Leptocera parallelipennis Buck, 2009
|
publication ID |
https://doi.org/10.11646/zootaxa.2039.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/BB4C084E-FF8B-A77D-0CE0-FEBCFEC0A498 |
|
treatment provided by |
Felipe |
|
scientific name |
Leptocera parallelipennis Buck |
| status |
sp. nov. |
Leptocera parallelipennis Buck , new species
( Figs. 14–15 View FIGURES 10–15 , 18 View FIGURES 16–29 , 109 View FIGURE 109 , 131–137 View FIGURES 131–137 , 144 View FIGURES 138–146 )
Leptocera ellipsipennis View in CoL auctt., nec Richards, 1955: Richards, 1955 (in part).
Taxonomy. This species has been confused with L. ellipsipennis View in CoL (see Taxonomy section under the latter).
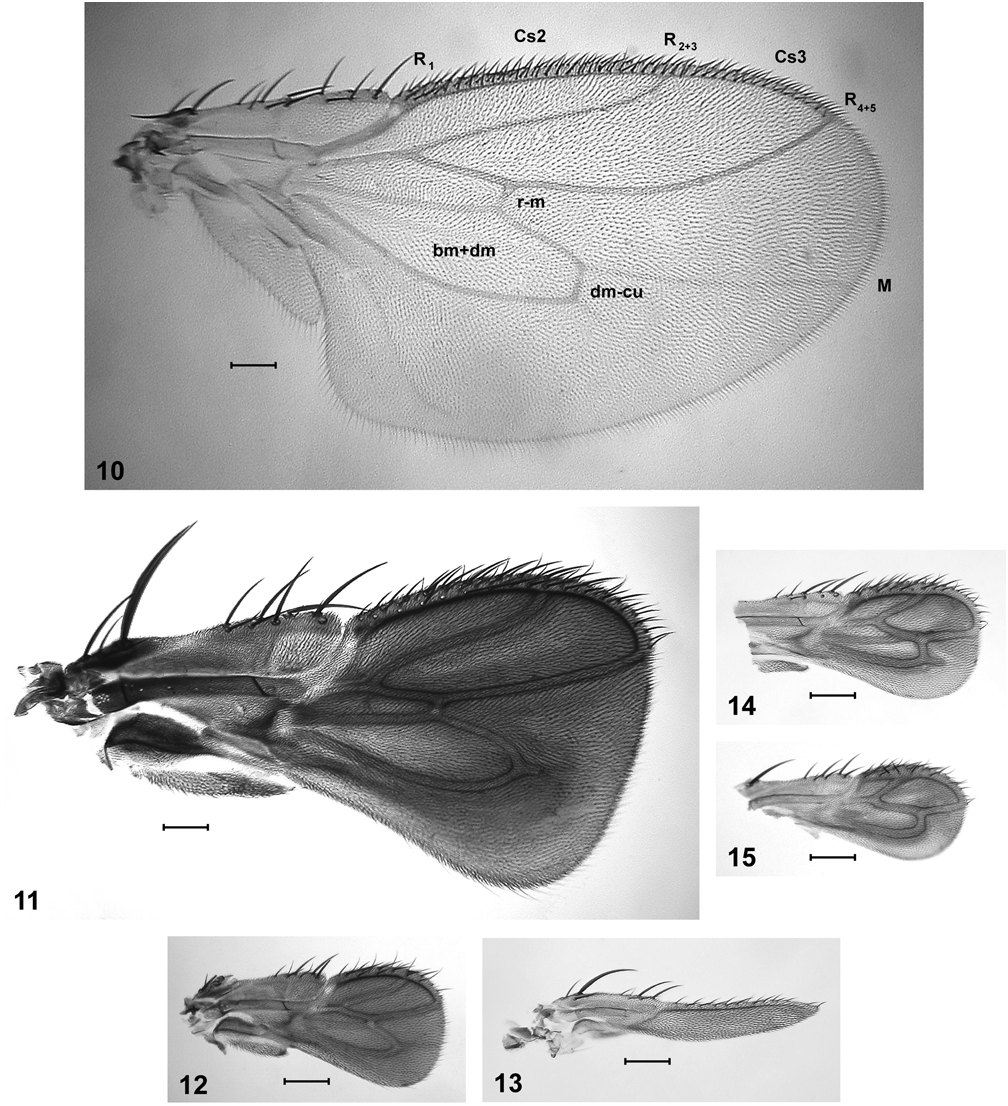
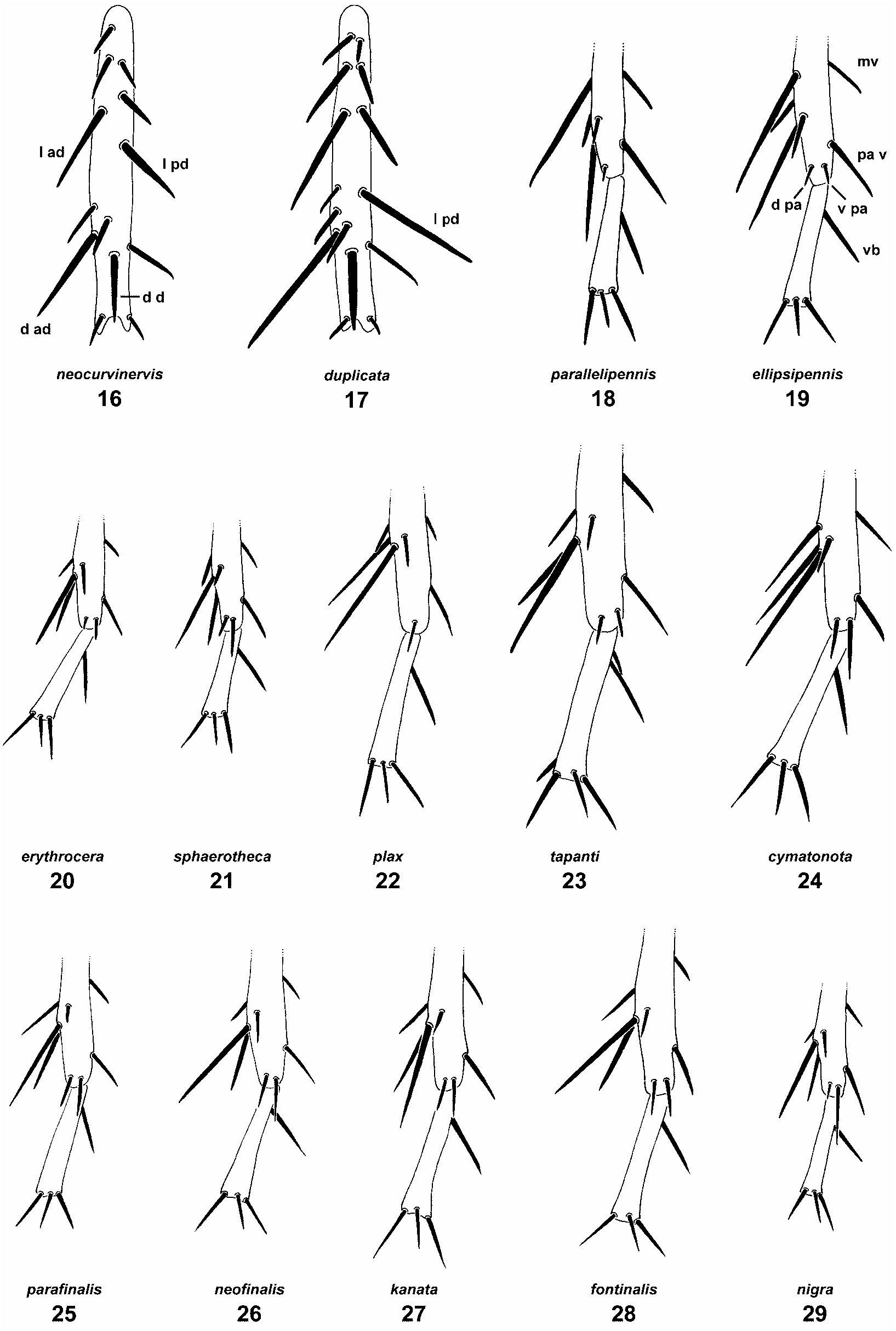
Description. Smaller than L. ellipsipennis , body length 1.6–2.5 mm. Habitus as in Fig. 109 View FIGURE 109 . Coloration as in L. ellipsipennis but posterior part of postpronotal lobe also often pale. Scutum with 4(–5) dorsocentral bristles (fifth one from behind, if present, less than half as long as fourth one; in L. ellipsipennis always present and more than half as long). Third dorsocentral bristle from behind shifted anteriorly: its socket on, or slightly behind a line connecting sockets of presutural supra-alar and presutural intra-alar bristles. Wing rudiment similar to L. ellipsipennis but usually less infuscated, less expanded distally, and wing venation slightly more reduced ( Figs. 14 View FIGURES 10–15 ). Third costal sector usually longer than second. Vein M more or less fused to R 4+5 and thus obliterating crossvein r-m, the point of connection between the two always very close to or at level of apex of discal cell. In specimens with very small wings the part of M basad of the fused section may be partially or completely absent ( Fig. 15 View FIGURES 10–15 ). Discal cell with truncate apex, usually appendiculate. Mid tibia with bristle above distal dorsal bristle longer than corresponding bristle above distal anterodorsal, 0.4–0.5x as long as distal anterodorsal; dorsal posteroapical bristle short, ventral posteroapical not differentiated (i.e., hair-like and indistinguishable from surrounding hairs; Fig. 18 View FIGURES 16–29 ).
Male terminalia ( Figs. 131–134 View FIGURES 131–137 ): Sternite 5 with small, microtrichose posteromedial area; hind margin without enlarged scales. Surstylus with anterior process of anterior section rounded apically and with small, darkened, anterior point; ventral lobe relatively short, with concave ventral margin bearing relatively long bristles. Posterior section of surstylus with two strong bristles close to apex, both with fine tips (outer one shorter and less tapered), inner margin broadly desclerotized, transparent and membranous; posterior surface with few bristles. Cercus simple, with microtrichose medial surface, preapically with a small tuft of enlarged microtrichia. Postgonite with deep and narrow posterobasal notch, shank thickened toward base ( Fig. 144 View FIGURES 138–146 ).
Female terminalia ( Figs. 135–137 View FIGURES 131–137 ): Tergite 7 often paler than previous tergite (at least posteriorly), relatively long, moderately convex, pruinose except for slightly shining hind margin; enlarged paramedian bristles of moderate length and strength, convergent or cruciate. Sternite 7 dark, with shining, paler (reddish) hind margin. Sternite 8 not emarginate on each side of posteromedial lobe. Tergite 10 + cerci very short, with few short hairs. Spermathecae elongate, dilated apical portion finely striate.
Type material. Holotype ♂ ( MNNC) and 5 ♂♂ paratypes ( DEBU): CHILE, Reg. Aconcagua, Juan Fernández Is., Robinson Crusoe I., above Plazoleta , 1–9.i.1993, open forest, pans, S.A. Marshall. Other paratypes: 1 ♂, same as previous but lower El Yunque trail, 31.xii.1992, aspirated, S.A. Marshall ( DEBU); 1 ♂, 1 ♀ (both also paratypes of L. ellipsipennis , misidentification), Masatierra [= Robinson Crusoe I.], 1952 ( BMNH); Masatierra, Plazoleta del Yunque, 200 m, 1 ♀ ( allotype of L. ellipsipennis , misidentification), 2.i.1952 ( IESC), 1 ♀ ( paratype of L. ellipsipennis , misidentification), 20.ii.1951 ( IESC), 1 ♂, 28.xii1954 ( BMNH), P.G. Kuschel; same as previous but 220 m, 1 ♂, 28.xii.1954, 1 ♂, 1 ♀, 3.ii.1955 ( BMNH, ♀: USNM); 1 ♀ ( paratype of L. ellipsipennis , misidentification), Masatierra, no other data [probably collected by G. Kuschel 1951–5] ( IESC). The following specimens are labelled as “ paratypes ” of L. ellipsipennis (misidentification by Richards) but do not represent true paratypes (see note under Type material of L. ellipsipennis ): 1 ♂, 1 ♀, “63.”, 1 ♂, 1 ♀, “69.”, no other data [probably collected by G. Kuschel on Robinson Crusoe I., 1951–5] ( IESC).
Etymology. This species is named for the shape of its wing rudiment (Lat. penna: feather, wing), which is usually more parallel-sided and less wedge-shaped than in the similar L. ellipsipennis .
Discussion. This species is similar to L. ellipsipennis with which it has been confused in the past. Besides characters mentioned in the key L. parallelipennis sp.n. can be distinguished from the latter as follows: third dorsocentral bristle from behind shifted forward (compare species descriptions), fifth dorsocentral bristle from behind small if present (compare species descriptions), ventral lobe of anterior section of surstylus with concave margin (slightly convex in L. ellipsipennis ), male cercus simple (with transparent, lamellate inner portion in L. ellipsipennis ), female tergite 7 pruinose (posterior half with shining medial stripe in L. ellipsipennis ), female sternite 8 not emarginate posteriorly (with deep emarginations on each side of posteromedial lobe in L. ellipsipennis ).
Conservation. Due to their extremely restricted distribution and association with well preserved native forests all species of the L. cultellipennis subgroup have to be considered highly vulnerable. Parts of Robinson Crusoe I. have been subject to deforestation and extreme soil erosion, leaving an irreversibly altered landscape that is unsuitable for the native Leptocera fauna as well as many other native animals and plants. Based on collection data it appears that a shift in the native Leptocera fauna has already taken place: G. Kuschel’s 1951–5 material which was examined by O.W. Richards included L. ellipsipennis and L. parallelipennis sp.n. at a ratio of 1: 3.2. The junior author’s material from 1992/3 included the same species at a ratio of 48: 1. Despite a targeted and extensive collecting effort over a three week period the junior author was only able to obtain 7 specimens of L. parallelipennis sp.n. (compared to 13 collected by Kuschel) even though 20 times more specimens of the two species were collected (341). It appears that L. parallelipennis sp.n. has become very rare and might be close to extinction. Further evidence of faunal change is the new discovery of the cosmopolitan and hemisynanthropic L. caenosa on the island in 1993 (see Material examined under L. caenosa ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.