Polycydora intermedia Diez, Sanjuan, Reygel & Artois, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4514.1.9 |
|
publication LSID |
lsid:zoobank.org:pub:C4BCDC9D-BCB2-4DF0-8EFB-7DBC61CB3E4 |
|
DOI |
https://doi.org/10.5281/zenodo.5992805 |
|
persistent identifier |
https://treatment.plazi.org/id/27E54D7D-337A-4B8B-BACD-21D56EE0E0EB |
|
taxon LSID |
lsid:zoobank.org:act:27E54D7D-337A-4B8B-BACD-21D56EE0E0EB |
|
treatment provided by |
Plazi |
|
scientific name |
Polycydora intermedia Diez, Sanjuan, Reygel & Artois |
| status |
sp. nov. |
Polycydora intermedia Diez, Sanjuan, Reygel & Artois View in CoL sp. n.
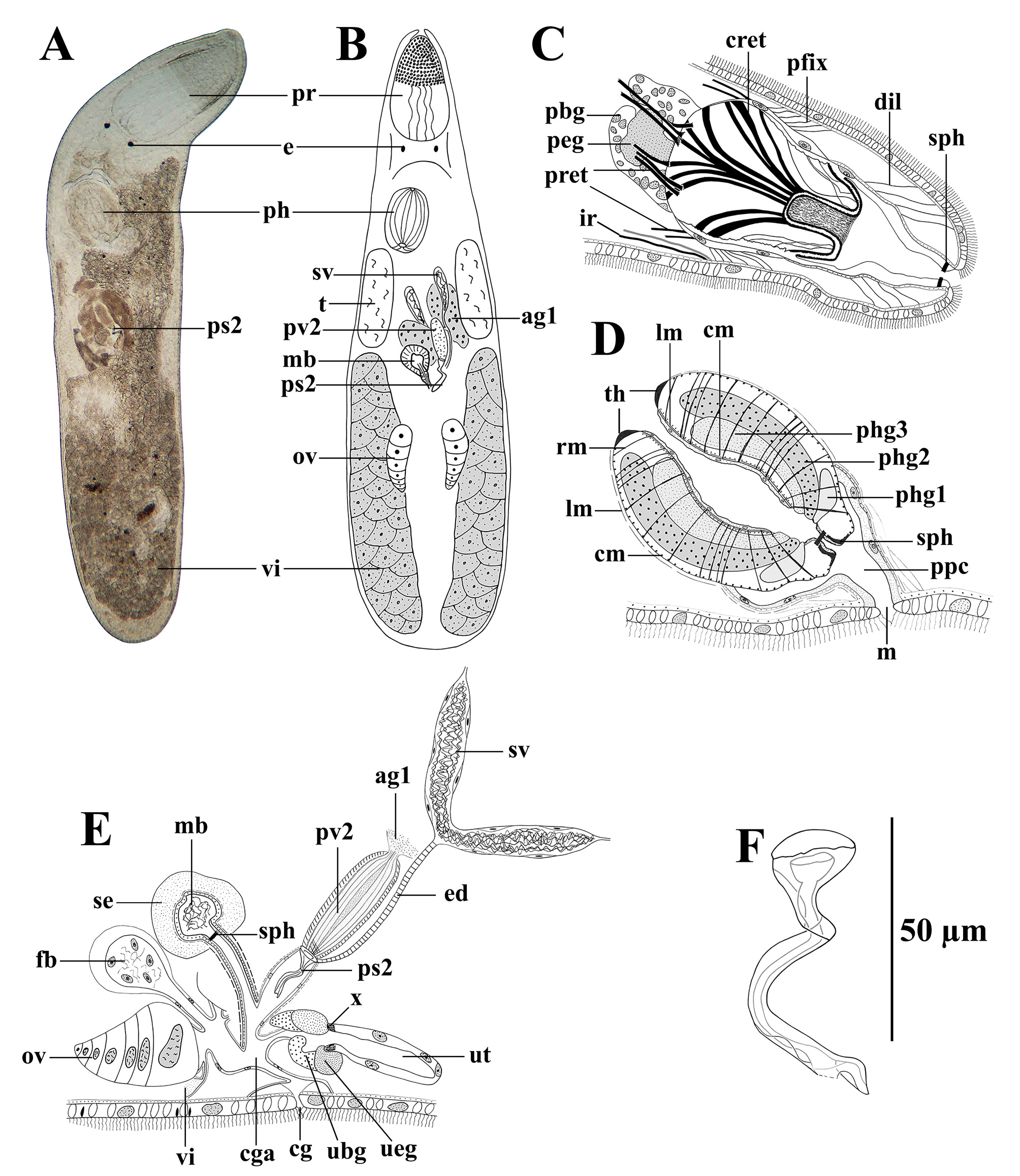
( Fig. 4 View FIGURE 4 )
Material and distribution in Cuba. Observations on live animals. Five whole mounts, one of which is designated holotype (FMNH http://id.luomus.fi/KV.604), and ten serially-sectioned specimens collected in Bahía de Gibara (type locality) (March 26, 2017), sublittoral, in coarse sand, 0.6 m depth, salinity 35 ‰. Three whole mounts from Siboney (May 15, 2016 & June 5, 2017), intertidal, upper 10 cm of fine sand with organic matter, salinity 35 ‰. One whole mount from Bueycabón (February 6, 2018), intertidal, upper 10 cm of fine sand, salinity 33 ‰. All reference material in HU (VIII.3.15–VIII.3.32).
Etymology. The epithet refers to the fact that the new species is morphologically intermediate between Polycystis and Paulodora .
Diagnosis. Species of Polycydora gen. n. with a 75-µm-long prostate stylet type II. The outer stylet is proximally funnel shaped, ±18 µm wide, then is constricted to a tube 4 µm wide. At one-third of its length it shows a 180° turn. Stylet with an asymmetrical distal opening.
Description. Living animals 1.5 mm long. Length measured on whole mounts 0.9–1.5 mm (x̄ = 1.2 mm; n = 4). Translucent, with a pair of eyes. Syncytial epidermis 7 µm thick and ciliated, cilia 6–7 µm long. Two types of vacuoles present in the epidermis: empty ones (support structures) and others filled with small granules. Rhabdites as large as the height of the epidermis, only present in the posterior two-third of the body.
Proboscis of the typical polycystidid type ( Fig. 4 View FIGURE 4 A–B: pr, Fig. 4C View FIGURE 4 ; for a detailed description see Meixner, 1925), 1/5 of the body length, with six pairs of fixator muscles ( Fig. 4C View FIGURE 4 : pfix). With four pairs of proboscis retractors ( Fig. 4C View FIGURE 4 : pret). Cone retractors well-developed ( Fig. 4C View FIGURE 4 : cret). As in most Polycystididae with a pair of ventral integument retractors ( Fig. 4C View FIGURE 4 : ir). Proboscis sheath surrounded by an internal circular and an outer longitudinal muscle layer. Without nuclei at the junction between sheath and cone epithelium. Thin dilator muscles are present in the distal half of the proboscis sheath ( Fig. 4C View FIGURE 4 : dil). Proboscis opening surrounded by a sphincter ( Fig. 4C View FIGURE 4 : sph). Proboscis glands can be observed just caudally from the proboscis: one central large gland producing an eosinophilic fine-grained secretion ( Fig. 4C View FIGURE 4 : peg), and surrounding it, coarse-grained basophilic glands ( Fig. 4C View FIGURE 4 : pbg).
Pharynx located at about 35% ( Fig. 4 View FIGURE 4 A–B: ph, Fig. 4D View FIGURE 4 ), measuring 153–174 µm in diameter (x̄ = 162 µm; n = 4). Prepharyngeal cavity ( Fig. 4D View FIGURE 4 : ppc) lined with a nucleated epithelium surrounded by an internal layer of circular muscles and an external layer of longitudinal muscles, opening to the outside through the mouth ( Fig. 4D View FIGURE 4 : m). Pharynx bulb with the normal polycystidid construction, showing the four teeth surrounding the proximal pharynx opening, typical of the family ( Fig. 4D View FIGURE 4 : th). Three types of glands open in the distal part of the pharyngeal lumen, which are, from distal to proximal: eosinophilic glands (stained light pink) ( Fig. 4D View FIGURE 4 : phg1), basophilic glands with a coarse-grained secretion (stained dark blue-black) ( Fig. 4D View FIGURE 4 : phg2), and eosinophilic glands with a fine-grained secretion (stained brownish) ( Fig. 4D View FIGURE 4 : phg3). At its distal side the pharynx has a muscular sphincter ( Fig. 4D View FIGURE 4 : sph), just distal from the opening of the glands. The musculature of the pharynx consists of an external layer of circular muscles ( Fig. 4D View FIGURE 4 : cm), just inside of the septum and a longitudinal muscle ( Fig. 4D View FIGURE 4 : lm) layer just outside of it, which is continuous with the longitudinal muscle layer of the prepharyngeal cavity. The pharynx lumen is surrounded by a circular muscle layer and a longitudinal one. Radial muscles run between the internal and the external walls ( Fig. 4D View FIGURE 4 : rm).
Genital organs situated in the middle section of the body. Testes ( Fig. 4B View FIGURE 4 : t) situated caudally to the pharynx. Seminal vesicles ( Fig. 4B & 4E View FIGURE 4 : sv) located between the testes and delineated by a thin nucleated epithelium. Both seminal vesicles fuse to form a thin ejaculatory duct ( Fig. 4E View FIGURE 4 : ed), covered by circular muscles. The ejaculatory duct runs aside the prostate vesicle, entering the male genital atrium next to the proximal funnel of the prostate stylet type II. Prostate vesicle type II elongated ( Fig. 4B & 4E View FIGURE 4 : pv2), 63–77 µm long (x̄ = 69 µm; n = 4) and 18–30 µm wide (x̄ = 23 µm; n = 4), filled with an eosinophilic secretion. Prostate vesicle surrounded by two muscular layers: external oblique fibres, and internal longitudinal fibres. Prostate vesicle connected proximally to brownish accessory glands type I ( Fig. 4B & 4E View FIGURE 4 : ag1).
Prostate stylet type II double walled and sickle shaped ( Fig. 4 View FIGURE 4 A–B & 4E: ps2, Fig. 4F View FIGURE 4 ), 60–87 µm long (x̄ = 75 µm; n = 8), with a proximal funnel, 16–21 µm wide (x̄ = 18 µm; n = 8). Distally from the funnel, the stylet is 4–5 µm wide (x̄ = 4 µm; n = 8), showing a 180° twist. Male bursa ( Fig. 4B & 4E View FIGURE 4 : mb) well developed, surrounded by a thick muscular coat, filled with sperm and separated from its muscular stalk by a sphincter ( Fig. 4E View FIGURE 4 : sph). Epithelium with nuclei surrounds the bursal stalk and the male atrium. Bursal stalk entering the common genital atrium ( Fig. 4E View FIGURE 4 : cga) dorsally, at the same place as does the male genital atrium. The internal vesicle of the male bursa is surrounded by a tick sheath of syncytial epithelium ( Fig. 4E View FIGURE 4 : se)
Ovaries kidney shaped ( Fig. 4B & 4E View FIGURE 4 : ov) and located in the posterior third of the body. Oocytes organised in a row, diminishing in size from the most distal one to the most proximal one. Paired vitellaria extend at both sides from the middle to the end of the body ( Fig. 4 View FIGURE 4 A–B & 4E: vi). Oviducts very short, each receiving a vitelloduct. Both oviducts unite to form a short common female duct type I that enters the common genital atrium caudally. Oviducts and female duct are lined by a nucleated epithelium. Muscles are not well defined around the oviducts and the female duct, however, there seems to be an internal layer of circular muscles and an external layer of longitudinal ones. Large female bursa 50 µm long (measured in one sectioned specimen), contains a central space with sperm and nuclei. It connects to the female duct by a bursal stalk at the junction of the two oviducts. Bursal stalk lined by a nucleated epithelium. Muscle layers are not observable around the female bursa and the stalk. A uterus ( Fig. 4E View FIGURE 4 : ut) with nucleated epithelium connects at the anterior side of the common genital atrium. Two rings of glands can be observed at the distal end of the uterus: one coarse-granulated basophilic gland ( Fig. 4E View FIGURE 4 : ubg), and one fine-granulated eosinophilic gland ( Fig. 4E View FIGURE 4 : ueg) separated from the nucleated epithelium by a dense structure, which could be a thick sphincter ( Fig. 4E View FIGURE 4 : x). Common genital atrium opens externally by a common gonopore ( Fig. 4E View FIGURE 4 : cg).
Discussion. The new species shows all diagnostic features of Polycystididae , the most typical of which are the presence of four teeth around the proximal pharynx opening and of three pairs of proboscis fixators ( Karling 1964). It lacks the typical features of the subtaxa Gyratricinae [unpaired ovaries; terminal (male) gonopore; unpaired seminal vesicle], Scanorhynchinae (position of external muscles of proboscis sheath inverted), Phonorhynchoidinae (proboscis sheath without circular muscles, subterminal gonopore combined with a very long genital atrium), Psammopolycystidinae (syncytial epidermis) and Typhlopolycystidinae (unpaired ovarium, enlarged proximal dilators of the proboscis sheath, typical seminal receptacle on the bursal stalk) (for details see Tessens et al. 2014 and references therein). Therefore, it can be included in Polycystidinae , a morphologically heterogeneous group, comprising most of the polycystidid taxa for which at this moment no clear apomorphies can be given (see Tessens et al. 2014).
The male atrial organs of P. intermedia gen. n. sp. n. include a double-walled prostate stylet type II, found in several representatives of Polycystidinae ( Antiboreorhynchus Karling, 1952 , Austrorhynchus Karling, 1952 , Cincturorhynchus Evdonin, 1970 , Paraustrorhynchus , Phonorhynchus Graff, 1905 , Porrocystis Reisinger, 1926 , Progyrator Sekera, 1901 , and Pygmorhynchus Artois & Schockaert, 1999 (see Artois & Schockaert 2003). In these genera the prostate stylet is connected to a prostate vesicle type II. However, all these genera (except Progyrator and Pygmorhynchus ) include an accessory stylet in the male atrium, a structure lacking in P. intermedia gen. n. sp. n. The prostate vesicle type II of P. intermedia gen. n. sp. n. contains only an eosinophilic secretion, while in the other genera of the subfamily with this kind of prostate vesicle the secretion is basophilic ( Artois & Schockaert 2003). Only in species of Gyratrix , Gyratricella Karling, 1955 , and Papia Karling, 1956 does the prostate vesicle type II contain an eosinophilic secretion ( Artois & Schockaert 2003), but these taxa differ from P. intermedia gen. n. sp. n. by many other diagnostic characters, as they belong to different subfamilies ( Tessens et al. 2014).
Polycydora intermedia View in CoL gen. n. sp. n. resembles species of Polycystis View in CoL as well as of Paulodora View in CoL . However, representatives of these two genera have a prostate stylet type I and a prostate vesicle type I. The prostate stylet type I in Polycystis View in CoL is short and wide, with the distal rim of the outer stylet toothed [except in P. gabriellae (Marcus, 1948) Karling, 1952 View in CoL ]. In Paulodora View in CoL the outer stylet is rather elongated and complex, with folds and gutters, probably to drain the sperm. The prostate stylet of P. intermedia View in CoL gen. n. sp. n. is (probably) of type II, as it is connected to a typical prostate vesicle of type II. It is rather long, but does resemble that of some species of Paulodora View in CoL (e.g. P. subcontorta Schockaert, 1982 View in CoL ) as to its general construction, i.e. with gutter-like folds, but without further ornamentations. Apart from the above-mentioned differences/resemblances in the construction of the stylet, P. intermedia View in CoL gen. n. sp. n. lacks several of the typical, diagnostic features of the other two genera.
Diagnostic features of species of Polycystis View in CoL lacking in P. intermedia View in CoL gen. n. sp. n. are the presence of accessory glands type I (also present in species of Paraustrorhynchus View in CoL ) and a large, asymmetrical, mostly excentric muscle bulb on the stalk of the male bursa (Artois & Schokaert 1998, 2003; Willems et al. 2006). The stalk of the male bursa is heavily muscular in P. intermedia View in CoL gen. n. sp. n., but it is never asymmetrical, let alone excentric. Furthermore, the tick syncytial epithelium surrounding the internal vesicle of the male bursa in P. intermedia View in CoL gen. n. sp. n. is not found in any species of Paulodora View in CoL or Polycystis View in CoL . Moreover, most of the species of Polycystis View in CoL are darkly pigmented ( Artois & Schockaert 1999), which is not the case in P. intermedia View in CoL gen. n. sp. n. . Most species of Paulodora View in CoL typically have elongated, kidney-shaped ovaries (see Artois & Tessens 2008), as is the case in P. intermedia View in CoL gen. n. sp. n., but P. intermedia View in CoL gen. n. sp. n. lacks the hard, umbrella-shaped "nozzles" at the connection between male bursa and each ovary that are so typical of species of Paulodora (Artois & Schockaert 1998) View in CoL . Moreover, in species of Paulodora View in CoL the oviducts (mostly) merge with the male bursal tissue, and the ejaculatory duct makes a 270° turn around the prostate vesicle before it ends in the male atrium next to the stylet. In P. intermedia View in CoL gen. n. sp. n., the male bursal stalk is free, and the ejaculatory duct runs straight.
A typical feature of P. intermedia gen. n. sp. n. is the presence of two bursae, a male and a female one, with the female bursa being large and with a long stalk. In species of Paulodora a female bursa is lacking, whereas in species of Polycystis it is very small (or absent, as in P. gabriellae ) and almost without a stalk.
Because of the issues discussed above, the new species cannot unambiguously be placed in any of the existing genera within Polycystididae , and, therefore, the erection of a new genus is warranted.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Polycystidinae |
|
Genus |
Polycydora intermedia Diez, Sanjuan, Reygel & Artois
| Diez, Yander L., Hernández, Claudia Sanjuan, Reygel, Patrick, Roosen, Paulien & Artois, Tom 2018 |
Polycydora intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
P. intermedia
| Diez & Hernández & Reygel & Roosen & Artois 2018 |
Paulodora
| Artois & Schockaert 1998 |
P. subcontorta
| Schockaert 1982 |
Paraustrorhynchus
| Karling & Schockaert 1977 |
P. gabriellae (Marcus, 1948)
| Karling 1952 |
Paulodora
| Marcus 1948 |
Paulodora
| Marcus 1948 |
Paulodora
| Marcus 1948 |
Paulodora
| Marcus 1948 |
Paulodora
| Marcus 1948 |
Polycystis
| Kolliker 1845 |
Polycystis
| Kolliker 1845 |
Polycystis
| Kolliker 1845 |
Polycystis
| Kolliker 1845 |
Polycystis
| Kolliker 1845 |