Halisarca desqueyrouxae, Willenz, Philippe, Ereskovsky, Alexander V. & Lavrov, Dennis V., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4208.6.1 |
|
publication LSID |
lsid:zoobank.org:pub:A61B3C3D-B5E0-45BE-BEEC-B32E17CA7796 |
|
DOI |
https://doi.org/10.5281/zenodo.6090128 |
|
persistent identifier |
https://treatment.plazi.org/id/B676932D-FF89-0809-67E5-FC3BFBD0CB32 |
|
treatment provided by |
Plazi |
|
scientific name |
Halisarca desqueyrouxae |
| status |
sp. nov. |
Halisarca desqueyrouxae sp. nov.
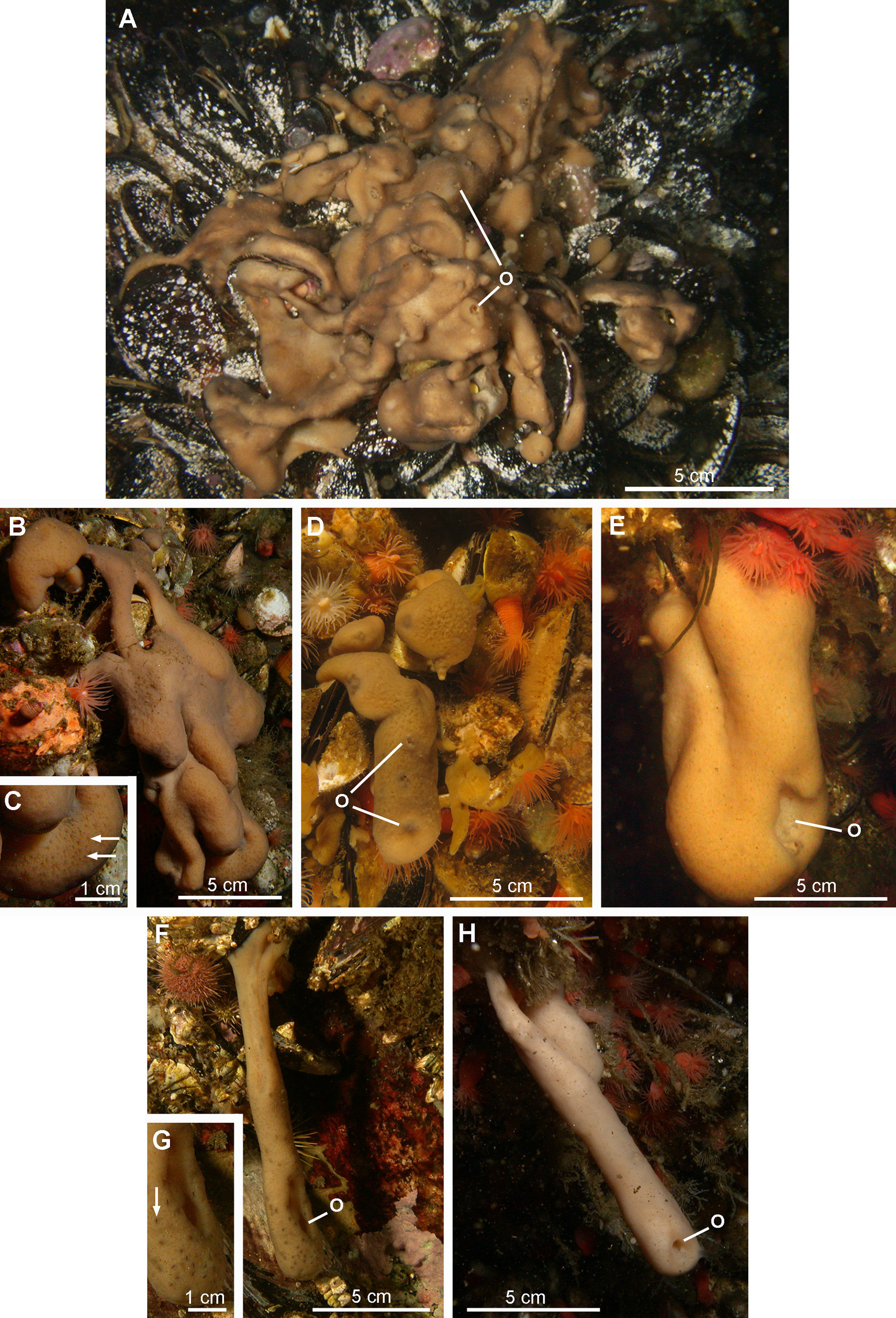
( Figs 11–18 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 , Table 1)
Material examined. Holotype: RBINS-IG 32236- POR. 10803 (Fragments: MNRJ 10803 View Materials , MHNG 90139 View Materials ), Reñihue Fjord , Palena Province, Chile (42°31'55.02"S –72°35'30.72"'W), 3 m depth, coll . Ph. Willenz & E. Hajdu, 22.v. 2007. Paratypes: RBINS-IG 3 2235- POR. 10754 (Fragments: MNRJ 10754 View Materials , MHNG 90094 View Materials ), Liliguapi Island, Comau Fjord , Palena Province , Chile (42°09’43.32”S – 72°35’54.90”W), 14 m depth, coll GoogleMaps . Ph. Willenz & E. Hajdu, 07.v.2007. RBINS-IG 3 2236- POR. 10757 (Fragments: MNRJ 10757 View Materials , MHNG 90097 View Materials ), Liliguapi Island, Comau Fjord , Palena Province , Chile (42°09’43.32”S – 72°35’54.90”W), 13 to 16 m depth, coll GoogleMaps . Ph. Willenz & E. Hajdu, 07.v.2007. RBINS-IG 3 2238- POR. 12917 (Fragments: MNRJ 12917 View Materials , MHNG 90251 View Materials ), Liliguapi Island, Comau Fjord , Palena Province , Chile (42°09'43.32"S – 72°35'54.90"W), 26 m depth, coll GoogleMaps . Ph. Willenz & J. Biro, 01.ii.2009. RBINS-IG 32238- POR. 1 2919 (Fragments: MNRJ 12919 View Materials , MHNG 90253 View Materials ), Liliguapi Island, Comau Fjord , Palena Province , Chile (42°09'43.32"S – 72°35'54.90"W), 26 m depth, coll GoogleMaps . Ph. Willenz & J. Biro, 01.ii.2009. RBINS-IG 32238- POR. 12924 (Fragments: MNRJ 12924 View Materials , MHNG 90258 View Materials ), Liliguapi Island, Comau Fjord , Palena Province , Chile (42°09'43.32"S – 72°35'54.90"W), 26 m depth, coll GoogleMaps . Ph. Willenz & J. Biro, 01.ii.2009.
Additional paratypes not observed in Electron Microscopy. RBINS-IG 32 232- POR. 8795 ( Fragment : MNRJ 8795 View Materials ) Liliguapi Island, Comau Fjord, Palena Province , Chile (42°09’44.23”S – 72°35’42.99”W), 20 m depth, coll. Ph. Willenz & E. Hajdu, 24.ii.2005 GoogleMaps . RBINS-IG 32236- POR. 9193 ( Fragment : MNRJ 9193 View Materials ), Estero Farquhar , Bernardo Fjord, Capitán Prat Province, Chile (48˚29'18,70"S–74˚12'25,70"W), 4 m depth, coll. V. Häusssermann & G. Försterra, 29.iii.2005 . RBINS-IG 32236- POR. 9214 ( Fragments : MNRJ 9214 View Materials , Estero Farquhar , Bernardo Fjord, Capitán Prat Province, Chile (48˚29'18,70"S–74˚12'25,70"W), 5 m depth, coll. V. Häusssermann & G. Försterra, 29.iii.2005 . RBINS-IG 32236- POR. 9215 ( Fragments : MNRJ 9215 View Materials , Tempano Fjord, Capitán Prat Province, Chile (48°42'59.70"S – 74°00'18.80"W), 8 m depth, coll. V. Häusssermann & G. Försterra, 26.iii.2005 GoogleMaps . RBINS-IG 32233- POR. 9961 (Fragments: MNRJ 9961 View Materials , MHNG 90662 View Materials ), Isla Lavinia, Última Esperanza Province , Chile (49°00'48.10"S – 74°58'37.50"W), 4 m depth, coll. Ph. Willenz & L. Atwood, 13.iii.2006 GoogleMaps . RBINS-IG 32 238- POR. 12923 ( Fragments : MNRJ 12923 View Materials , Liliguapi Island , Comau Fjord, Palena Province, Chile (42°09’44.23”S – 72°35’42.99”W), 11 m depth, coll. Ph. Willenz & J. Biro, 1.ii.2009 GoogleMaps .
External morphology. Polymorphic sponge. The first morphotype corresponding to the holotype is the most frequently encountered. It consists of an encrusting wrinkled mat, up to 2 cm thick, composed of ridges and short digitations with well-defined oscula (morphotype 1, Figs 11 View FIGURE 11 A–11D). The surface is slimy with clear and evenly spread ostia. The consistency is resistant to tearing. The second morphotype is represented by individuals of tubular form varying in length, with a single and sometimes stretched out osculum (morphotype 2, Figs 11 View FIGURE 11 E–11H).
Colour. Both morphotypes are light brown when exposed to the light and beige when hidden under overhangs.
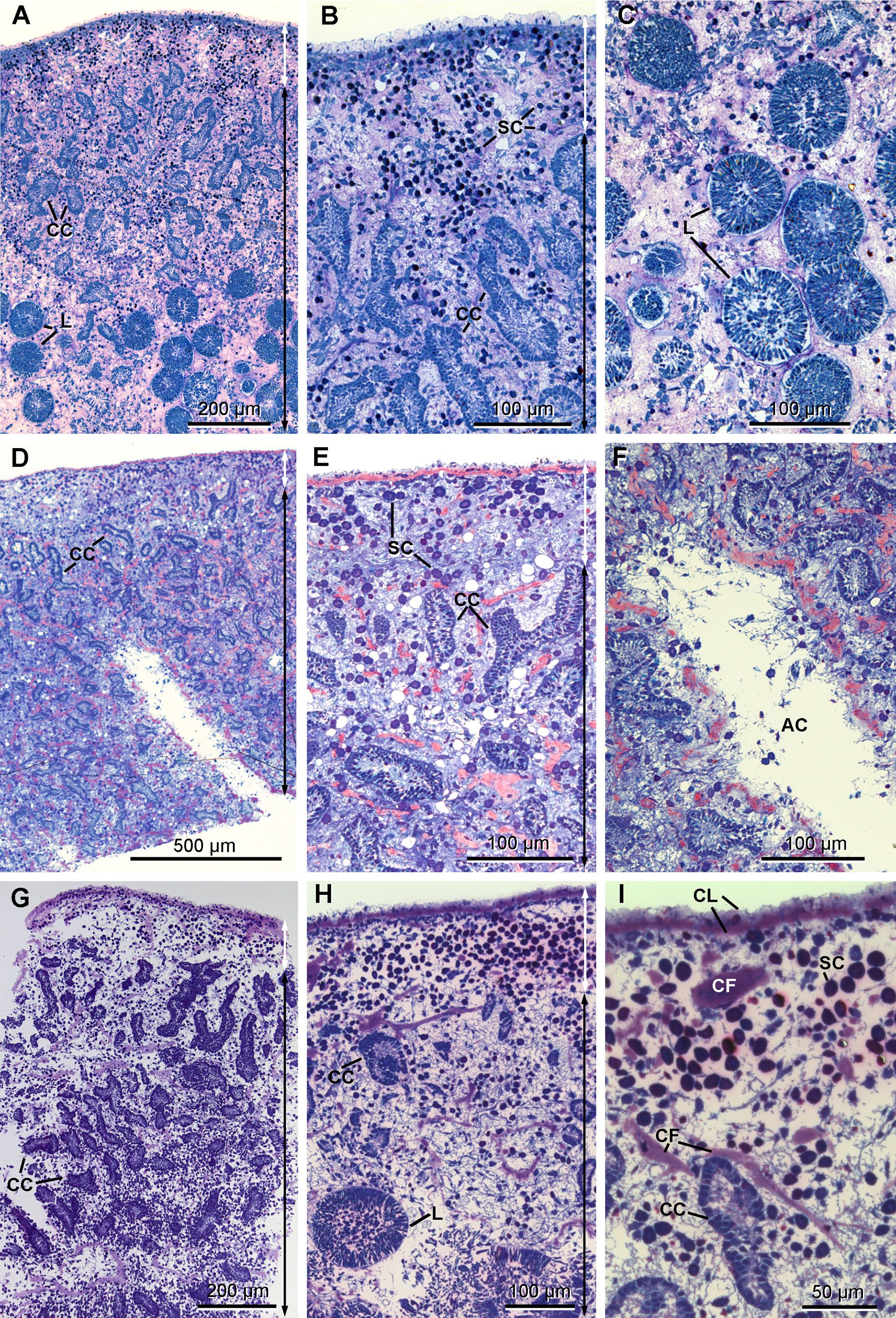
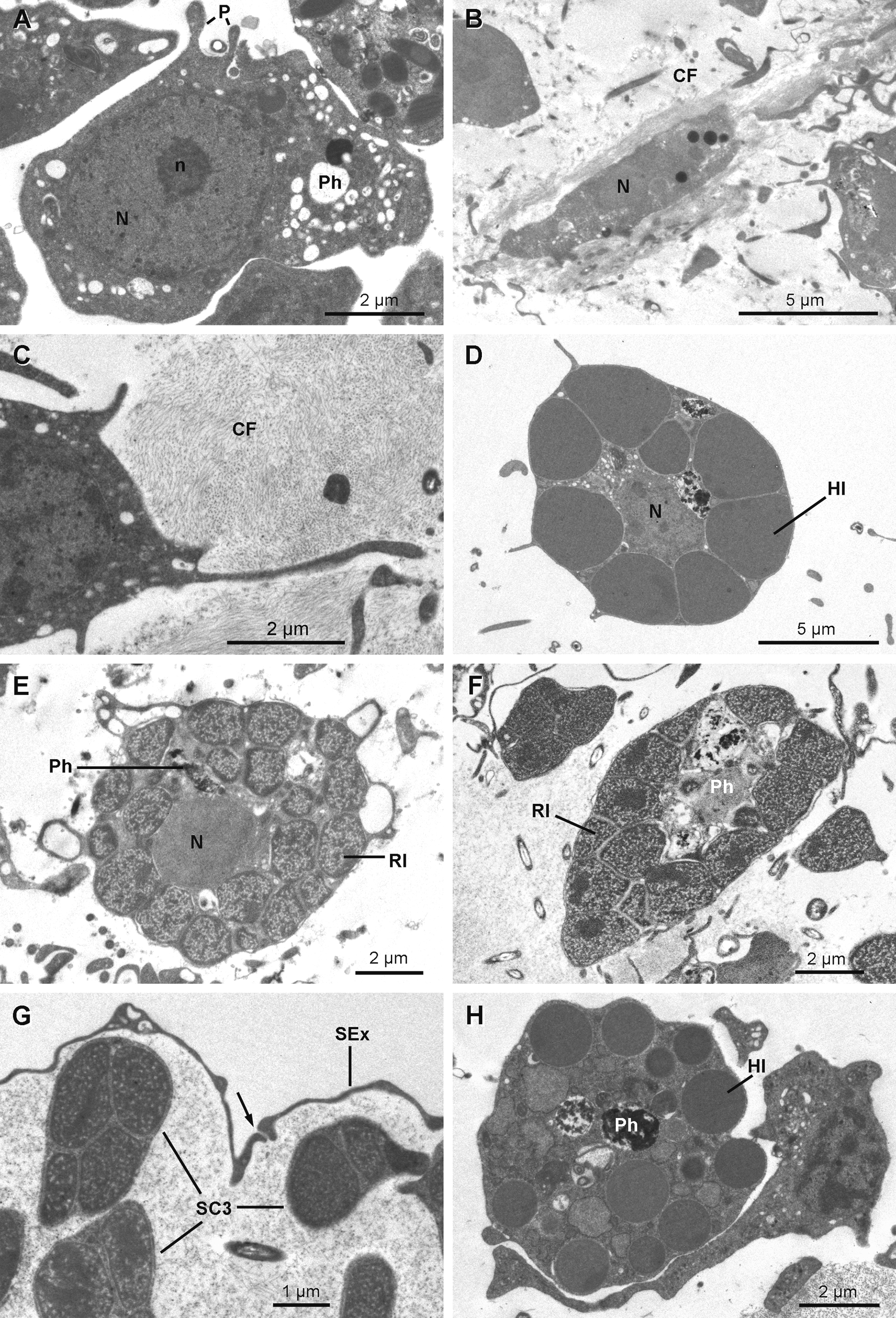
Anatomy. The ectosome varies from 80 to 250 µm in thickness ( Figs 12 View FIGURE 12 A, 12B, 12D, 12E & 12G–12H). On its outside, it is delimited by a superficial layer made of plane extensions of exopinacocytes connecting through thin processes with their cell bodies, which are located under a thick complex collagenous layer ( Figs 13 View FIGURE 13 B & 13C). No glycocalyx covers the external membrane of the exopinacocytes in any of the observed samples. A complex collagenous layer contains two distinct regions: its outward side has a loose and diffuse unorganized structure, 1 to 14 µm thick; its inward side, 3 to 5 µm thick, is made of collagen fibrils arranged in dense tracts, which are in contact with the underlying choanosome ( Figs 13 View FIGURE 13 A, 13C & 13E). The global thickness of the collagenous layer is not consistent and ranges between 6 to 19 µm among the samples, but without any particular widening. Spherulous cells are scattered in the collagenous layer and are abundantly concentrated in the ectosome. They are more dispersed in the choanosome ( Figs 12 View FIGURE 12 A– 12I). Symbiotic bacteria are also present from the outermost collagenous layer to the deepest regions of the mesohyl.
The choanosome includes typical elongate and convoluted choanocyte chambers (50 to 260 µm long x 20 to 60 µm wide) surrounded by numerous aquiferous canals of various diameters ( Figs 12 View FIGURE 12 A– 12I).
The mesohyl is very lax and remarkably clear, lacking dense granules ( Figs 14–17 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 ), but contains collagen tracts ( Figs 12 View FIGURE 12 D–12F) and different wandering cell types at low density.
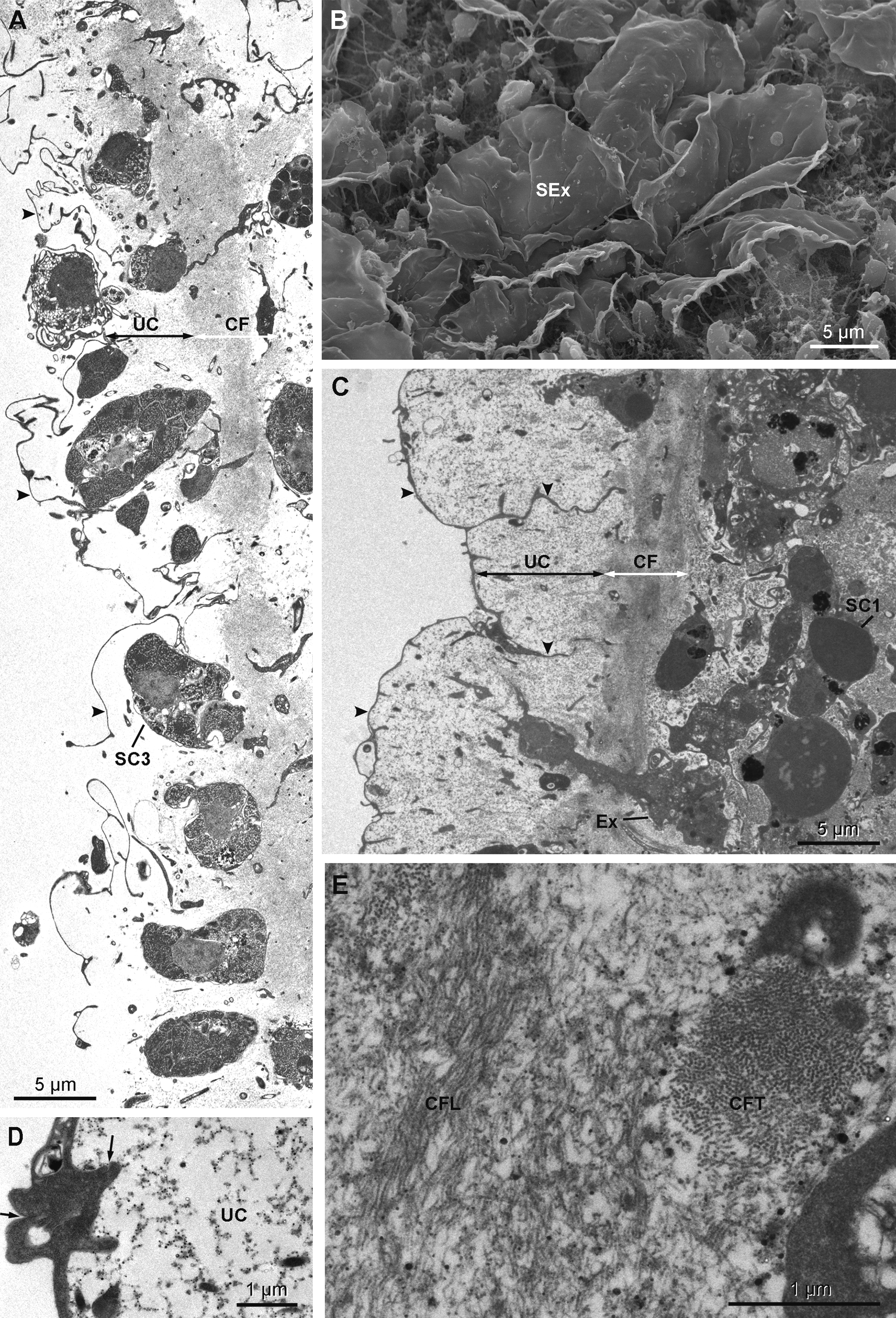
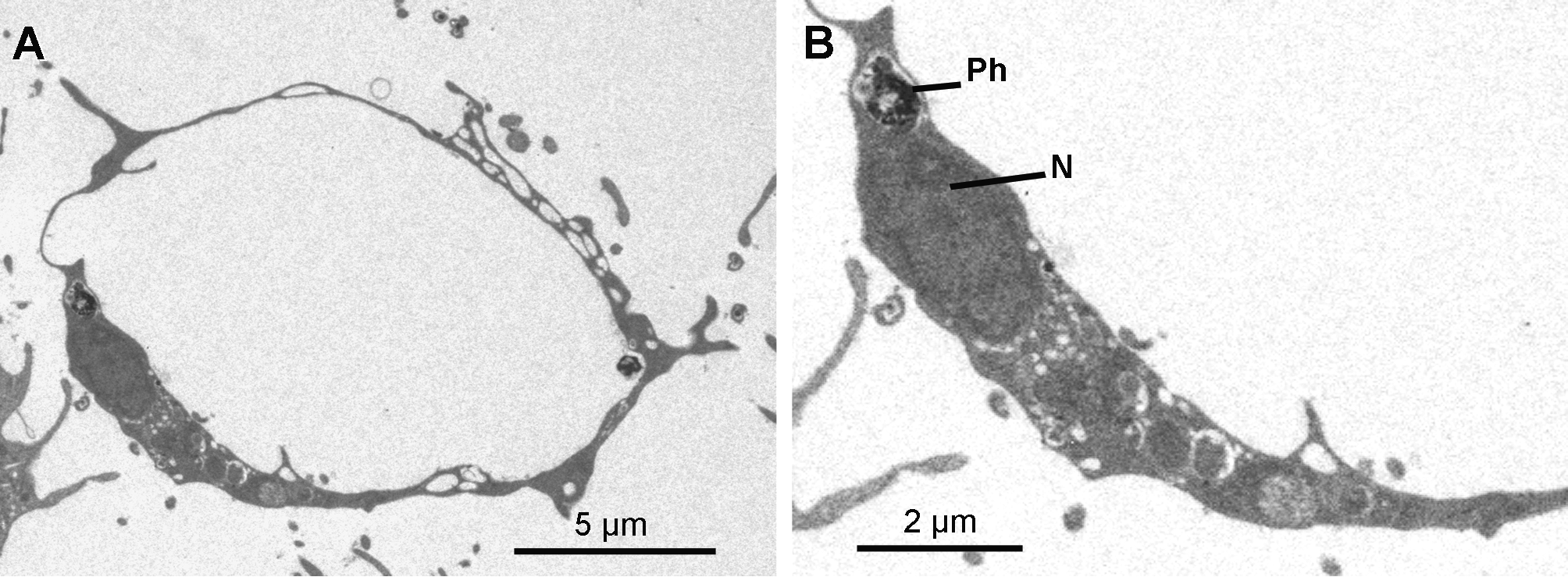
Cytology. Exopinacocytes ( Figs 13 View FIGURE 13 A–13E) are "T-shaped" in transverse section. Their cell bodies (2.8 to 6.7 µm in diameter), with basal nucleolated nuclei, are deeply rooted under the complex collagenous layer, and connect with thin flake-like superficial extensions, revealed in SEM, through outstretched projections ( Figs 13 View FIGURE 13 A– 13C). Inter-cellular junctions between superficial extensions connect exopinacocytes to each other ( Fig. 13 View FIGURE 13 D). Choanocytes ( Figs 14 View FIGURE 14 A–14D) are stretched and cylindrical with a roughly pyramidal base (6.9 to 10.5 µm long x 2.1 to 8.3 µm wide) provided with basal pseudopods extending toward the mesohyl. The cytoplasm comprises electron dense inclusions and phagosomes, mainly concentrated on the basal side of the cell, and an apical nucleus. A periflagellar sleeve surrounds the base of the flagellum.
Endopinacocytes ( Figs 15 View FIGURE 15 A & 15B) are flat with a prominent oval nucleus and constitute the walls of the canals.
Basopinacocytes were not observed.
Archaeocytes ( Fig 16 View FIGURE 16 A) are few and have a conventional amoeboid shape (6.2–8.0 µm) with a spherical nucleolated nucleus (3.7 to 4.3 µm in diameter). The cytoplasm is dense with phagosomes and vacuoles of different densities varying from 270 to 670 nm in diameter.
Lophocytes ( Figs 16 View FIGURE 16 B & 16C) are elongated (ranging 2.3 x 9.6 µm) with tufts of collagen fibrils emerging from their posterior side or surrounding them. The nucleus is located in the anterior part of the cell. The cytoplasm is dense, contains phagosomes and small osmiophilic inclusions (330 x 730 nm).
Spherulous cells of two different types are distinguished in TEM ( Figs 13 View FIGURE 13 A & 13C).
Spherulous cells Type I ( Figs 16 View FIGURE 16 D & 17C) are oval cells (7.8–10.9 x 11.5–12.0 µm in diameter) with short pseudopodial extensions. Large dense osmiophilic homogeneous membrane-bound inclusions of variable diameter (1.0 to 3.8 µm) squeeze the central nucleolated nucleus, leaving exiguous space for a cytoplasm containing phagosomes and small electron translucent vesicles (100 to 200 nm). They are the most abundant cells in the mesohyl.
Spherulous cells Type III ( Figs 13 View FIGURE 13 A, 16E–16G & 17F) are large cells (5.0–7.4 x 8.8–10.6 µm) with irregular shape. Large inclusions (0.8 to 2.0 µm in diameter) of porous texture with a denser periphery occupy most volume of the cytoplasm that contains large phagosomes, except for the cells occurring near the exopinacocytes ( Fig. 16 View FIGURE 16 G). These cells are common and mainly located in the upper part of the ectosome and occur less often in the choanosome; they are not found in all specimens examined (Table 1).
Granular cells ( Fig. 16 View FIGURE 16 H) are round cells (4.5–7.0 x 6.7–7.7 µm in diameter) with homogeneous spherical inclusions of variable size and density (0.6–1.0 x 1.6–3.6 µm in diameter) and large phagosomes. These cells are found in the choanosome but do not occur in all specimens (Table 1).
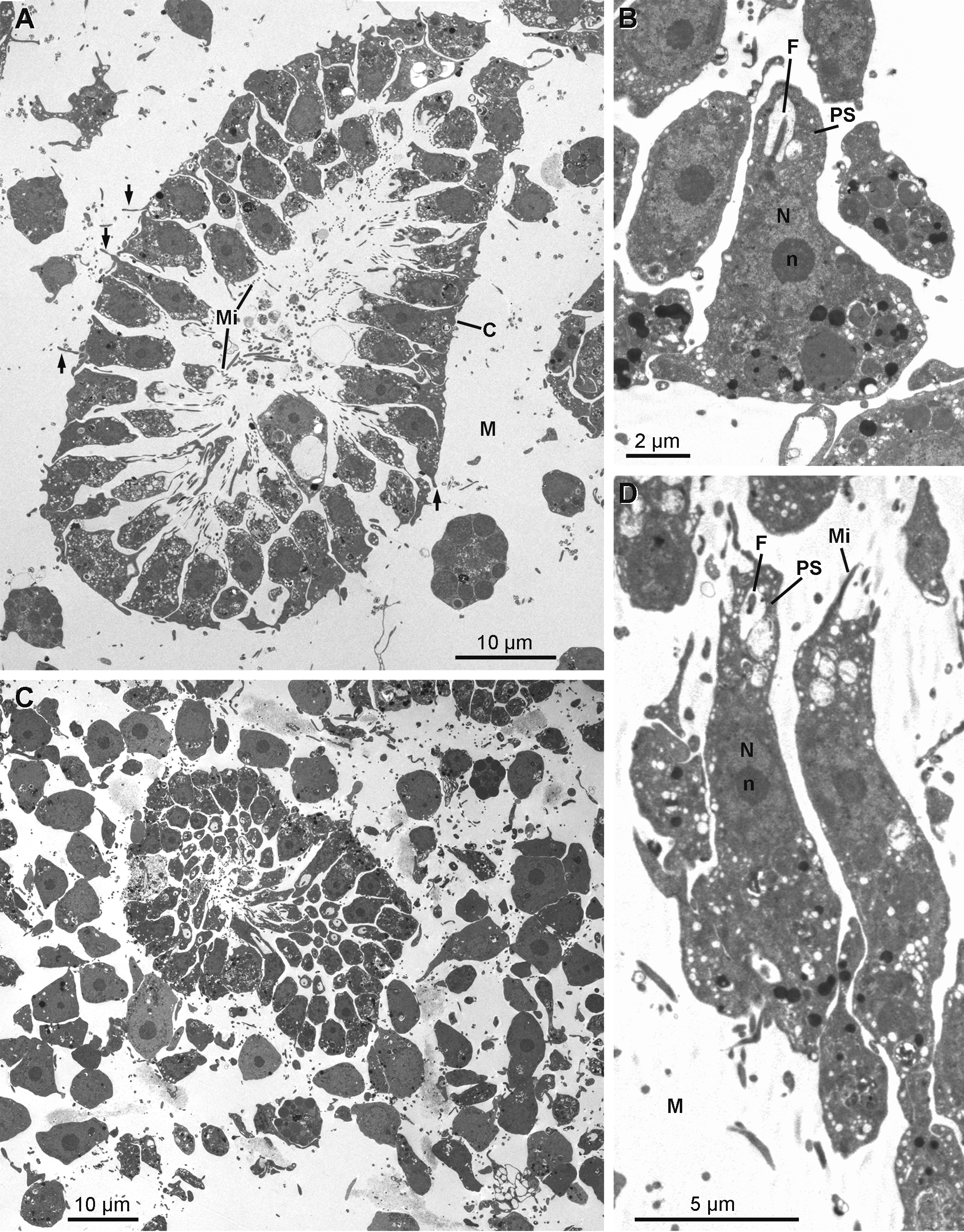
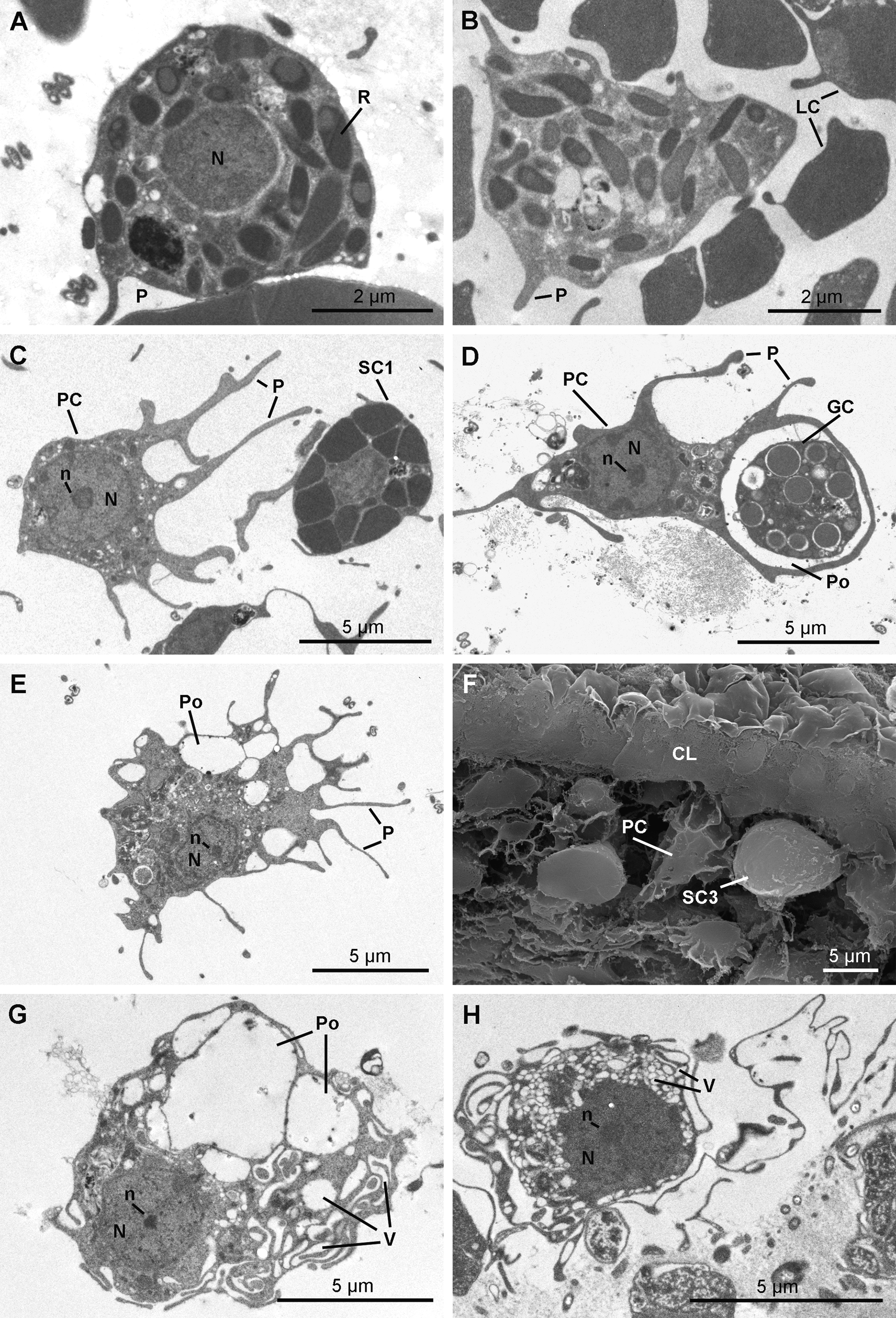
Rhabdiferous cells ( Figs 17 View FIGURE 17 A & 17B) are irregularly shaped cells (7.9–4.0 x 6.3–1.7 µm) with short pseudopodia. The nucleus is spherical and anucleolated (1.7–2.6 µm in diameter). The cytoplasm contains abundant osmiophilic elliptical membrane bound rhabdites (up to 1.3 µm in length and 0.8 µm in diameter), which include a less dense spherical sub-inclusion. Rhabdiferous cells occur in the ectosome and in the choanosome, where they are either free in the mesohyl or occasionally enclosed within choanocyte chambers and even larvae.
Pocket cells ( Figs 17 View FIGURE 17 C–17H) are amoeboid cells (6.3–13.3 x 3.9–7.2 µm) with long pseudopods extending actively around the cell to phagocytize components of the mesohyl, including cells from other types and bacteria, forming large "pockets". The nucleolated nucleus is round to ovoid (2.9 to 4.0 x 2.6 to 3.5 µm). These cells occur everywhere in the sponge, from the choanosome to the ectosome where they appear to be more developed. Their membrane is folded in many thin and puffed layers, forming small electron translucent microvesicles located at the periphery of the cell. Some pocket cells with numerous pockets and smaller electron translucent vacuoles are crossing the collagen layer to be rejected from the sponge as they are found at the surface of the exopinacocytes.
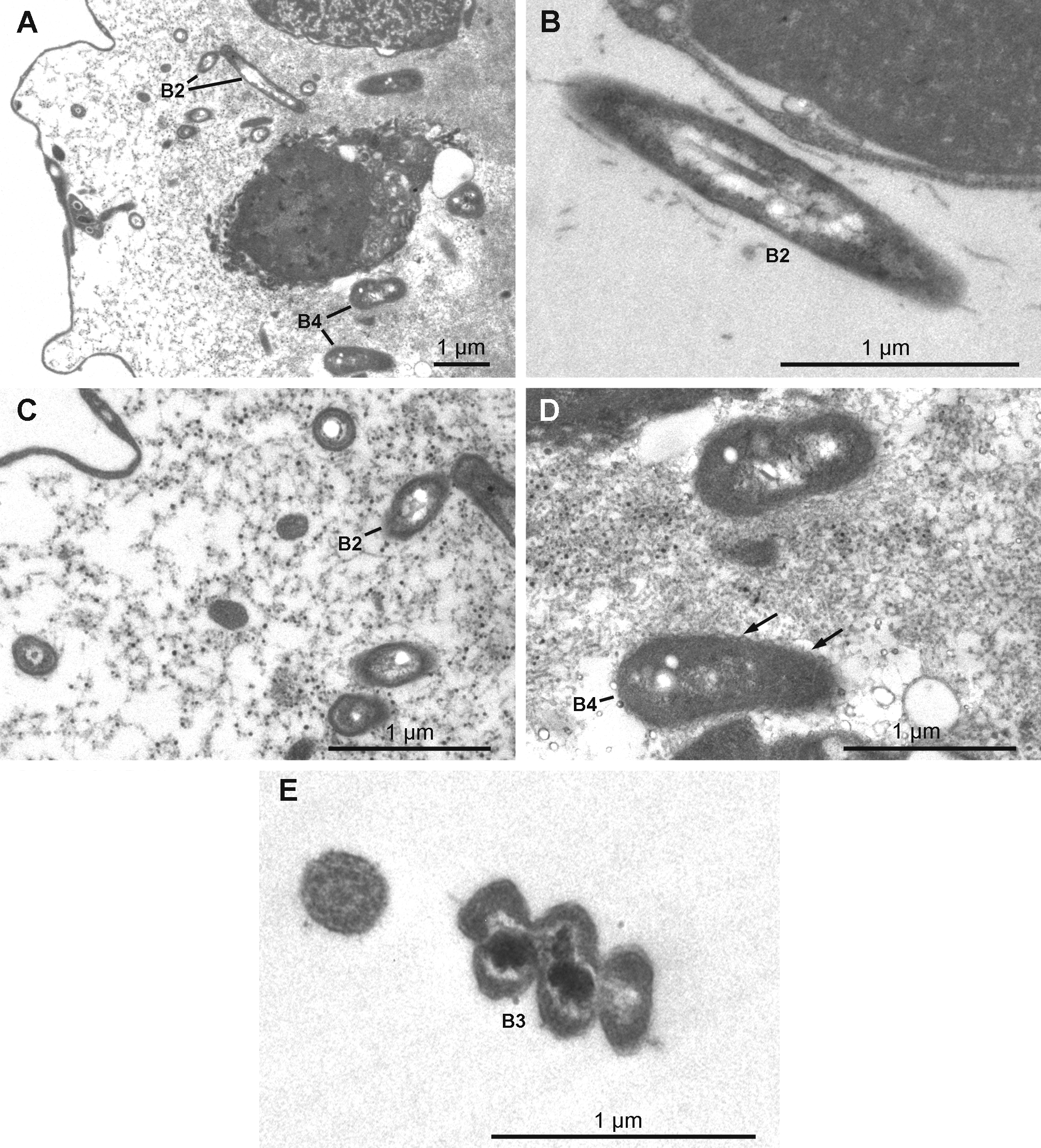
Extracellular symbiotic bacteria ( Figs 18 View FIGURE 18 A–18E) of three different morphotypes occur in the mesohyl. The nomenclature used here corresponds to the one used above for H. magellanica . Type B1 is absent. Type B2 is rod shaped and reaches 1.9 µm in length and 0.3 µm in diameter with an electron dense thick wall and an electron translucent cytoplasmic matrix. The electron translucent nucleoid presents a central rod surrounded by filamentous material. Type B2 occurs only in H. desqueyrouxae sp. nov. morphotype 2 (Table 1). Type B3 is spiral-shaped and thick walled, its width reaches 0.4 to 0.5 µm. The cytoplasmic matrix is clear and the nucleoid is electron dense. Type B3 occurs in the choanosome of all specimens of both morphotypes where it is evenly distributed. Type B4 is larger in diameter than Type B2 (1.1 to 1.4 x 0.5 µm), and has a more ovoid shape and a wrinkled capsule. No central rod appears in the clear nucleoid. This type was found in only one specimen of each morphotype (Table 1). Types B2 and B4 are exclusively found in the collagenous layer of the ectosome where they are frequent.
Reproduction. One of the three specimens collected in early February 2009 contained embryos and prelarvae. Larvae appeared in two out of the three specimens collected in May 2007 ( Figs 12 View FIGURE 12 A, 12C & 12H).
Distribution. Halisarca desqueyrouxae sp. nov. is presently known from Chilean Patagonia (42°S).
Habitat. Halisarca desqueyrouxae sp. nov. is commonly found at depths ranging from 3 to 26 m, on mussel beds at shallow depths or covering rocks exposed to the light and under overhangs below 10 m.
Etymology. The specific epithet is a tribute to Dr. Ruth Desqueyroux-Faúndez who inspired our first sponge collections in Chilean Patagonia and actively participated in several expeditions in that region.
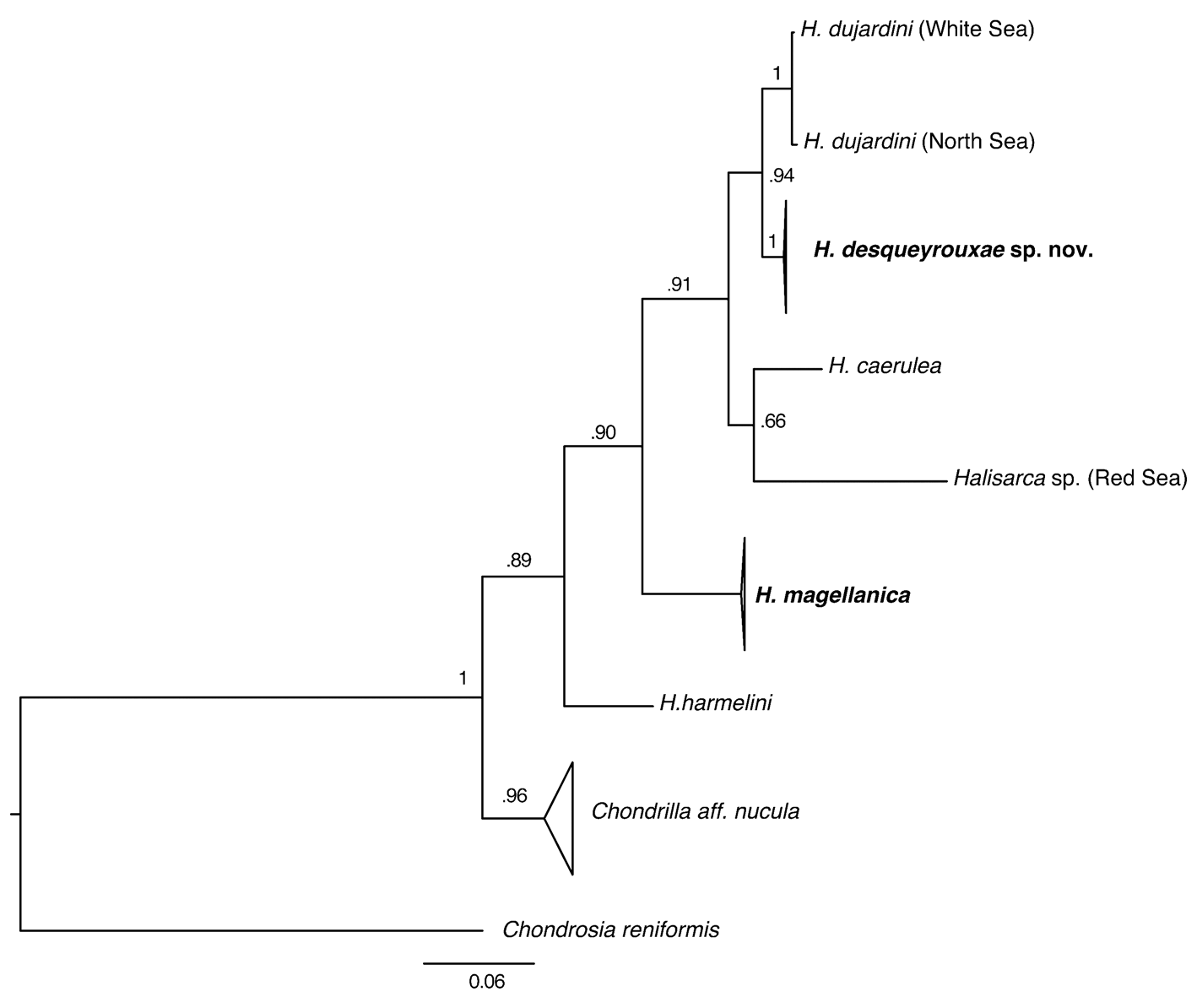
Phylogenetic analysis. Partial cox1 sequences were determined for seven specimens of H. desqueyrouxae sp. nov. (RBINS 10803, 10757, 12917, 12919, 12924 and 12923 = morphotype 1; RBINS 10754 = morphotype 2) and were found to be identical. Phylogenetic analysis placed H. desqueyrouxae sp. nov. as a sister group to H. dujardini , but revealed 2.3% difference between their cox1 sequences ( Fig. 19 View FIGURE 19 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |