Scelidosaurus, Norman, 2020
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlaa061 |
|
DOI |
https://doi.org/10.5281/zenodo.10541469 |
|
persistent identifier |
https://treatment.plazi.org/id/B66BDD2A-0835-FF8A-E0A3-72B0FD52E72D |
|
treatment provided by |
Felipe |
|
scientific name |
Scelidosaurus |
| status |
|
SCELIDOSAURUS : POSTCRANIAL BIOLOGY
DIGESTIVE SYSTEM
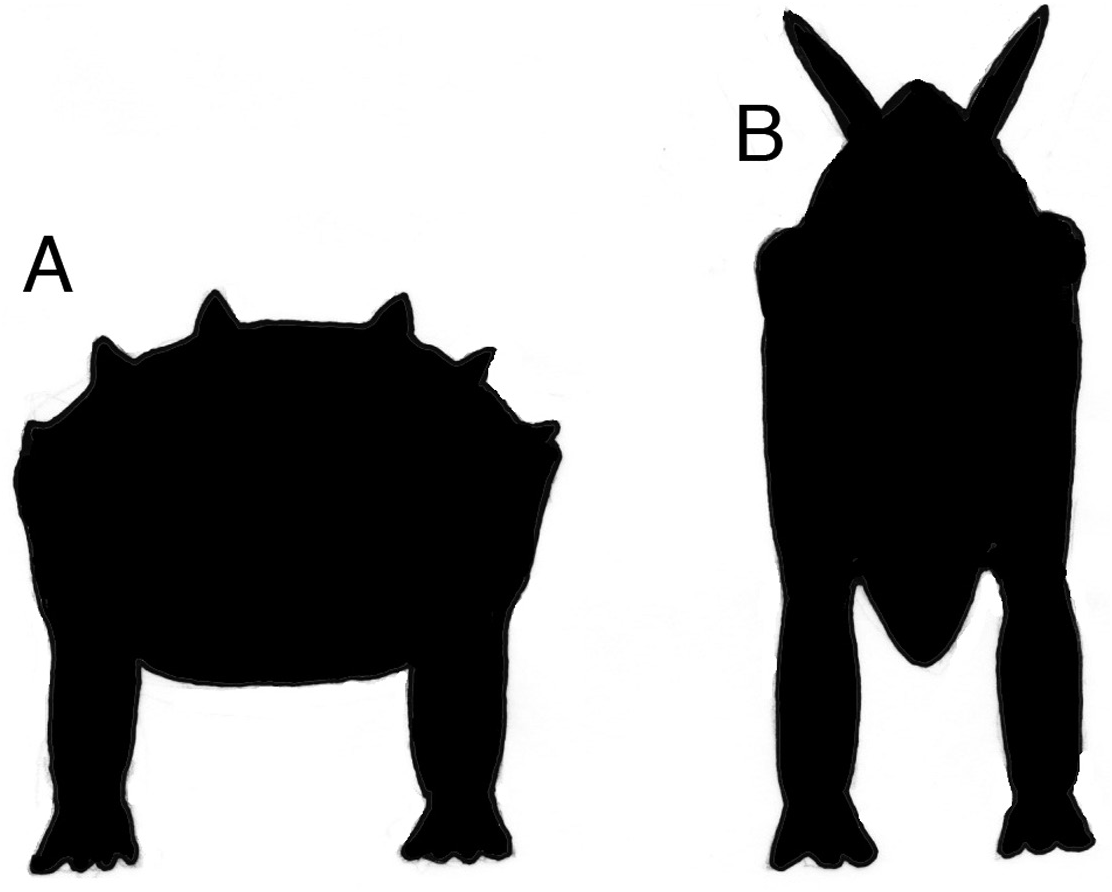
A diet of terrestrial plants requires a variety of modifications to be made to the digestive system ( King, 1996; Sues, 2000). Land plants have a skeletal scaffold formed by a combination of structural polymers: lignin, hemicellulose and cellulose, none of which can be hydrolysed by vertebrate gut enzymes. To release the soluble contents of plant cells, their lignin/ cellulose fabric needs to be broken down. This process is started in the mouth using teeth and jaw muscles. There is structural evidence in Scelidosaurus for a small, sharp keratinous beak that was narrow so that the animal was capable of cropping plant material (perhaps more succulent items) selectively. Once in the oral cavity, a modest amount of pulping and shearing of plant tissue occurred prior to swallowing, judged by the morphology of the dentition. The structure of the gut into which the browse was passed is unknown in Scelidosaurus but the wide span of the ribcage ( Norman, 2020b) indicates that the torso was broad. In its proportions and general body shape, Scelidosaurus more closely resembles those seen in ankylosaurs ( Fig. 22A View Figure 22 ), than the vertically extended (narrow and deep) torso morphology exhibited by stegosaurs ( Fig. 22B View Figure 22 ).
It is reasonable to assume that a modest amount of chewing of ingested plant material occurred in the mouth. However, the residence time in the gut to allow for the enzymatic breakdown of lignin and digestion of cellulose would be expected to be long, prior to absorption and assimilation of the plant breakdown products. There are additional factors to be considered, such as the presence of antipredation chemical defences produced by the plants, such as alkaloids, terpenoids, condensed and hydrolysable tannins ( Swain, 1976), as well as the relative succulence and physical texture of the browse. A crop can be inferred (because it is present in living archosaurs) as a specialized sac-like compartment at the base of the oesophagus, adjacent to the stomach. The crop can store and chemically prepare the browse for subsequent digestion by softening and enzymatically detoxifying plant tissue. Herbivorous reptiles and birds have a far greater tolerance of alkaloids than, for example, crop-less herbivorous mammals ( King, 1996). This may in part be attributed to the ability of the former groups to chemically neutralize these poisons in the crop before they enter the absorptive part of the digestive system.
The stomach of living birds and crocodiles is also modified by the presence of a muscular gizzard whose walls are abrasive and used to physically pulverize the plant tissues (or large bones in the case of crocodiles) in preparation for digestion. Birds and crocodiles are known to swallow grit or stones (gastroliths) that become lodged in the walls of the gizzard and assist in the physical breakdown of food in the stomach (gastroliths are also known to serve as ballast in crocodiles – Taylor, 1987). Beyond the stomach and gizzard, the intestine has an absorptive section (small intestine) that can remove soluble plant cell contents released by the crushing of their tissues. In herbivorous birds, this region of the gut contains a series of blind-ended pouches (caecae). The caecae are diverticulae in the gut (sometimes spirally coiled) into which the partly digested and crushed plant material passes for further digestion mediated by symbiotic microbes (prokaryotes and protistans). Unlike their vertebrate hosts, these microbes are capable of producing enzymes that hydrolyse plant cell walls by converting them to breakdown products, such as sugars and volatile fatty acids ( McBee, 1977). Enzymatic breakdown of the plant cell walls releases sugars, proteins, minerals and vitamins that can be absorbed through the lining of the caecum and small intestine. The process of providing nutrition to the population of symbiotic microbes boosts their population, which in turn allows the host to absorb amino acids and other breakdown products derived from cell death among symbionts. In living herbivorous lizards (and mammals), the more distal region of the gut accommodates a voluminous caecum that arises at the junction of the small and large intestines ( Romer & Parsons, 1980).
The size of the abdominal cavity simply reflects the storage capacity of the gut and its ability to cater for the lengthier phases of digestion and absorption inherent in a vegetarian diet. Only in exceptional circumstances are traces of the soft tissues of the gut (cololites) preserved ( Dal Sasso & Signore, 1998; Ji et al., 1998), but in the case of Scelidosaurus there is, to date, no known preservation of gut tissues or gastroliths in association with the abdominal cavity that might illuminate gut structure and function.
RESPIRATORY SYSTEM
The respiratory systems of dinosaurs are not preserved but they are so central to the development of an understanding of the physiology and metabolic status of these animals that they have become a persistent subject of investigation. Carrier & Farmer (2000a, b), Perry (2001) and Perry & Sander (2004) did much to promote debate on this topic by focusing on the respiratory potential in dinosaurs, given what was then known about the skeletal mechanics and respiratory physiology of extant squamates, crocodiles and birds. The close relationship between theropod dinosaurs and birds ( Huxley, 1868; Ostrom, 1976; Xu et al., 2014) focused much of the subsequent discussion about dinosaur lung structure on the osteological correlates identifiable in theropods: pneumatized bones, uncinate ribs and gastralia ( Claessens, 2004; O’Connor & Claessens, 2005; Codd et al., 2008; Benson et al., 2012) and, to a lesser extent, sauropods ( Britt, 1997; Perry & Reuter, 1999). Benson et al. (2012: 188), in an article that focused solely upon skeletal pneumaticity and its implications for dinosaurian (including bird) physiology, noted that Ornithischia is a clade of diverse and abundant dinosaurs that is deeply nested within ornithodiran archosaurs and yet lacks a pneumatic postcranium, implying that this factor needed to be reconciled in any model of dinosaurian biology.
Aspiratory respiration became established in amniotes ancestral to Archosauria, resulting in the potential to increase the overall efficiency of gas exchange among these animals ( Perry & Sander, 2004). Most models of amniote respiration were understood to be driven by muscle-induced repositioning of the ribs to change the volume of the thoracic cavity (costal aspiration). However, it has become clear that respiration can be augmented by cuirassal aspiration (indicated by the presence of an abdominal skeleton of gastralia – belly ribs) and pelvic aspiration (dependent upon an ability to flex either the entire pelvis against the dorsal vertebral column or specialized parts of a fixed pelvis – Carrier & Farmer, 2000a, b).
In living birds, highly compliant air-sacs evolved in association with a unidirectional (‘flow-through’) lung structure. A strictly comparable respiratory system was hypothesized for theropods ancestral to birds ( O’Connor & Claessens, 2005; Benson et al., 2012). Abdominal wall compliance probably increased in birds with the loss of gastralia. However, cross-current (unidirectional) gas-exchange systems were then identified by Farmer & Sanders (2010) in the lungs of alligators. The strong similarity in air-flow patterns in the lungs of birds and crocodilians suggests that these features are plesiomorphic for Archosauria ( Schachner et al., 2013a). Flow-through lungs are also now recognized more widely among diapsid amniotes, including squamates ( Schachner et al., 2013b; Cieri & Farmer, 2016), so the inference of the existence of this type of lung in dinosaurs cannot seriously be doubted.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.