Phyllodesmium rudmani, Burghardt, Ingo & Gosliner, Terrence M., 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.173797 |
|
DOI |
https://doi.org/10.5281/zenodo.5662435 |
|
persistent identifier |
https://treatment.plazi.org/id/BB368798-FFB8-FF98-3476-FAE35D77075A |
|
treatment provided by |
Plazi |
|
scientific name |
Phyllodesmium rudmani |
| status |
sp. nov. |
Phyllodesmium rudmani sp. nov.
Figs. 1 View FIGURE 1 –5
Type material: Holotype: PHILIPPINES, LUZON ISLAND, Batangas Province, Calumpan Peninsula, Arthur’s Rock, 12 m depth, 22 February,1995, (Michael Miller) ( CASIZ 103747). (specimen #4; see Table 1 View TABLE 1 ). Paratype: INDONESIA, NORTH SULAWE SI, Pulau Talise, close to the village of Aerbanua, 1 m depth, 23 July 2003 (Ingo Burghardt) ( ZSM Moll 20050285).
Other material examined: two specimens, INDONESIA, NORTH SULAWESI, south side of Bunaken Island, Bunaken Islands National Park, in the lagoon in front of Papa Boa Bungalows, GPS: 01°37’3.1’’N, 124°45’51.1’’E; 0.5 m depth, 19, 21 July 2003, (Ingo Burghardt).
Distribution: Known only from the Philippines and northern Indonesia.
Etymology: This species is dedicated to Dr. Bill Rudman, a great scientist and colleague who described the vast majority of Phyllodesmium species and is a pioneer in working on “solar powered” nudibranchs.
Description
Colour and external morphology of living animal:
Body of living animals, including rhinophores, oral tentacles, foot translucent white ( Figs. 1 View FIGURE 1 A–C, 1E). Animals elongate, up to approximately 45 mm in length (without cerata), with few short cerata (especially at margin of foot), several long cerata (longer than rhinophores and oral tentacles), covering whole notum ( Figs. 1 View FIGURE 1 A, 1E). Cerata (40–100) arranged in up to 7 double clusters with up to 10 cerata on each pad. Lateral cerata very small, short. Cerata elongate, circular in crosssection. Upper third of ceras bulbous, broadened, mimicking closed Xenia polyp. Basal creamcoloured part of ceras smooth with several slight longitudinal grooves. Basal end of ceras appearing clubbed. Cream basal part of ceras continuing in up to 10 ridges of apical part. Most ridges continuing and meeting in apex of ceras, some only partly developed. Between cream ridges broad brownish depressions, composed of very small brownish dots, probably representing clusters of zooxanthellae within digestive gland.
Smooth oral tentacles shorter than rhinophores, usually directed laterally ( Fig. 1 View FIGURE 1 B). Rhinophores wrinkled but not lamellate, similar in shape, surface texture to oral tentacles. Rhinophores standing close together. Oral tentacles, rhinophores blunt, rounded. Anterior foot margin angular, slightly extended ( Fig. 1 View FIGURE 1 B). Posterior end of foot tapering, but not acutely pointed.
Description of preserved animals
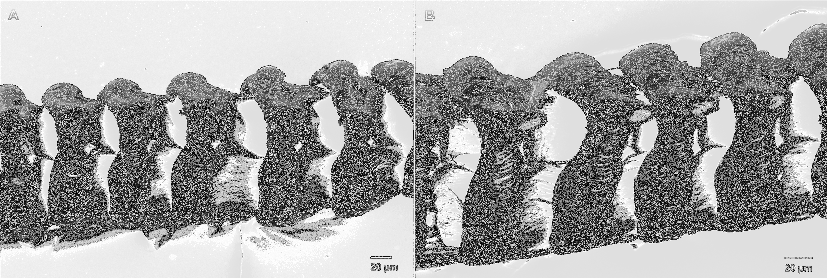
External morphology: No distinct notal rim. Foot anteriorly angled without propodial tentacles; posterior portion with pointed extension. Oral tentacles wrinkled, shorter than rhinophores. Rhinophores also wrinkled, sometimes appearing lamellate, standing close to each other. Directly behind rhinophores, in some specimens, distinct white patch of pigmentation present. Cerata appear shrunken, without colour. Insertion of cerata arranged in incomplete arches, lying on distinct pads. Apical portion of arches absent, two adjacent sloping pads not contiguous. Internally, digestive gland duct of each sloping pair of cerata limbs uniting with other half, connecting to primary axis of digestive gland. Four to seven pads or patches present on each side, lying opposite each other. First cerata slightly anteriolateral of rhinophores. Anal papilla cleioproctic, located dorsolaterally on right
side between first, second postcardiac pads. Genital aperture ventral to anterior edge of anterior limb of precardiac arch. Whole epidermis composed of specialised vacuolated cells ( Figs. 4 View FIGURE 4 B–C).
Central nervous system: Central nervous system similar to all described Phyllodesmium species, highly concentrated with fused cerebralpleural ganglia. Pedal ganglia situated laterally as distinct lobes. Small eyes adjacent to junction of cerebropleural ganglia with pedal ganglia. Pair of small buccal ganglia situated on posterior side of buccal mass, immediately ventral to junction of mass with oesophagus.
Digestive system (including radula): Buccal mass large, highly muscular with large chitinous jaws occupying much of external surface of mass. Radular sac short, readily visible. Pair of thin, simple, elongate salivary glands entering buccal mass near junction of mass with esophagus. Esophagus expanding into thinwalled stomach on left side of body, adjacent to genital mass. Intestine emerging from right side of stomach, terminating at lateral anus. Paired jaws ( Fig. 2 View FIGURE 2 B) thick, coriaceous with thickened anterior cusp. Masticatory border containing 6–7 irregular denticles. More anterior denticles shorter, more irregular in shape. Posteriormost denticle longest. Radular formula of holotype (CASIZ 103747): 62 X 0.1.0. Radular teeth ( Figs. 3 View FIGURE 3 A–B) wide, triangular in shape with short primary cusp. Series of 39–51 small, congested, pointed denticles present along either side of primary cusp. Some denticles bifurcated deeply near common base of denticles.
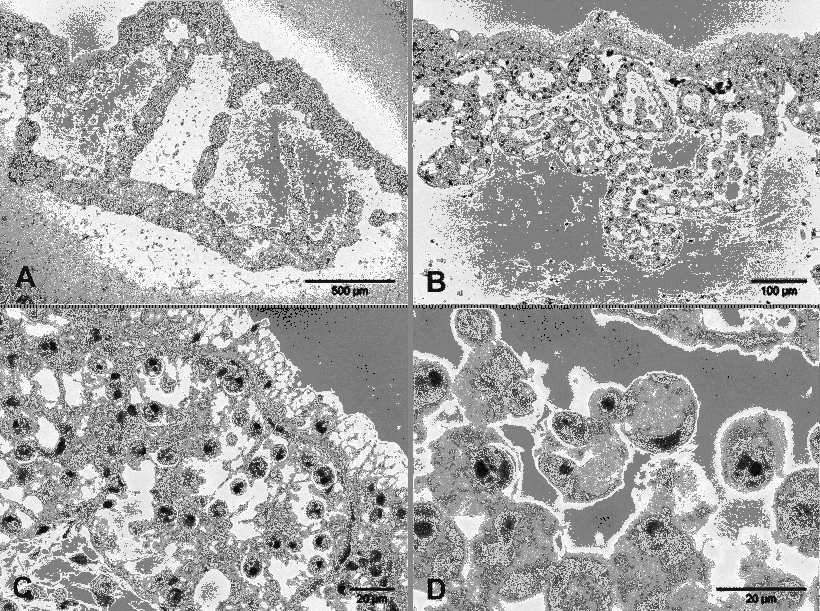
Digestive gland in cerata: Single central narrow digestive gland duct extending through each ceras. Apical, bulbshaped part, central duct ramifying into primary, secondary branches, with secondary branches radiating towards ceratal wall ( Fig. 4 View FIGURE 4 A).
Each secondary branch terminating in one or multiple saclike structures ( Fig. 4 View FIGURE 4 B). Intact zooxanthellae present in digestive glandular cells of these sacs, epithelium of branches ( Figs. 4 View FIGURE 4 B–C). High concentration of zooxanthellae present especially in apex of ceras. Highest concentration of zooxanthellae in cells close to epidermis ( Figs. 4 View FIGURE 4 A–B). Zooxanthellae also present in lumen of branches, mostly enclosed in circular, free floating digestive glandular cells ( Figs. 4 View FIGURE 4 B, 4D). Cnidosac in largest cerata not detectable. In basal part of each ceras less branching of digestive gland observed, in general lower concentration of zooxanthellae present. Smaller cerata not investigated. Whole epidermis composed of specialised vacuolated cells ( Figs. 4 View FIGURE 4 B–C; similar to Phyllodesmium jakobsenae ).
Reproductive system: Reproductive system ( Fig. 2 View FIGURE 2 A) androdiaulic. Short, narrow preampullary duct widening into elongate, slightly convoluted ampulla. Postampullary duct narrow, bifurcating into vas deferens and oviduct. Vas deferens initially thin, gradually widening, curving into wider prostatic portion. Prostatic portion continuing to widen, curving twice again before entering more muscular penial sac. Inside penial sac, short, blunt, unarmed penial papilla present. Penial sac exiting adjacent to opening of mucous gland at common genital aperture. Short oviduct entering elongate receptaculum seminis. Receptaculum highly muscularized with nodular surface. Nodular surface uncommon for genus. From distal base of receptaculum, second duct emerging, entering small albumen gland. Albumen gland encased in larger membrane gland. Mucous gland forming largest portion of female gland mass. Female gland mass with many folds, second more pale coloured portion present. Mucous gland terminating at genital aperture.
Biological notes:
Most specimens were found deeply burrowed into an unknown species of Xenia (Ehrenberg, 1831; Xeniidae, Alcyonaria, Octocorallia ). The bodies of these specimens were not visible, only the cerata were slightly surpassing Xenia’ s tentacles. Phyllodesmium rudmani was found in the same location as P. jakobsenae , even in the same Xenia species, but on different colonies ( Burghardt & Wägele 2004).
Phyllodesmium rudmani is very well camouflaged: A ceras mimics a whole closed polyp of Xenia in colour and shape. In each coral colony only one specimen was present. Figure 1 View FIGURE 1 F shows the emerging slug on top of the Xenia colony; in Figure 1 View FIGURE 1 C slug and coral are separated. Unfortunately there are no ecological data available for the type locality.
The site close to Bunaken Island is a shallow lagoon, fringed by mangroves. The two specimens were found in the intertidal zone in approximately 0.5 m depth. The substrate is a mixture of sand, mud, coral rubble and living hard corals. On the coral rubble there are single colonies of Xenia . Most of the sandy patches around the Xenia colonies are covered with seagrass. Water temperature was 31°C on average (July 2003).
The second Indonesian locality close to Pulau Talise is an intact coral reef with some big dead coral blocks. The big specimen #3 of P. rudmani was sitting isolated on top of one of these blocks resembling a whole Xenia colony. There were no Xenia colonies in the vicinity.
FIGURE 5. Measurements of photosynthetic activity of zooxanthellae in situ: A: Yield (IIemax) values measured against irradiance (light intensity) of Phyllodesmium rudmani , P. jakobsenae and Xenia sp. Data based on measurements of eight specimens of P. jakobsenae , three specimens of P. rudmani and one Xenia colony. Note the higher values for Xenia at same irradiances. B: Yield values of one specimen (#2) of P. rudmani measured over time during starvation period. The average line indicates a slight decrease of the yield values. C: Groundfluorescence (F0; [mV]) of one specimen (#2) of P. rudmani during starvation. Decline of values indicates the decrease of numbers of photosynthetic active zooxanthellae. Average lines calculated by Windows Excel 2000 (Microsoft). P. rudmani ; P. jakobsenae ; Xenia sp.
All Indonesian specimens were immediately put into aquaria in order to perform longterm starvation experiments. The cerata easily autotomised when the animals were disturbed, probably because of the high water temperatures in the aquaria. As in P. jakobsenae , the detached cerata exuded a sticky secretion and moved for some minutes. This secretion may have come from the few mucous cells found especially in the epidermis of the proximal part of the ceras and probably not from the “cellules spéciales” ( Edmunds 1966: 34) since they have not been observed in the cerata.
There was no diurnal behaviour observed in P. rudmani as in P. jakobsenae . The appearance of P. rudmani depends on its activity state. During resting periods it spreads its cerata and looks like an isolated Xenia colony ( Fig. 1 View FIGURE 1 D). Almost the whole body is well camouflaged in this state. Similar behaviour can be seen in specimens sitting on Xenia ( Fig. 1 View FIGURE 1 F). Slugs that are in motion look quite different ( Figs. 1 View FIGURE 1 A, 1E): The notum, parts of the foot and particulary the rhinophores and oral tentacles are visible.
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.