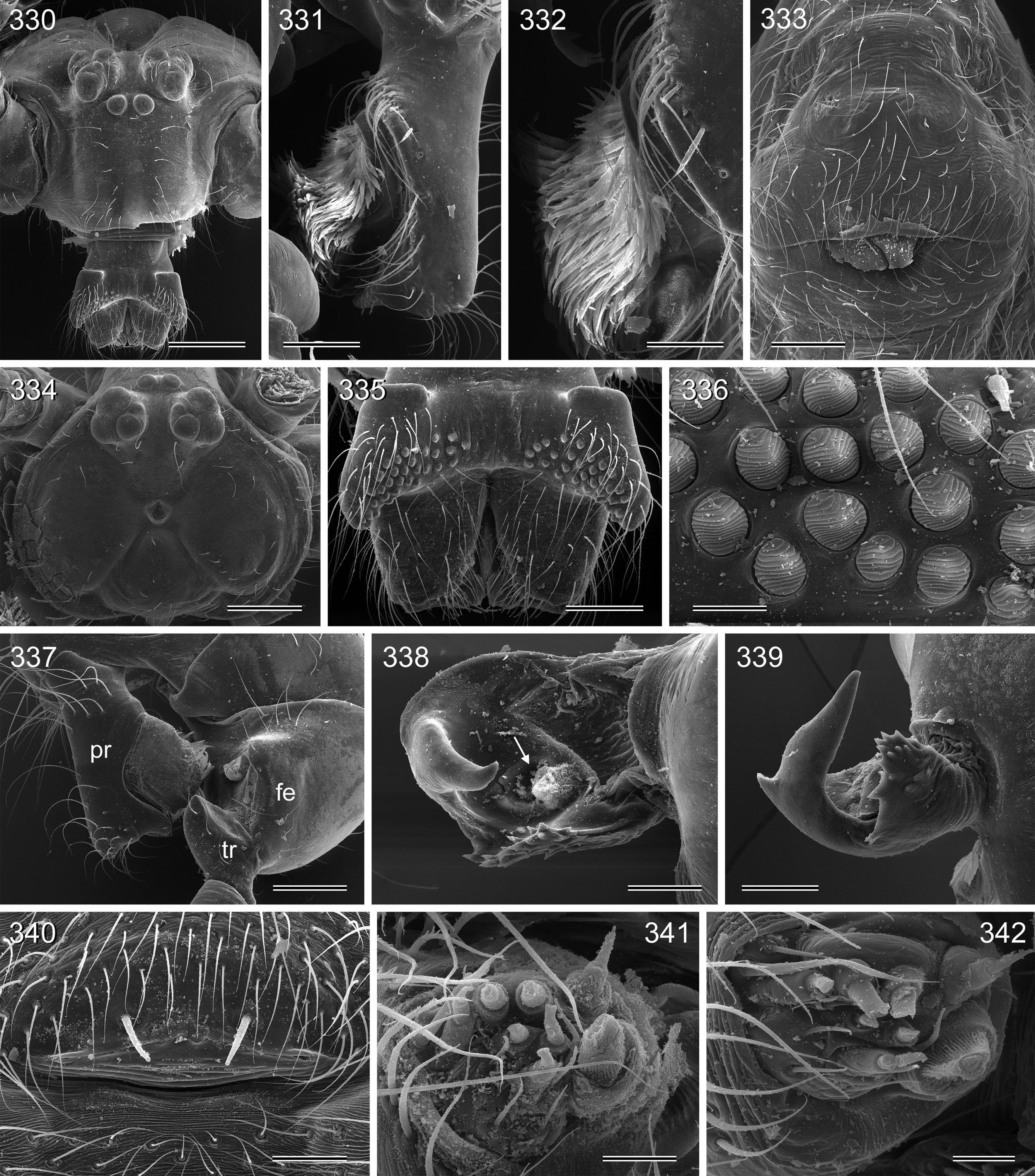
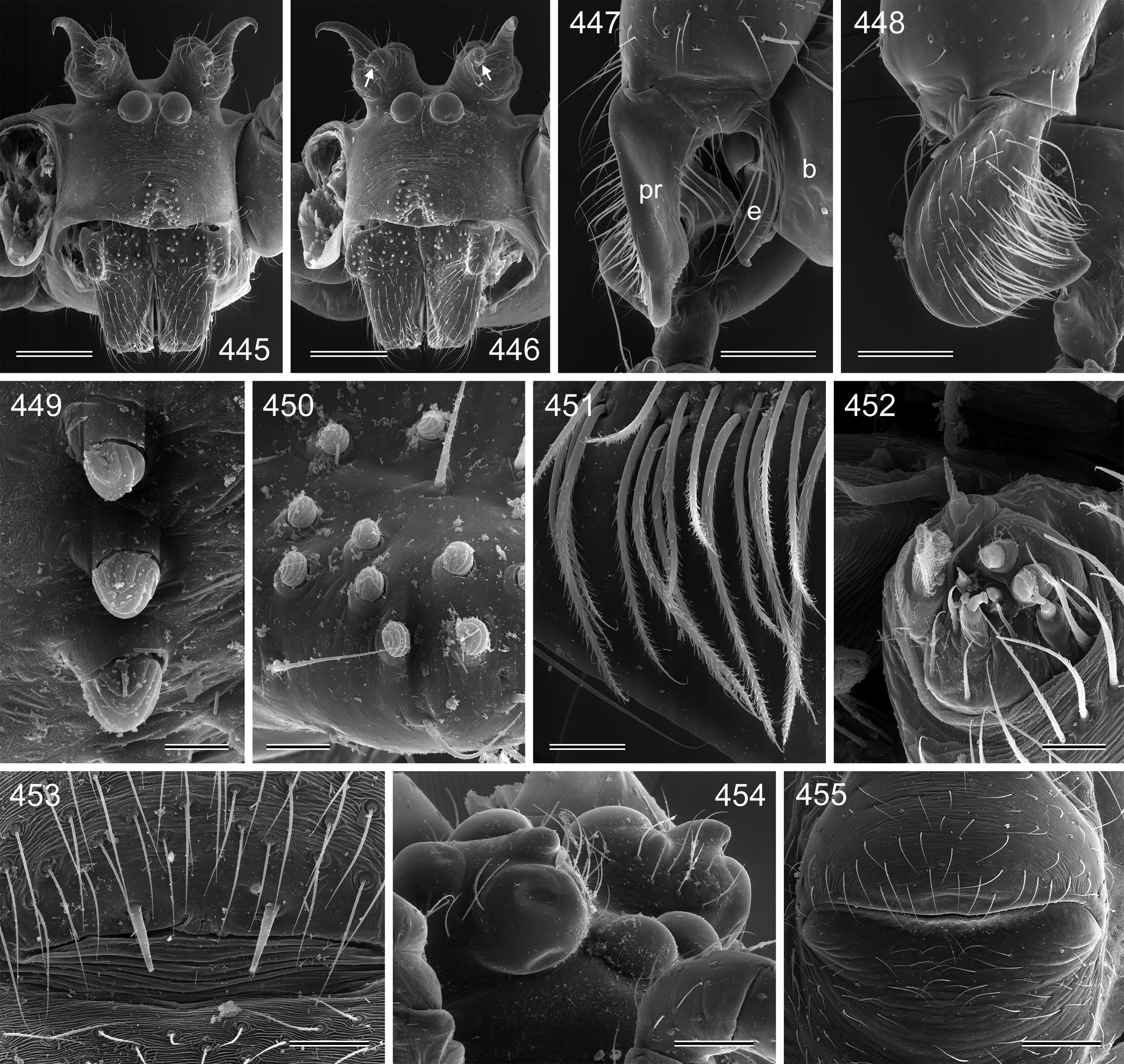
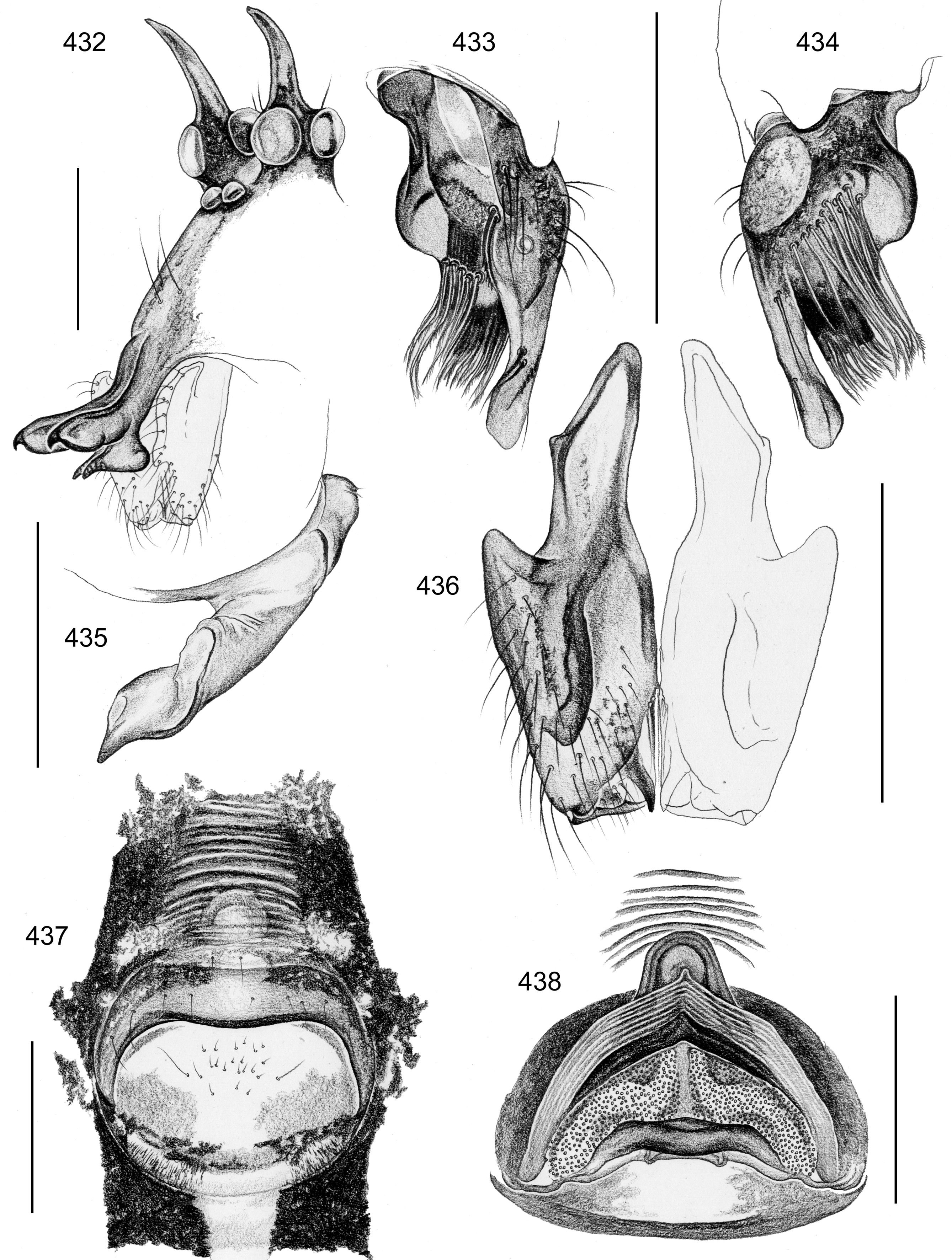
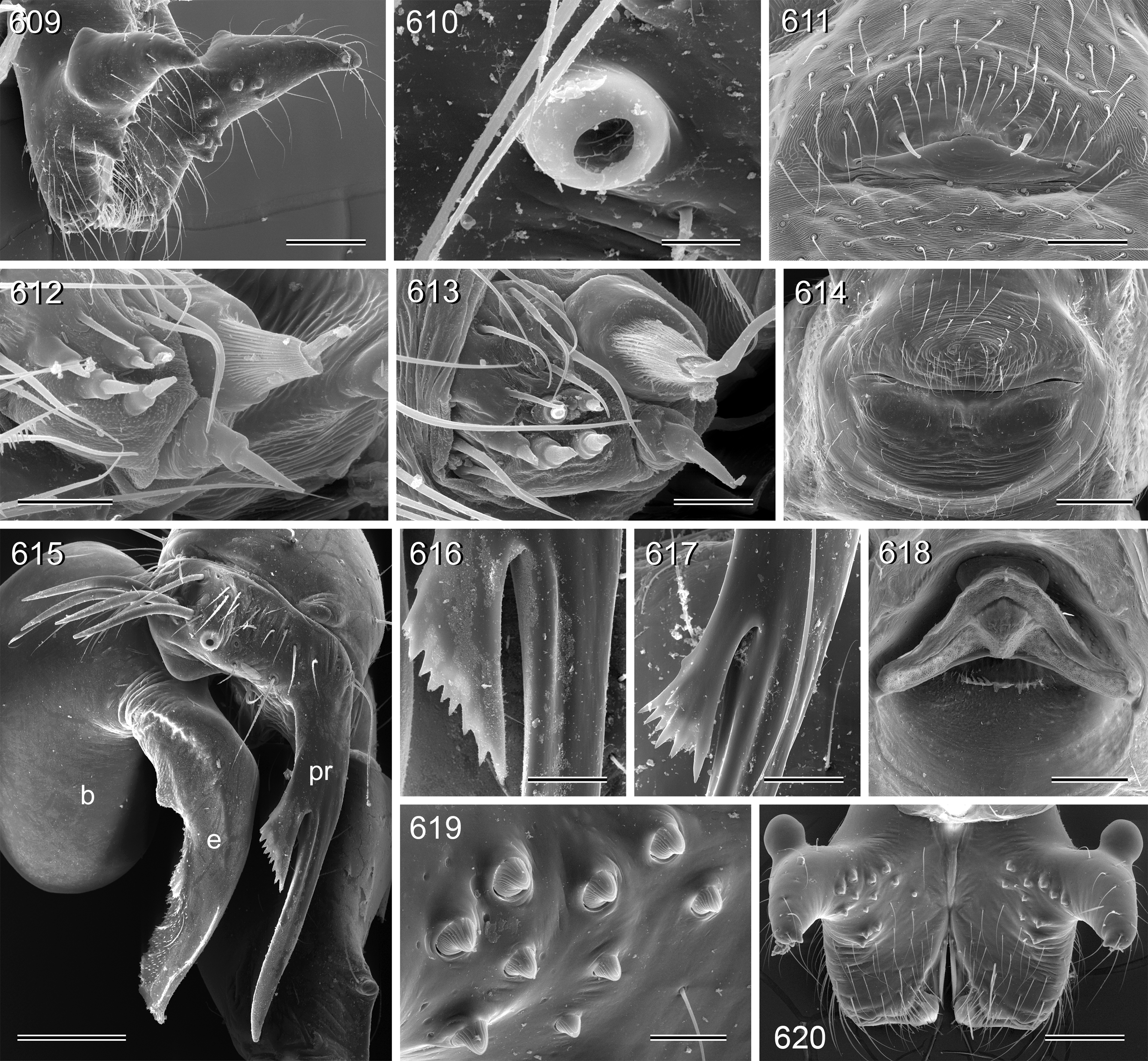
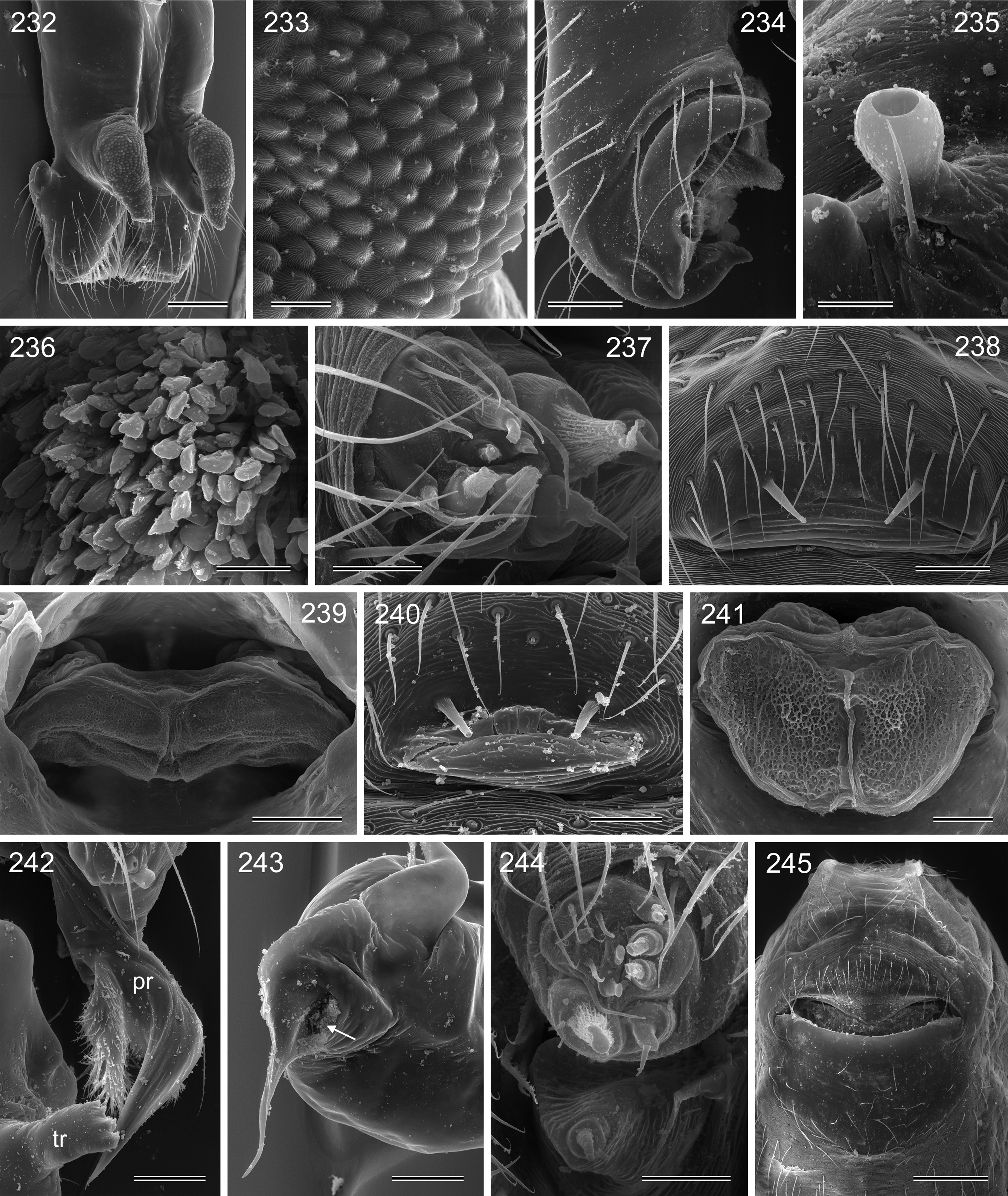
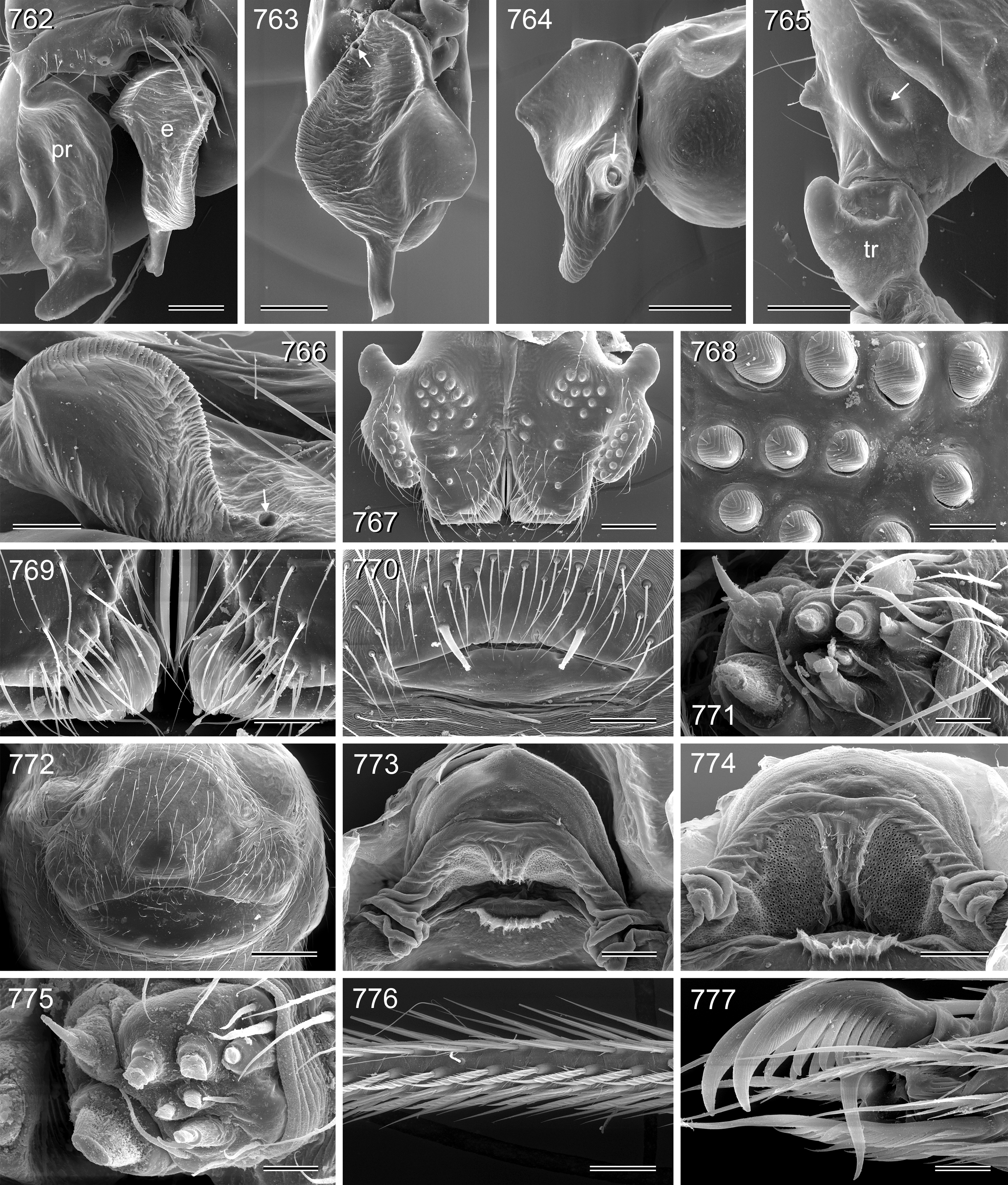
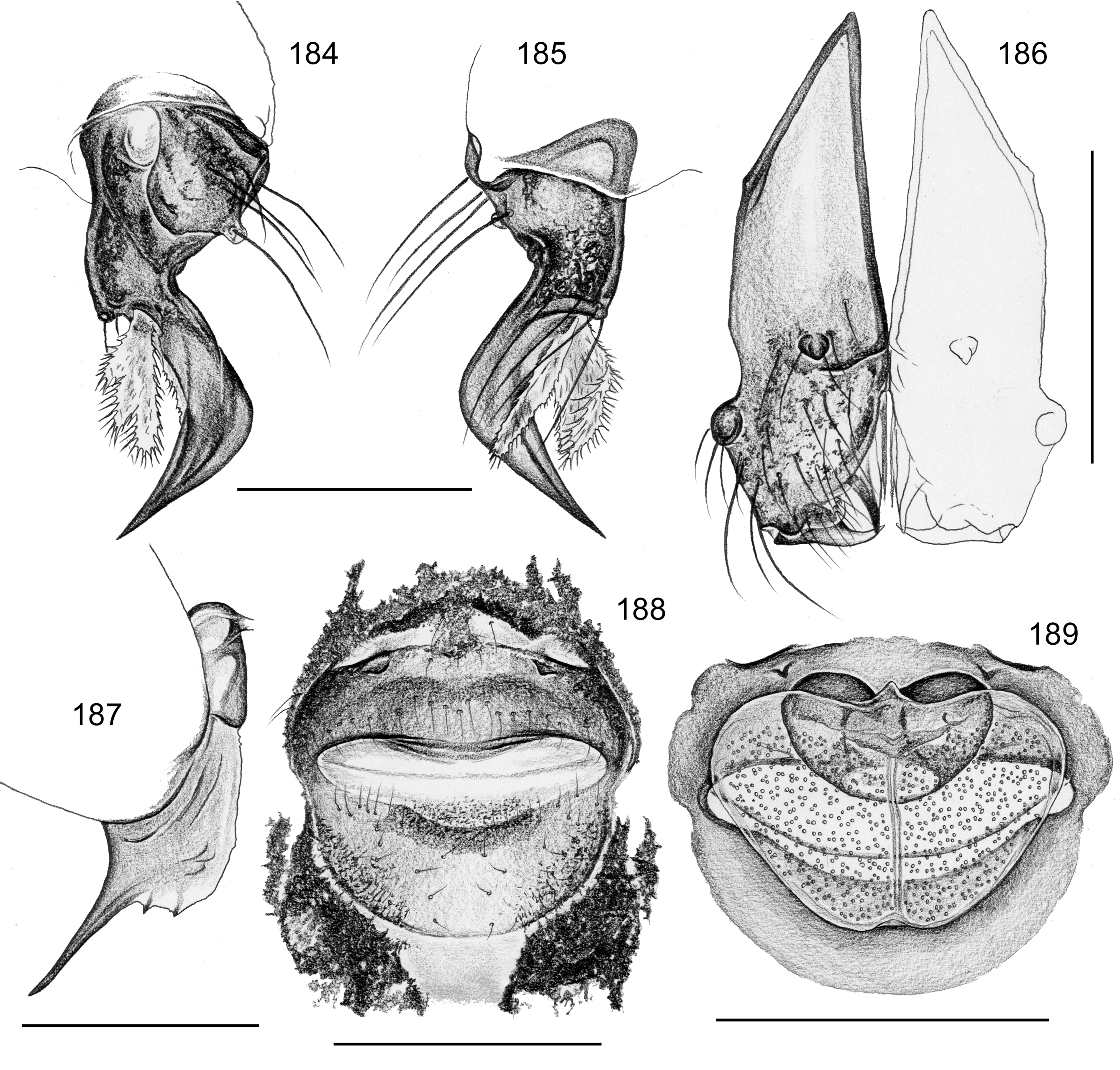
Diagnosis. Small to large pholcids (body length about 2.5–10) with oval to elongate abdomen ( Figs. 2–31 View FIGURES 2 – 16 View FIGURES 17 – 31 ), deep thoracic pit ( Figs. 65 View FIGURES 63 – 75 , 330, 334 View FIGURES 330 – 342 , 726 View FIGURES 725 – 737 ), and long legs (leg 1 about 7–12 x body length). Distinguished from other Smeringopinae genera by presence of proximal lateral apophyses on male chelicerae (e.g. Figs. 59 View FIGURES 56 – 62 , 380 View FIGURES 378 – 383 , 782 View FIGURES 778 – 784 ) and by presence of ventral or retrolateral apophysis on male palpal trochanter; from most Smeringopinae (except Smeringopus ) also by male gonopore with only two epiandrous spigots (instead of four; e.g. Figs. 72 View FIGURES 63 – 75 , 219 View FIGURES 211 – 224 , 453 View FIGURES 445 – 455 , 736 View FIGURES 725 – 737 ) and by absence of stridulatory ridges on chelicerae; from most Smeringopinae (except Smeringopus and Cenemus ) also by absence of spines on male femora; from most species groups of its putative sister genus Smeringopus also by male palpal femur without deep retrolateral furrow (cf. figs. 344, 411 in Huber 2012), and legs without curved hairs on tibiae and metatarsi.
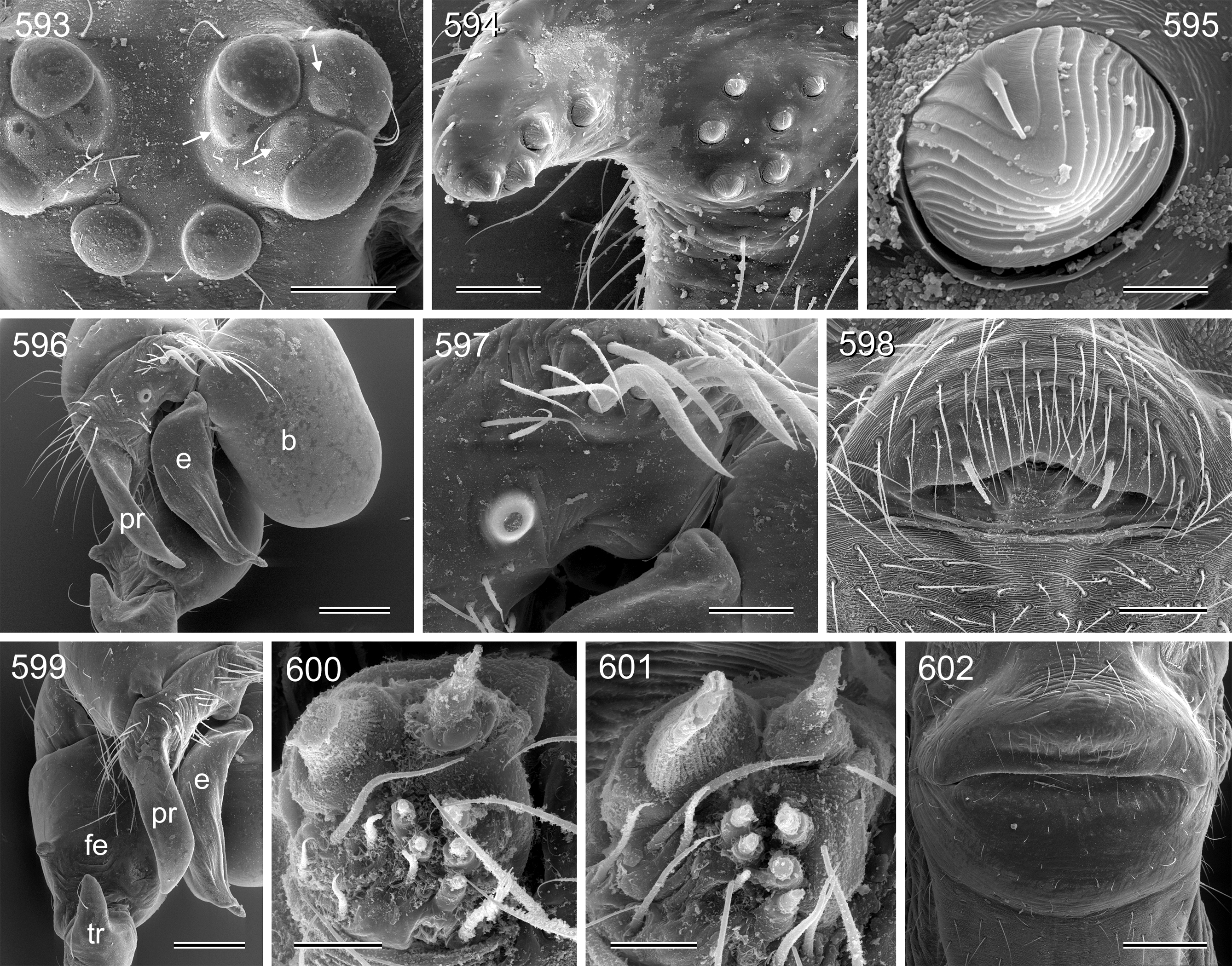
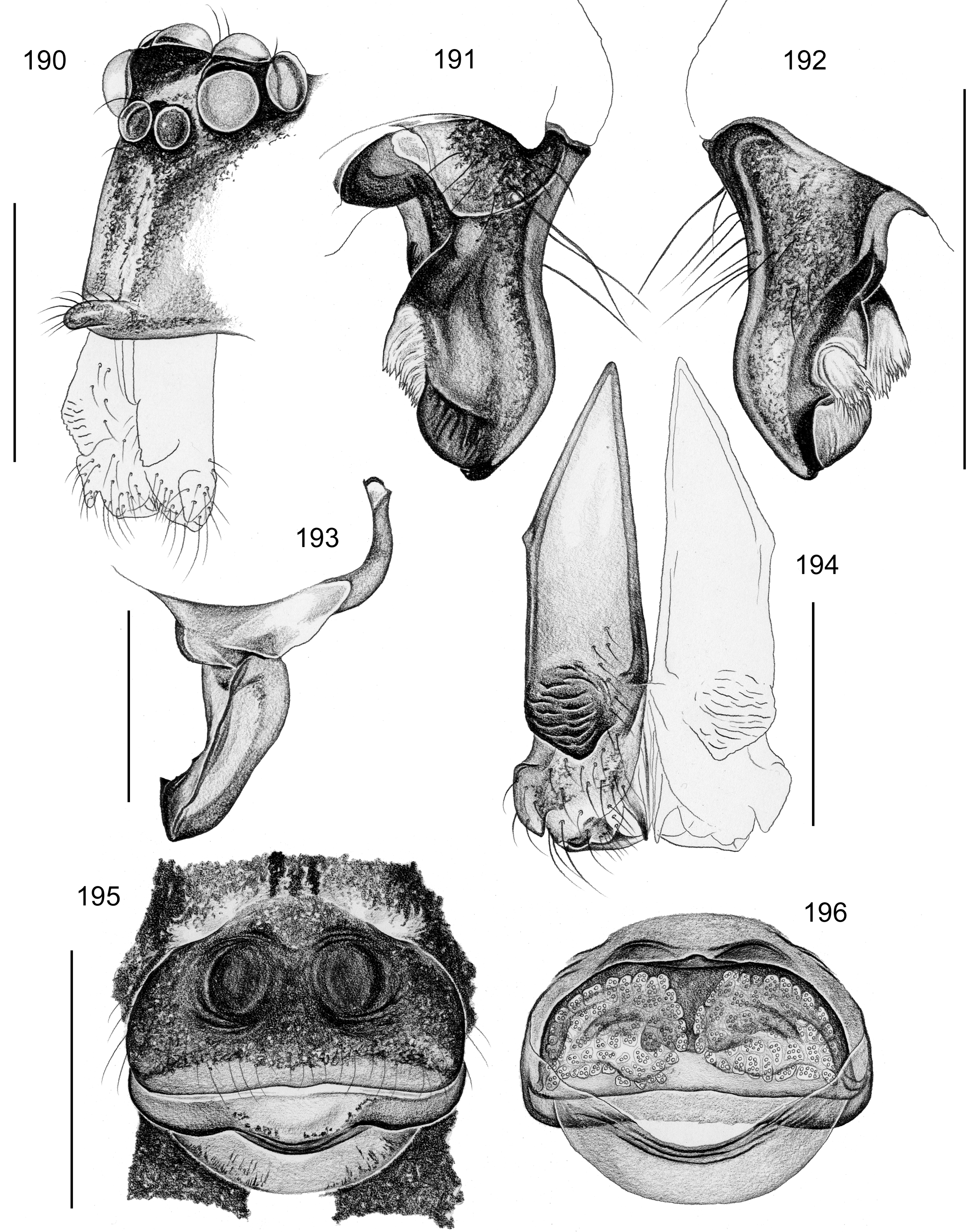
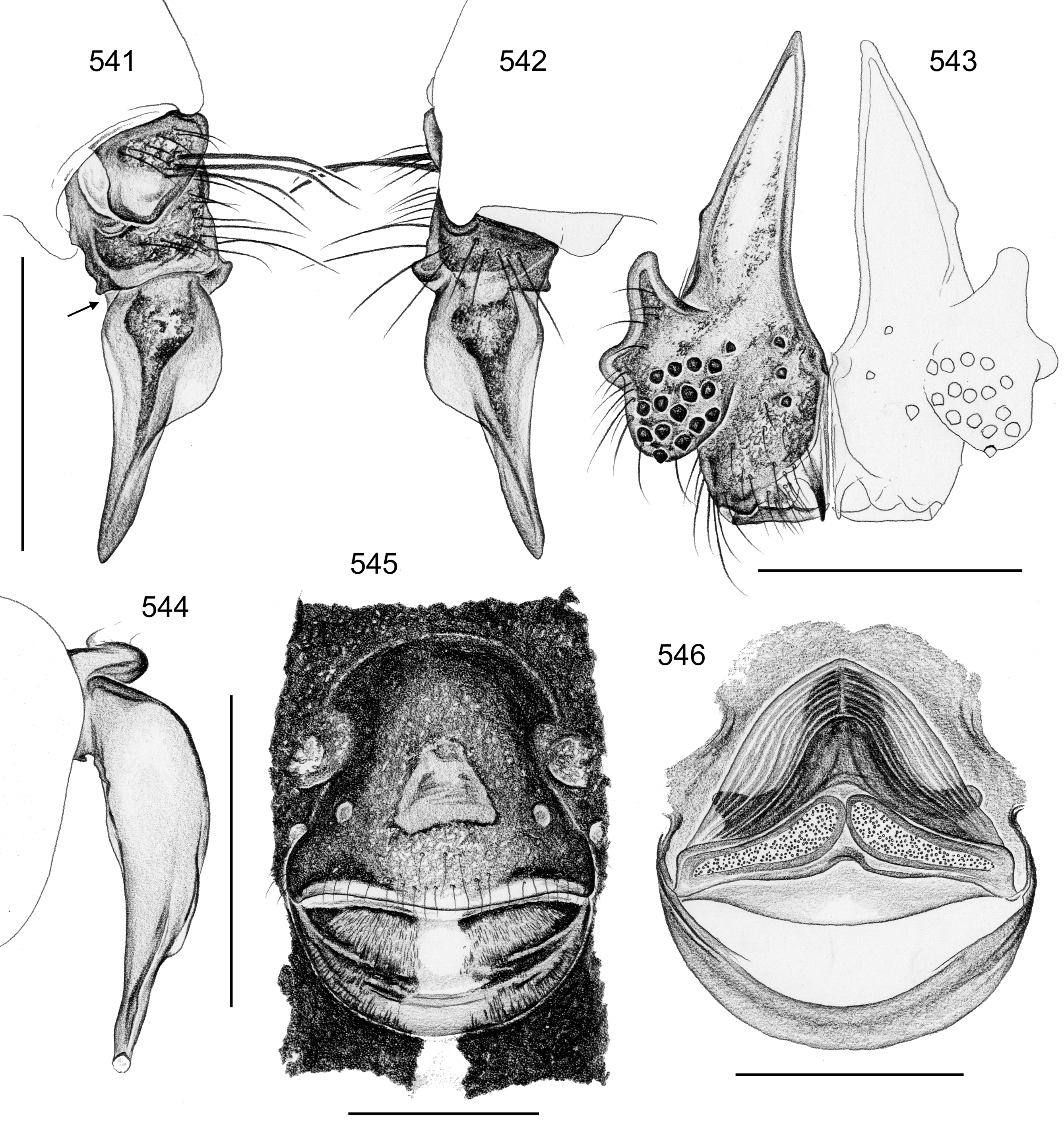
Description. Male: Total body length 2.4–10.2 (usually ~3–8); carapace width 0.9–2.1. Carapace with deep pit and pair of shallow furrows diverging toward posterior rim; ocular area weakly raised, eye triads relatively close together (distance PME-PME about 0.8–1.8 x PME diameter), each secondary eye accompanied by more or less distinct elevation ( Figs. 67 View FIGURES 63 – 75 , 87 View FIGURES 83 – 95 , 593 View FIGURES 593 – 602 ; ‘pseudo-lenses’; cf. Huber 2009), AME relatively large, in low position. Clypeus high, either unmodified or with various kinds of simple to highly complex modifications (modified hairs, median or paired projections; e.g. Figs. 190 View FIGURES 190 – 196 , 211 View FIGURES 211 – 224 , 246 View FIGURES 246 – 252 , 271 View FIGURES 271 – 280 , 427 View FIGURES 427 – 431 , 432 View FIGURES 432 – 438 , 446 View FIGURES 445 – 455 , 725 View FIGURES 725 – 737 , 778 View FIGURES 778 – 784 , 850 View FIGURES 850 – 855 ). Chelicerae never with stridulatory ridges, usually with pair of proximal lateral apophyses (shifted to a more distal position in the lekoni group), often with modified hairs on distal cheliceral apophyses and/or frontal cheliceral face (e.g., Figs. 68 View FIGURES 63 – 75 , 335– 336 View FIGURES 330 – 342 , 446 View FIGURES 445 – 455 , 619 View FIGURES 609 – 620 , 733–735 View FIGURES 725 – 737 ). Palpal coxa with or without retrolateral hump or apophysis; trochanter with ventral or retrolateral apophysis; femur rarely simple, usually with various kinds of modifications (e.g., ventral rim, ventral pocket, retrolateral apophysis, retrolateral flap bordering whitish ventral area, distal ventral projection), without deep retrolateral furrow; prolateral femur-patella joint often strongly shifted toward ventrally (e.g. Figs. 127, 130 View FIGURES 125 – 132 ); palpal tarsus sometimes with dorsal macrotrichia ( Figs. 476 View FIGURES 469 – 478 , 481, 488 View FIGURES 479 – 488 , 493 View FIGURES 489 – 498 , 597 View FIGURES 593 – 602 , 615 View FIGURES 609 – 620 ), palpal tarsal organ capsulate ( Figs. 69 View FIGURES 63 – 75 , 216 View FIGURES 211 – 224 , 235 View FIGURES 232 – 245 , 597 View FIGURES 593 – 602 , 610 View FIGURES 609 – 620 ); procursus very variable from extremely simple to highly complex, sometimes with hinge dividing proximal from distal part ( Figs. 541 View FIGURES 541 – 546 , 785), tip usually with sclerotized and membranous structures; bulb with only one process, sperm duct either traversing this process completely (embolus; e.g. Figs. 728 View FIGURES 725 – 737 , 764 View FIGURES 762 – 777 ) partly (e.g. Fig. 338 View FIGURES 330 – 342 ) or opening at basis of process (e.g. Figs. 218 View FIGURES 211 – 224 , 243 View FIGURES 232 – 245 ).
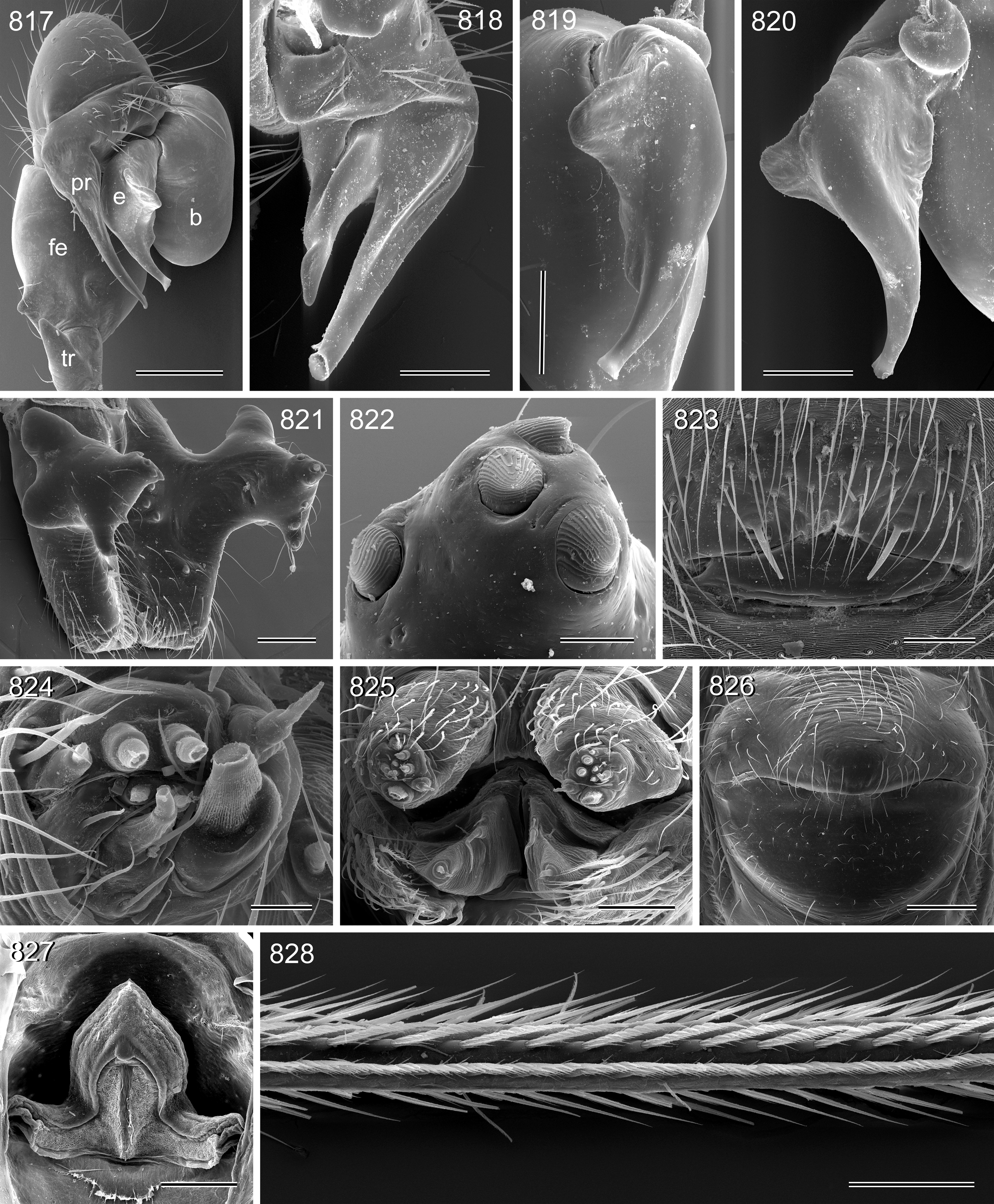
Legs long and thin, leg 1 length 17–91 (usually ~20–85), tibia 1 length 4.2–20.9 (usually ~8–20), tibia 2/tibia 4 length 0.8–1.2 (tibia 2 tends to be relatively longer in large space-dwelling species; tibia 4 relatively longer in small litter-dwelling species). Tibia 1 L/d usually ~65–115, lower only in the two representatives of the attuleh group (55). Legs without spines on femora, without curved hairs on tibiae and metatarsi, retrolateral trichobothrium very proximal (at 1.0–2.5%), prolateral trichobothrium always present (also on tibiae 1). Tarsal pseudosegments very indistinct, apparently never regular rings but rather indistinct irregular rings or platelets ( Figs. 94 View FIGURES 83 – 95 , 828 View FIGURES 817 – 828 ). Tarsus 4 with two rows of prolatero-ventral comb-hairs ( Figs. 93–95 View FIGURES 83 – 95 , 828 View FIGURES 817 – 828 ).
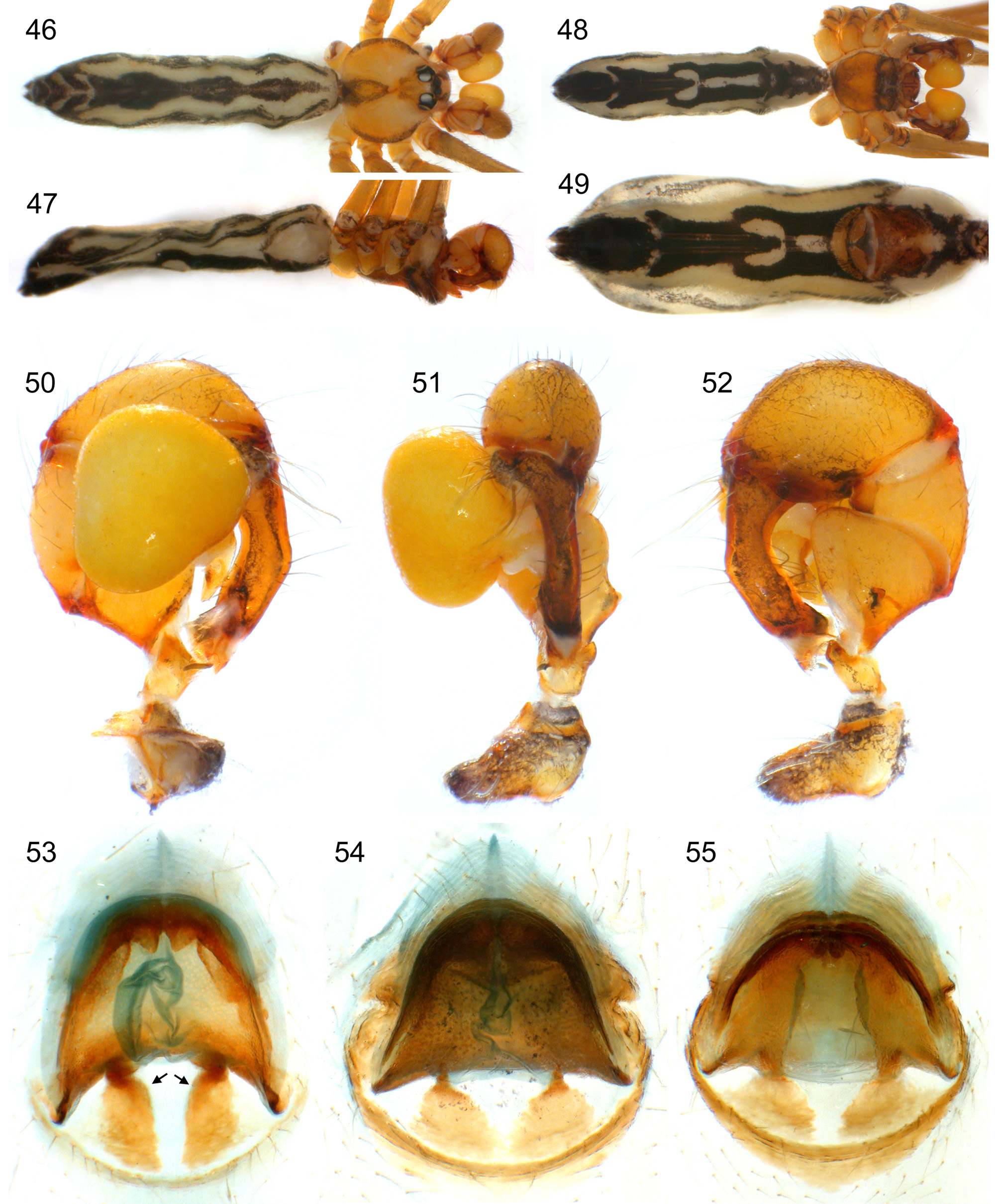
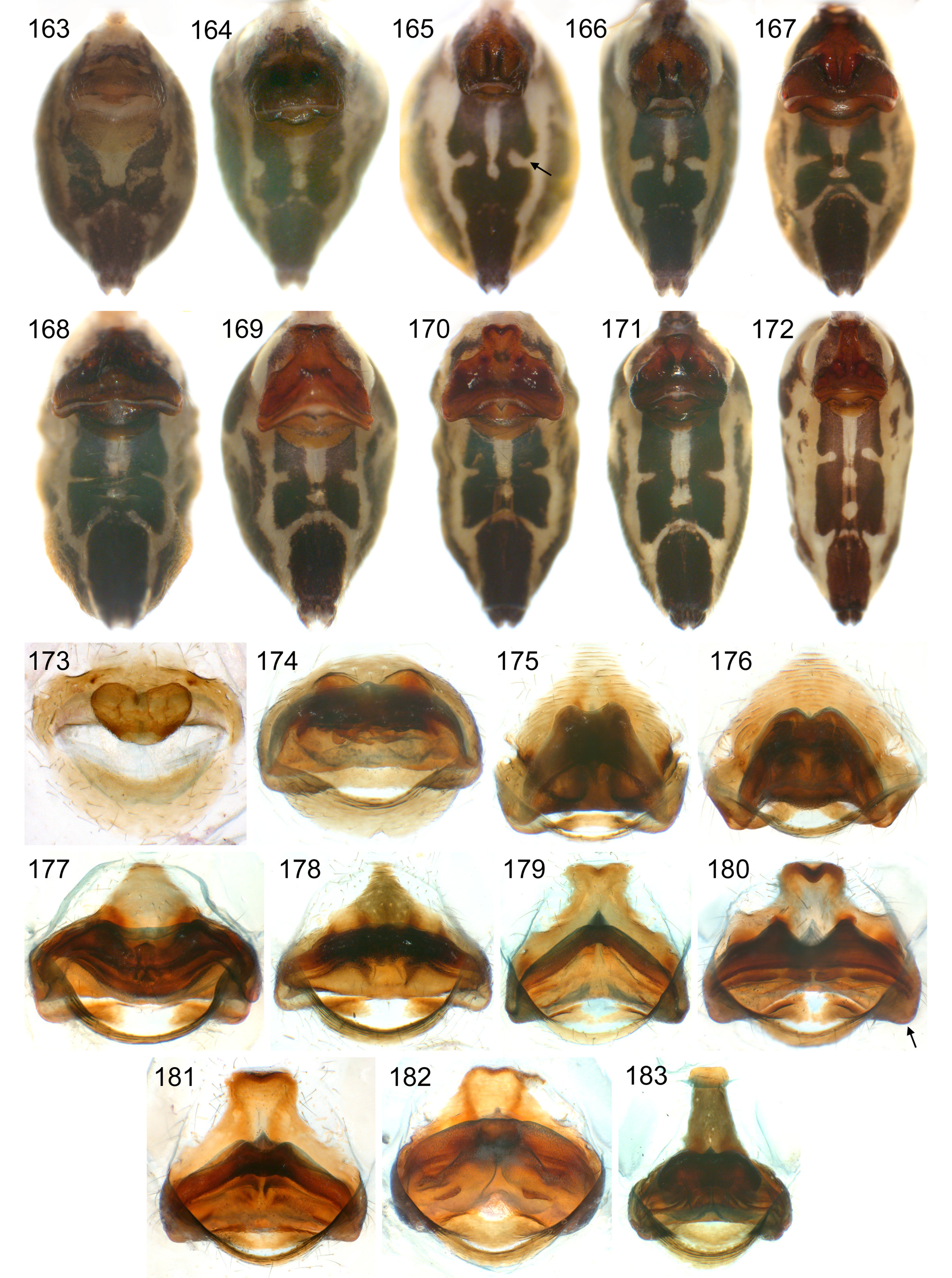
Abdomen oval to cylindrical, posteriorly pointed at spinnerets, never elevated above spinnerets, usually with distinct dark pattern dorsally, oblique lines or marks laterally, and distinctive ventral pattern (e.g., Figs. 46–49 View FIGURES 46 – 55 , 163–172 View FIGURES 163 – 183 , 678–693 View FIGURES 678 – 693 ). Male gonopore always with only two spigots (e.g. Figs. 72 View FIGURES 63 – 75 , 219 View FIGURES 211 – 224 , 453 View FIGURES 445 – 455 , 736 View FIGURES 725 – 737 ), each ALS with large widened spigot, pointed spigot, and 5–6 cylindrically shaped spigots (e.g. Figs. 71 View FIGURES 63 – 75 , 244 View FIGURES 232 – 245 , 278 View FIGURES 271 – 280 , 566 View FIGURES 559 – 568 ); PMS with two spigots each ( Fig. 566 View FIGURES 559 – 568 ).
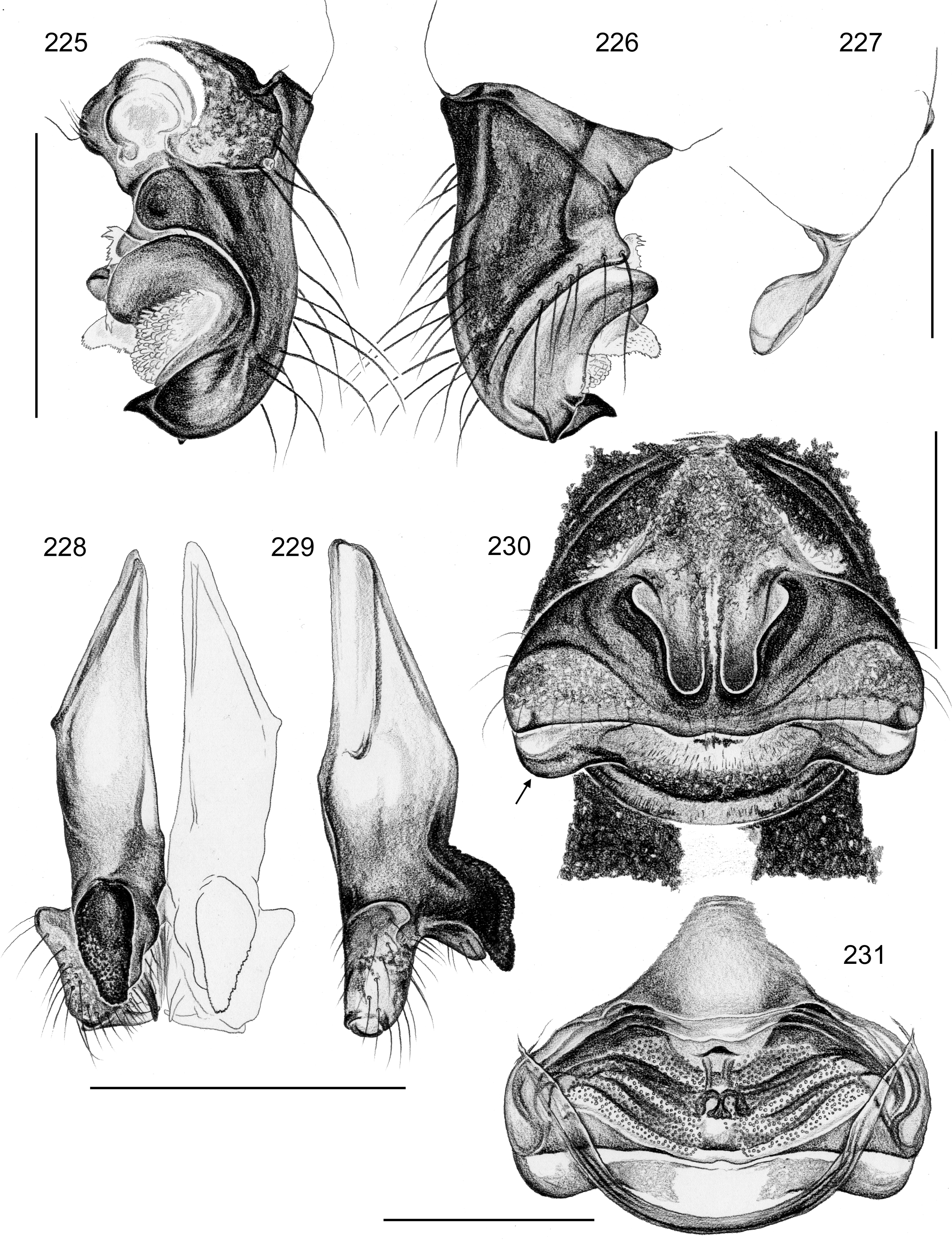
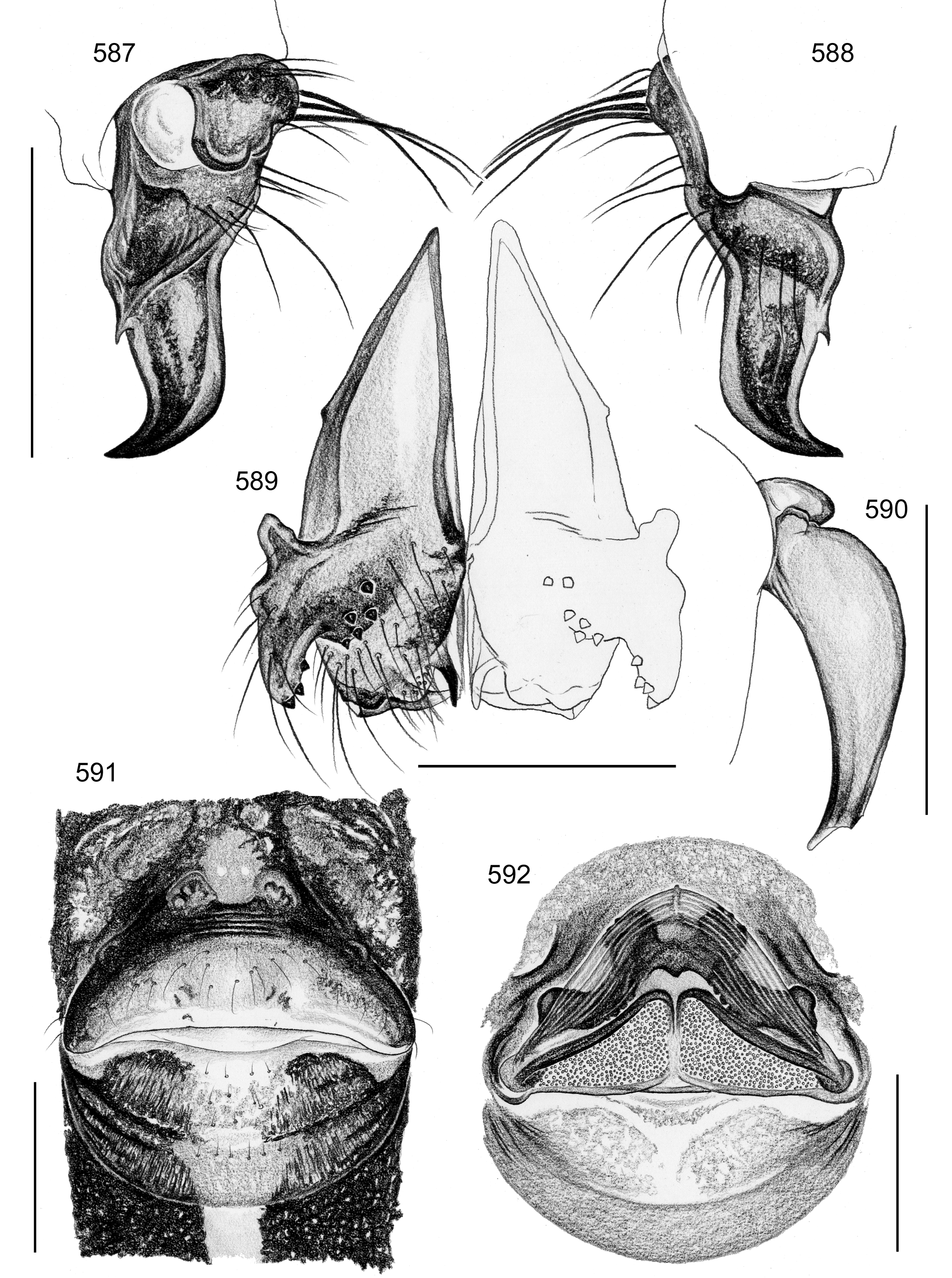
Female usually very similar to male, clypeus and chelicerae unmodified, ocular area unmodified or with much smaller horns than male ( Fig. 454 View FIGURES 445 – 455 ); legs usually slightly shorter than in male. Epigynum usually consisting of large, simple anterior plate and relatively large posterior plate; anterior plate sometimes with simple paired or unpaired projections ( Figs. 206 View FIGURES 205 – 210 , 520 View FIGURES 509 – 524 , 689 View FIGURES 678 – 693 ), rarely with pair of pockets ( Fig. 230 View FIGURES 225 – 231 ). Internal genitalia with frontal valve that is sometimes hidden behind other structures in dorsal view; pores of pore plates mostly homogeneously distributed ( Figs. 62 View FIGURES 56 – 62 , 189 View FIGURES 184 – 189 , 592 View FIGURES 587 – 592 ), rarely in groups ( Figs. 196 View FIGURES 190 – 196 , 210 View FIGURES 205 – 210 ).
Monophyly. The cladistic analysis identifies four synapomorphies for Smeringopina : (1) abdomen ventrally with one pair of dark lines (char. 7); (2) male chelicerae with lateral apophyses directed toward proximally (char. 17); (3) presence of modified hairs on male distal lateral apophyses (char. 23); (4) male palpal trochanter with ventral to retrolateral apophysis (char. 31). The first character is problematic (see Appendix 3). The second and fourth characters may be functionally related (Huber 1995, 2002; Uhl et al. 1995). Preliminary analyses of molecular data that included representatives of four species groups ( guineensis group; attuleh group; cornigera group; beninensis group) and several representatives of Smeringopus , mostly resolved Smeringopina as monophyletic (Dimitrov, Astrin & Huber 2013).
Generic relationships. The present analysis supports the close relationship between Smeringopus and Smeringopina proposed in Huber (2012) and confirmed by molecular data (Dimitrov, Astrin & Huber 2013). Since the characters considered informative among Smeringopinae genera are basically the same as those in Huber (2012), relationships among genera are unchanged and not further discussed here.
Specific relationships. Based on the cladistic analyses above, Smeringopina is here divided into nine operational species groups, two of them monospecific. Most species groups appear well supported by synapomorphies, but relationships among species groups are partly poorly supported and dubious. In the descriptive section below, species are ordered according to species groups in the order used here (which in turn is derived from the cladogram in Fig. 1 View FIGURE 1 ).
1. guineensis group. This group includes the three West African ( Fig. 33 View FIGURE 33 ) species described by Millot (1941): S. guineensis , S. bineti , and S. pulchra . The monophyly of the group is strongly supported by several synapomorphies, some of them unique among Pholcidae : ventral abdominal pattern with light U-shaped element (char. 9); palpal femur with large round bulge ventro-distally (char. 38); procursus with ventral hairs curved around it retrolaterally and pointing toward dorsally (char. 48); procursus with sclerite embedded in dorso-distal membranes (char. 52); posterior epigynal plate with pair of sclerotized areas forming distinct anterior sclerite (char. 62); and internal female genitalia with globular structures between ‘valve’ and epigynal plate (char. 67).
2. lekoni group. This group includes nine new species ( S. kinguele ; S. mohoba ; S. fang ; S. tebe ; S. lekoni ; S. iboga ; S. moudouma ; S. ndjole ; S. kikongo ). Three characters support the entire group: proximal lateral cheliceral apophyses shifted to distal position (char. 18); absence of original pair of distal lateral apophyses (char. 22); and strong retrolateral apophysis on palpal femur bent toward ventrally (char. 35). S. kinguele is quite exceptional (shape of procursus; paired clypeus modification) and its position requires further study. All species except S. kinguele share three further synapomorphies: dark ventral abdominal bands with lateral constriction (char. 10); palpal coxa with retrolateral apophysis (char. 30); and posterior epigynal plate with lateral overhanging folds (char. 61). The species group includes mostly small, litter-dwelling species and is most diverse in Gabon but ranges further south at least until western Congo D.R. ( Fig. 114 View FIGURE 114 ).
3. attuleh group. The two species included in this group ( S. attuleh ; S. mbouda ) are very similar (e.g. shapes of male chelicerae, procursus, and embolus; these are the only species in Smeringopina with tibia 1 L/d <60) and geographically close ( Cameroon range; Fig. 293 View FIGURE 293 ). Even though the cladistic analysis identifies only one synapomorphy (other similarities were difficult to code across the entire matrix), the two species are very probably indeed sister taxa.
4. S. fon . This widely distributed ( Fig. 293 View FIGURE 293 ) species is problematic and requires further study. The male palpal femur (char. 34) places it in a group together with the attuleh , ankasa , and cornigera groups. The male clypeus and embolus seem to suggest an affinity with the lekoni group.
5. ankasa group. This West African group ( S. bomfobiri ; S. ankasa ; S. ibadan ; Fig. 293 View FIGURE 293 ) is characterized by a hinged procursus (char. 45). In contrast to other species groups with hinged procursus ( simplex and beninensis groups), the hinge is in a very distal position.
6. cornigera group. This group includes five species ( S. armata ; S. bamenda ; S. nyasoso ; S. cornigera ; S. kala ) whose monophyly appears well supported by the presence of ocular horns (char. 2) and the brush of hairs retrolaterally on the procursus (char. 47). Except for S. kala (southern Cameroon) and an undescribed species from Bioko Island (only females known, in CAS), the group is restricted to the Cameroon range ( Fig. 390 View FIGURE 390 ).
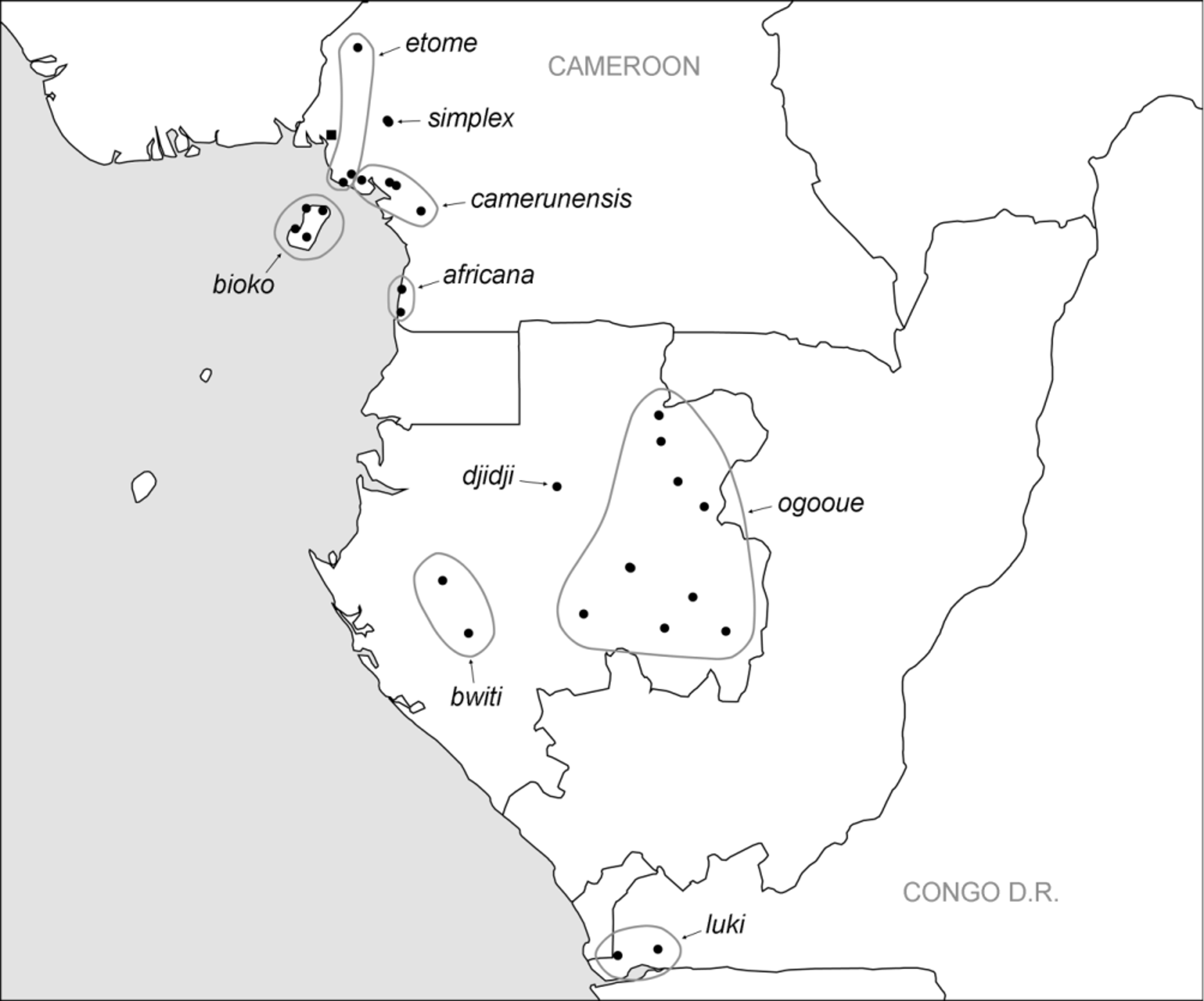
7. simplex group. This large group (nine species: S. camerunensis ; S. djidji ; S. ogooue ; S. bioko ; S. etome ; S. africana ; S. simplex ; S. bwiti ; S. luki ) is characterized by a highly simplified procursus with pointed tip (char. 43). It is here seen as the sister-group of the beninensis -group, supported primarily by the strong ventral ridge proximally on the male palpal femur (char. 36). Other characters supporting this relationship are partly not clearly visible in the simplex group (e.g. the procursus hinge, possibly due to the simplification of the procursus), or not easily coded (palpal tarsus macrotrichia, not easy to differentiate clearly from ‘normal’ hairs). The simplex group ranges from the Cameroon range to western Congo D.R. ( Fig. 468 View FIGURE 468 ).
8. beninensis group. This largest group (eleven species: S. ebolowa ; S. essotah ; S. mayebout ; S. simintang ; S. belinga ; S. beninensis ; S. kribi ; S. sahoue ; S. tchimbele ; S. bayaka ; S. chaillu ) is supported by two synapomorphies: palpal femur with proximal ventral pocket (char. 37); and proximally sclerotized and usually widened and sculptured embolus (char. 55). Together with the simplex group, it includes the largest species in the genus; the two groups also largely share the geographic distribution (western Cameroon and Gabon; the type species S. beninensis is a notable outlier; Fig. 627 View FIGURE 627 ).
9. S. ngungu . In preliminary cladistic analyses, this highly unusual species was either considered sister to all other Smeringopina species or was placed at other places close to basal nodes within Smeringopina . The species was thus deleted from the final matrix and is here considered incertae sedis.
Natural history. Most or all species of Smeringopina seem to be restricted to humid tropical forests. This is in contrast to many species of its sister genus Smeringopus (Huber 2012) , but also to most other Smeringopinae genera from the Mediterranean and Middle East that seem to support considerable levels of insolation and aridity. This probably explains the fact that no species of Smeringopina is known to be synanthropic, while several other Smeringopinae genera have highly synanthropic species (most notably Holocnemus , Crossopriza , and Smeringopus ).
Most species were collected in well preserved forests or forest fragments from sea level to 1800 m. Only the two representatives of the attuleh species group have been found beyond 1800 m, up to 2450 m. Large species require large sheltered spaces like those among and under rocks or between large tree buttresses. In forests where large trees and rocks were missing, juveniles where sometimes found among low vegetation but adult spiders were difficult to find (e.g. S. chaillu in the Massif du Chaillu , sites 1 and 3; S. simintang near Simintang ; S. ogooue at Lekoni River). Small species require relatively large dead leaves that are curved in a way to provide a protected space between leaf and ground. In addition, the leaf litter must provide sufficient humidity. In degraded forest fragments with very dry litter (e.g. NE Makokou and at hill near Mouanda, Gabon; lower site in Atewa Hills, Ghana) I could not find any litter-dwelling species.
In general, Smeringopina species tend to inhabit the lowest layers in the forest. Whenever they occur sympatrically with species of other genera that share the same type of microhabitat (especially Pholcus ), Smeringopina is closer to the ground and more difficult to access (in hollows, under roots, etc.). This was observed repeatedly, e.g. in Koumbaya, Guinea ( S. bineti near ground, Pholcus kindia slightly higher) or Bamboutos , Cameroon ( S. mbouda near ground, Pholcus bamboutos slightly higher).
There seems to be a tendency for species that tolerate disturbed habitats to have wider distributions. For example, S. bineti was found in cavities of roadcuts in a sparsely forested site near Marela in Guinea; S. pulchra was found in degraded forests in the Atewa Hills and near Booyem in Ghana; S. ebolowa was collected from manmade holes in the ground near Koukoué and near Nkoetye in Cameroon; S. ogooue was found in a highly degraded forest patch on a hill near Mouanda and in a degraded dry forest along the road NE of Makokou in Gabon. On the other hand, many of the species that were found at only one locality were found in well conserved, humid forests (e.g. S. essotah in Essotah ; S. tchimbele and S. kinguele in Monts de Cristal; S. nyasoso on Mt. Koupé; S. tebe near Tébé ; S. lekoni at Lekoni River; S. mohoba near Mohoba Mozeye).
While West African localities rarely provided more than one species of Smeringopina , localities in Cameroon, Gabon, and Congo D.R. usually provided two or even three species. In most cases, a large species ( simplex group or beninensis group) shared the forest with a small species ( cornigera group, lekoni group, attuleh group). In some cases, three species were found at one locality, as e.g. in Mayebout (two large and one small species: S. ogooue , S. mayebout , and S. fang ), Ndjolé (one large and two small species: S. bayaka , S. fang , and S. ndjole ), or Kakum N.P. (one large and two small species: S. pulchra , S. ankasa , S. bomfobiri ). In such cases it was usually impossible to see any difference in microhabitat among the two same-size species (also in the case of the two large species S. africana and S. kribi between Kribi and Campo in Cameroon). Only in Mayebout ( Gabon) was there a tendency for one of the large species ( S. mayebout ) to prefer hollow trees and cavities in the ground, while the other large species ( S. ogooue ) was rather found among tree buttresses.
As usual in Pholcidae , Smeringopina species usually vibrate their bodies when disturbed but do not or only reluctantly move away to hide (e.g. S. bineti , S. mbouda ). Another strategy to avoid predation was frequently observed in species living among large rocks or tree buttresses. The spiders quickly moved toward the rock and pressed their bodies flat on the rock which often made them very difficult to locate, especially when mosses covered the rock surface (e.g. S. essotah in Essotah ). In S. guineensis , both strategies were observed in Diéké Forest. In S. ogooue , strategies seemed to vary among localities: in the forests near Bongoville and Moudouma , the spiders fled extremely rapidly at the slightest disturbance, making capture very difficult; at the entrance to the Pahon Pira cave near Lastoursville they just vibrated and were easy to catch.
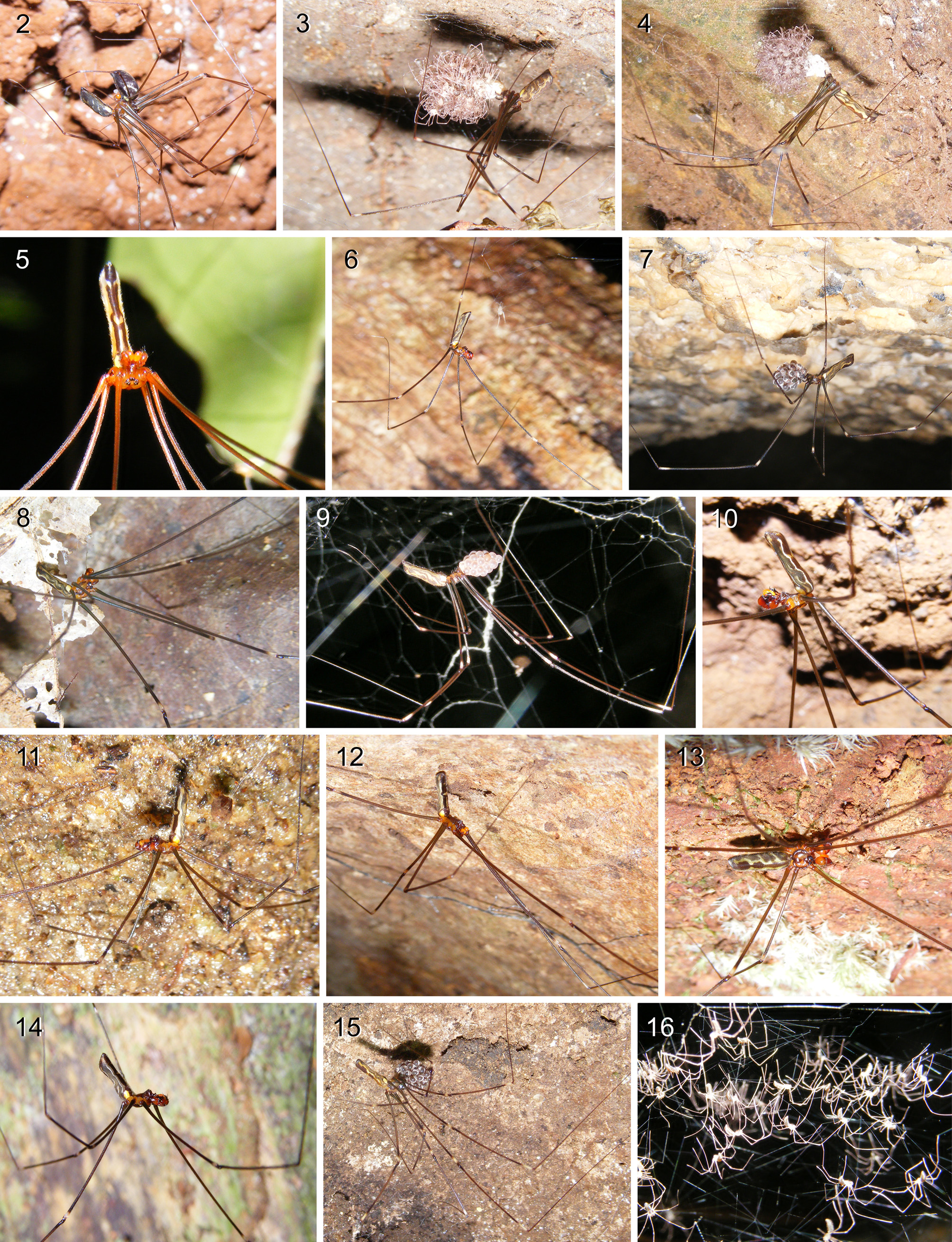
Males and females were often found to share a web. Females carry their round to slightly elongate eggsacs until the spiders hatch and even a short while after that (e.g. Figs. 3–4 View FIGURES 2 – 16 , 24–25, 31 View FIGURES 17 – 31 ). Most or all species of Smeringopina build the ‘typical’ pholcid dome-shaped webs with a tangle of lines above the sheet ( Figs. 17–18 View FIGURES 17 – 31 ). The dome may be strongly curved and in rare cases even spherical (seen in S. bineti near Koumbaya), a facultative web-type that has been reported for Holocnemus pluchei (Sedey & Jakob 1998) . Silk balls that are facultatively attached to the webs and that may be a synapomorphy of Smeringopinae (Huber 2012) have never been observed in Smeringopina .
In S. simplex , a pointed process ventrally on the procursus ( Fig. 588 View FIGURES 587 – 592 ) might be related to the scars found in the membranous part of the anterior genital plate of most females ( Fig. 521 View FIGURES 509 – 524 ). This might thus constitute a case of copulatory genital damage.
Small nematocerous flies were often found hanging from the webs of Smeringopina species, sometimes in high numbers ( Fig. 18 View FIGURES 17 – 31 ). This was most common in large webs (e.g. S. simplex above Nyasoso ; S. guineensis on Mt. Nimba; S. pulchra in Ghana; S. chaillu in the Massif du Chaillu ; S. kribi between Kribi and Campo), but also in the webs of litter-dwelling species (e.g., S. nyasoso at Mt. Koupé; S. moudouma in Moudouma ; S. bomfobiri in Bomfobiri W.S.).
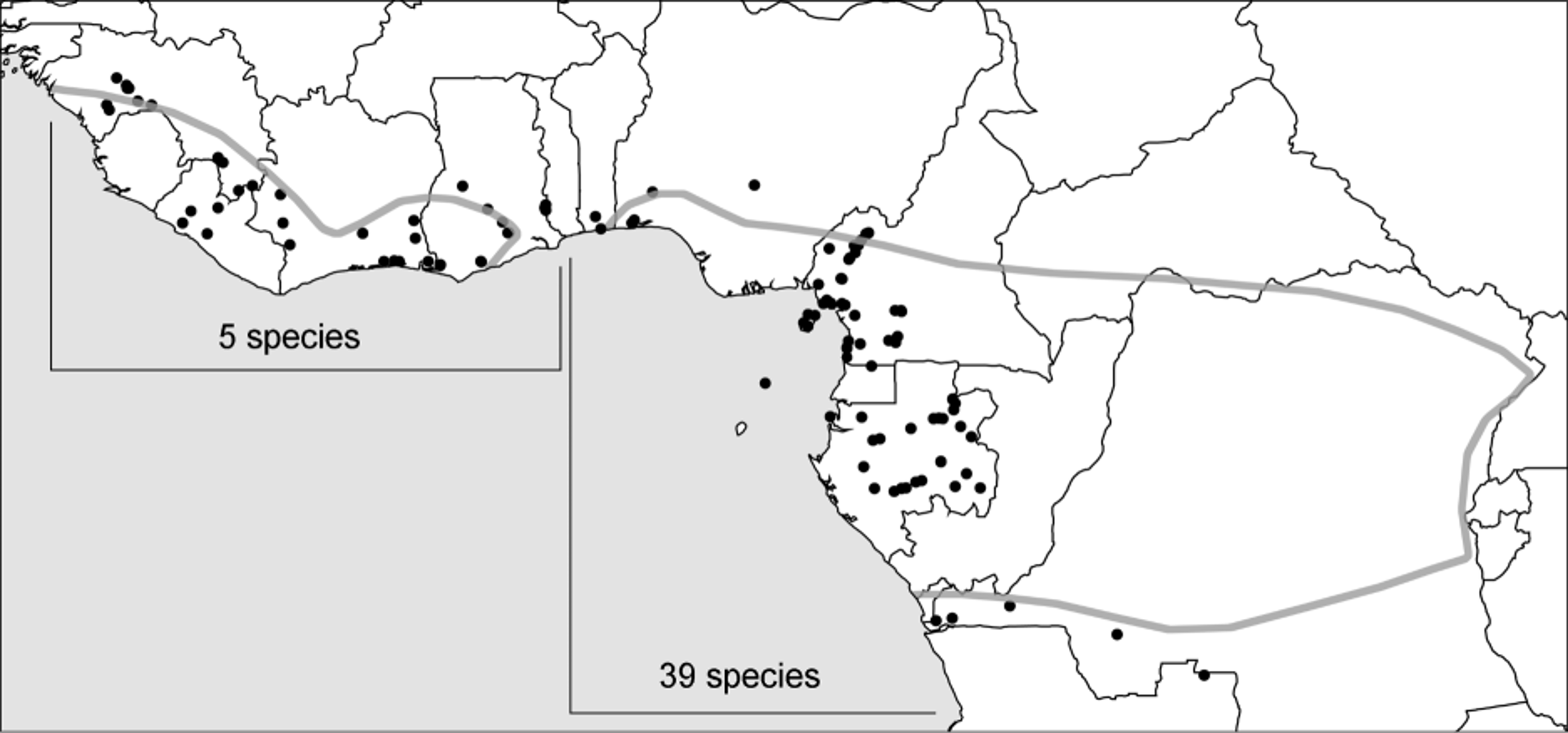
Distribution. Smeringopina seems restricted to West and Central Africa, ranging from Guinea to western Congo D.R. ( Fig. 32 View FIGURE 32 ). It is possible that the genus ranges into northern Angola and further east into the Central Congolian lowland forests but these regions are too poorly sampled to allow conclusions.
Composition. Smeringopina now includes 44 described species, 35 of which are newly described below. The collections seen include about five further undescribed species that are not treated because males are not known. Considering the patchiness of collecting efforts and known distribution patterns in the genus, it appears likely that 50% or more of the actual species remain undescribed. This is especially true for the Northwestern Congolian lowland forests, whose Gabonese part provided numerous species in a few days of collecting but whose Cameroonian and Congolese parts are essentially unexplored.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |