Capromys pilorides ( Say, 1822 )
|
publication ID |
https://doi.org/10.1093/mspecies/sead002 |
|
publication LSID |
lsid:zoobank.org:pub:A4B7822F-44FC-4EC6-B574-7DE2D7038407 |
|
persistent identifier |
https://treatment.plazi.org/id/BB5687AF-FFF1-FFD6-FC6E-30B4BB80F9C0 |
|
treatment provided by |
Plazi |
|
scientific name |
Capromys pilorides ( Say, 1822 ) |
| status |
|
Capromys pilorides ( Say, 1822) View in CoL
Desmarest’s Hutia
I [ sodon] pilorides Say, 1822:333 . Type locality “ South America , or one of the West Indian Islands. ”
Capromys Furnieri Desmarest, 1822b:43 View in CoL , plate I. Type locality “ Cuba View in CoL .”
Capromys Fournieri View in CoL : Waterhouse, 1848:150. Corrected spelling of Capromys furnieri Desmarest, 1822b .
Capromys pilorides View in CoL : Waterhouse, 1848:287. First use of current name combination.
Capromys Fourneiri View in CoL : Waterhouse, 1848:287. Incorrect subsequent spelling of Capromys fournieri Desmarest, 1822b .
Capromys geayi Pousargues, 1899:152 View in CoL . Type locality “ Mountains between La Guayra and Caracas, Venezuela.” Collecting locality incorrectly recorded; this specimen is likely a juvenile Capromys pilorides View in CoL erroneously reported from Venezuela ( Woods and Kilpatrick 2005).
Procapromys geayi View in CoL : Chapman, 1901:322. Name combination.
Capromys intermedius Arredondo de la Mata, 1958:48 . Type locality not given.
Capromys megas Varona and Arredondo, 1979:12 . Type locality “ Cueva Lamas, a 2 km aproximadamente al SW de Playa Santa Fe, Bauta , Habana, Cuba View in CoL .”
Capromys arredondoi Varona, 1984a:2 . Type locality “Cueva Insunsa, La Salud, Provincia Habana, Cuba View in CoL .”
Capromys pappus Varona, 1984b:2 . Type locality “ Cueva del Abuelo , Sierra de Caballos, Isla de la Juventud ( Isla de Pinos), Cuba View in CoL .”
Capromys fourniere : Woods and Kilpatrick, 2005:1594. Incorrect subsequent spelling of Capromys fournieri Desmarest, 1822b .
CONTEXT AND CONTENT. Context as for genus. Capromys pilorides View in CoL has six currently recognized subspecies, one extinct ( C. p. lewsi — Morgan et al. 2019) and five extant ( Silva Taboada et al. 2007; Borroto-Páez 2011a; Borroto-Páez and Mancina 2017).
C. p. ciprianoi Borroto, Camacho, and Ramos, 1992:87. Type locality “Punta del Este, on the south-eastern portion of the Isle of Youth, Cuba ;” (see “Nomenclatural Notes”).
C. p. doceleguas Varona, 1980:141. Type locality “Cayo Anclita (Miraflores), Laberinto de las Doce Leguas, Archipiélago Jardines de la Reina, Cuba .”
C. p. gundlachianus Varona, 1983:77 . Type locality “Cadena de cayos al oeste de Cayo Bahía de Cádiz , a unos 2 km al sur de la desembocadura del canal principal, Canal Blanca, en los manglares de los canalizos, Bahía de Santa Clara, Archipiélago de Sabana, Cuba .”
C. p. lewisi Morgan, MacPhee, Woods, and Turvey, 2019: 28. Type locality “ Stake Bay Cave, 0.25 miles west of Stake Bay, Cayman Brac.”
C. p. pilorides ( Say, 1822:333) . See above; arredondoi Varona , fournieri Desmarest , geayi Pousargues , intermedius Arredondo de la Mata , and megas Varona and Arredondo are synonyms.
C. p. relictus G. M. Allen, 1911:207. Type locality “ Casas Mountains , Nueva Gerona, Isle of Pines, Cuba ”; pappus Varona is a synonym .
NOMENCLATURAL NOTES. The genus name is a Latinized version of the Greek words capro (wild boar) and mys (mouse), this refers to its similarity to a wild boar in terms of its general appearance and manner of running ( Palmer 1904). The specific name was first mentioned as Mus pilorides Pallas, 1778:91 ; but Tate (1935) noted that it is not associated with the genus Capromys . An additional common name in English is Conga hutia; in Cuba its common name in Spanish is “jutía conga.”
Based on limited molecular divergence (0.4%), the subspecies C. p. ciprianoi was considered as junior synonym of C. p. relictus ( Woods et al. 2001). However, Borroto-Páez et al. (2005) justified maintaining it as a distinct subspecies based on morphological differences. Cytochrome-b divergence data have also been used to propose the existence of an undescribed subspecies from Cayo Campo, Archipiélago de los Canarreos ( Woods et al. 2001), and three more Cuban populations offshore have been proposed to also represent distinct but unnamed subspecies ( Borroto-Páez et al. 2012b). Using three mitochondrial and three nuclear genes, Upham and Borroto-Páez (2017) found evidence of a phylogeographic split from west to east in Capromys pilorides , with 5.2% of cytochrome-b sequence divergence between two major clades. The western clade includes Cuban island hutias and those on Isla de la Juventud plus Cayo Cantiles, and the eastern clade contains all Capromys east of Sierra del Escambray in central Cuba , including mainland and offshore forms. These authors, based on the observed high level of genetic divergence, suggested that the eastern clade could be a different species of Capromys . To date, due to lack of clear diagnostic characteristics and limited morphological and genetic data, these forms of genetic divergence have not yet been related to named taxonomic units within Capromys .
DIAGNOSIS
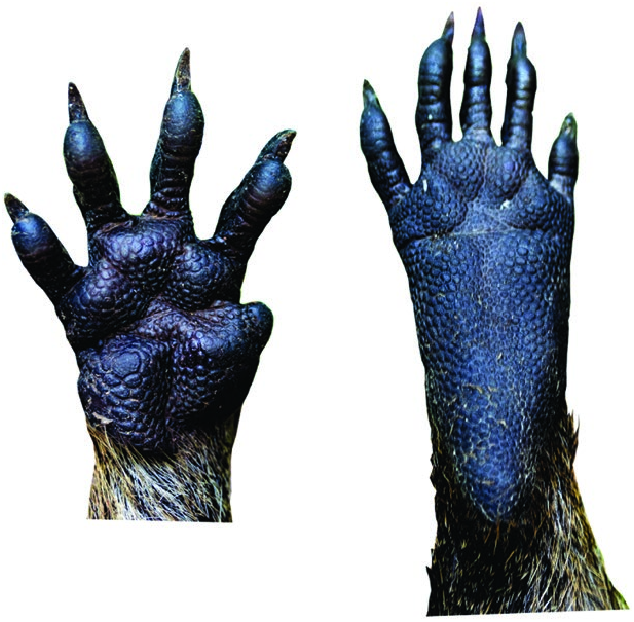
Capromys pilorides ( Fig. 1 View Fig ) is sympatric with the prehensile-tailed hutia Mysateles prehensilis , eared hutia Mesocapromys auritus , dwarf hutia Mesocapromys nana , Cabrera’s hutia Mesocapromys angelcabrerai , and black-tailed hutia Mesocapromys melanurus ( Silva Taboada et al. 2007; Borroto-Páez and Mancina 2011; Fabre et al. 2016). Capromys pilorides is distinguished from these five species by its large size; it is one of the world’s largest rodents and it is the largest capromyid species (adult mass> 3 kg, occipito-premaxillary length> 90 mm). All other capromyids have an adult mass < 3 kg and an occipito-premaxillary length < 75 mm. Capromys pilorides has the proportionally shortest tail length of the Cuban capromyids, approximately 50% of the head–body length, whereas the other Cuban species have a tail of about 70% of the head–body length. Capromys pilorides also has dark skin on the sole of the hind foot, whereas in other capromyid species, this skin is pale ( Fig. 2 View Fig ). The liver of C. pilorides is subdivided into reticulated lobules, in other Cuban capromyids these lobules are smooth ( Say 1822; Silva Taboada et al. 2007; Borroto-Páez 2011a).
GENERAL CHARACTERS
The pelage consists of moderately long and coarse guard hairs, its coloration varies from a whitish-gray to a reddish-brown, dark brown to almost black ( Berovides Álvarez and Smith 1982). Capromys pilorides shows predominantly three coloration patterns of pelage ( Fig. 3 View Fig ). The more common is a variety of pale brown to reddish-brown, known as “agouti,” there are individuals with whitish-gray heads and chests, coloration known as “furnieri ” and almost black individuals with a coloration known as “mandinga.” The underside is generally lighter than the rest of the body ( Gundlach 1877; Berovides Álvarez and Smith 1982; Pérez-Beato et al. 1982; Abreu and Manójina 1989; Borroto-Páez 2011a). Individual guard hairs are smooth and straight; these generally have a brown band that varies in intensity and amplitude, determining the phenotypic diversity in agouti coloration throughout the Cuban archipelago ( Berovides Álvarez and Smith 1982). Pelage color has no association with sex or age ( Angulo 1946).
The body is robust, the head is broad with snout truncated anteriorly, and the eyes and ears are relatively small. The limbs are short and there are prominent postdigital tubercles on the palmar and plantar surfaces. The claws are well-developed except on the rudimentary thumb ( Gundlach 1877; Angulo 1946; Silva Taboada et al. 2007; Borroto-Páez 2011a). There is no caudal autotomy and there are short hairs with brownish-orange or dark tones and scaly rings that decrease in size toward the distal extremity of the tail ( Gundlach 1877; Abreu and Manójina 1989; Silva Taboada et al. 2007).
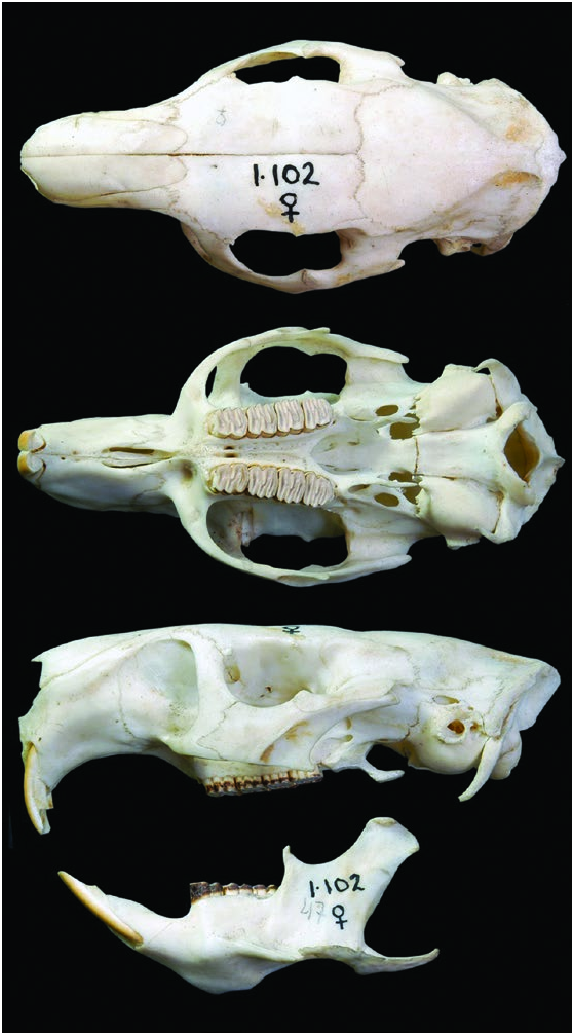
The skull is robust and slightly flat in lateral profile, the paroccipital process is long and thin, and the lateral process is flattened against the paroccipital process ( Fig. 4 View Fig ). The pterygoid plate is separated from the pterygoid process by a blade-like lateral pterygoid ridge. The zygomatic arch extends ventrally as a blade-like structure below the level of the molar toothrow and terminates in a large inferior jugal process; the superior and inferior zygomatic roots of the maxillary are relatively narrow. The nasals are anteriorly inflated and the incisors are strongly curved. The maxillary toothrows are slightly convergent anteriorly and all the open-rooted molariform teeth are nearly the same size. The mandible has a well-developed coronoid process, the masseteric crest and pterygoid shelf are shallow and the angular process is thin. The retromolar fossa is a narrow groove and the mandibular foramen is on its posterior margin. The incisors are delicate, the alveolar sheath terminates at the level of the mandibular foramen; the molariform teeth lie in a horizontal plane ( Woods and Howland 1979; Woods and McKeen 1989; Silva Taboada et al. 2007).
Mean external measurements (mm or kg; ± SD; parenthetical n) of western C. pilorides pilorides from Península de Guanahacabibes, Pinar del Río Province, for females and males, respectively, were: total length, 759.7 ± 15.5 (30) and 772.9 ± 18.1 (28); tail length, 235.6 ± 6.9 (30) and 239.6 ± 8.7 (28); ear length, 39.8 ± 0.9 (30) and 39.8 ± 0.8 (28); length of hind foot, 91.9 ± 6.2 (30) and 98.1 ± 1.3 (28); body mass, 4.233 ± 0.3035 (27) and 4.266 ± 0.3534 (24— Abreu and Manójina 1989). Mean external measurements (mm or kg; ± SD; parenthetical n; sexes combined) of northern C. pilorides relictus and southern C. pilorides ciprianoi from Isla de la Juventud, respectively, were: total length, 693.4 ± 97.5 (18) and (726.6 ± 57.8, 19); tail length, 240.1 ± 20.9 (18) and 244.4 ± 28.0 (19); ear length, 35.5 ± 3.1 (18) and 35.1 ± 4.9 (19); length of hind foot, 97.8 ± 4.5 (18) and 101.1 ± 6.5 (19); body mass, 3.51 ± 0.73 (18) and 4.86 ± 0.15 (19— Borroto-Páez et al. 1992).
Cranial and dental measurements (mm; mean ± SD; parenthetical range; n; sexes combined) for adults were: condyle-premaxillary length, 93.2 ± 5.24 (74.3–108.3, 86); occipito-premaxillary length, 98.6 ± 5.88 (80.7–115.4, 88); occipital height, 22.6 ± 1.3 (19.8–25.8, 88); acoustical breadth, 33.4 ± 1.54 (29.8–37.6, 86); mastoid breadth, 31.8 ± 1.65 (28.9– 36.2, 87); zygomatic breadth, 48.2 ± 2.96 (42.6–57.3, 82); zygomatic length, 34.6 ± 2.59 (21.3–40.4, 88); interorbital breadth, 26.9 ± 1.92 (22.3–32.8, 89); diastema length, 26.1 ± 1.66 (22.0– 30.7, 91); nasal breadth, 14.9 ± 1.46 (12.2–19.2, 88); nasal length, 28.6 ± 2.37 (22.8–34.7, 89); frontal length, 32.0 ± 2.75 (24.7–37.5, 89); frontal breadth, 31.9 ± 2.47 (24.7–38.9, 90); postfrontal length, 37.0 ± 2.82 (28.9–44.9, 89); temporal breadth, 29.2 ± 2.13 (23.7–36.7, 89); occipital condyle breadth, 17.7 ± 0.98 (14.9–19.9, 88); crown length of upper molariform toothrow, 21.3 ± 1.21 (17.9–24.8, 85); alveolar length of upper molariform toothrow, 21.4 ± 1.17 (18.0–24.5, 92); greatest length of mandible, 24.8 ± 0.98 (23.3–26.4, 19); mandibular diastema length, 4.1 ± 0.59 (2.9–7.0, 117); crown length of lower molariform toothrow, 7.0 ± 0.44 (6.4–8.2, 13); alveolar length of lower molariform toothrow, 7.2 ± 0.67 (6.3–10.2, 54); length of mandibular symphysis, 7.7 ± 0.57 (6.6–9.4, 99— Silva Taboada et al. 2007). Additional cranial measurements may be found in Abreu and Manójina (1989) and Borroto-Páez et al. (2005).
DISTRIBUTION
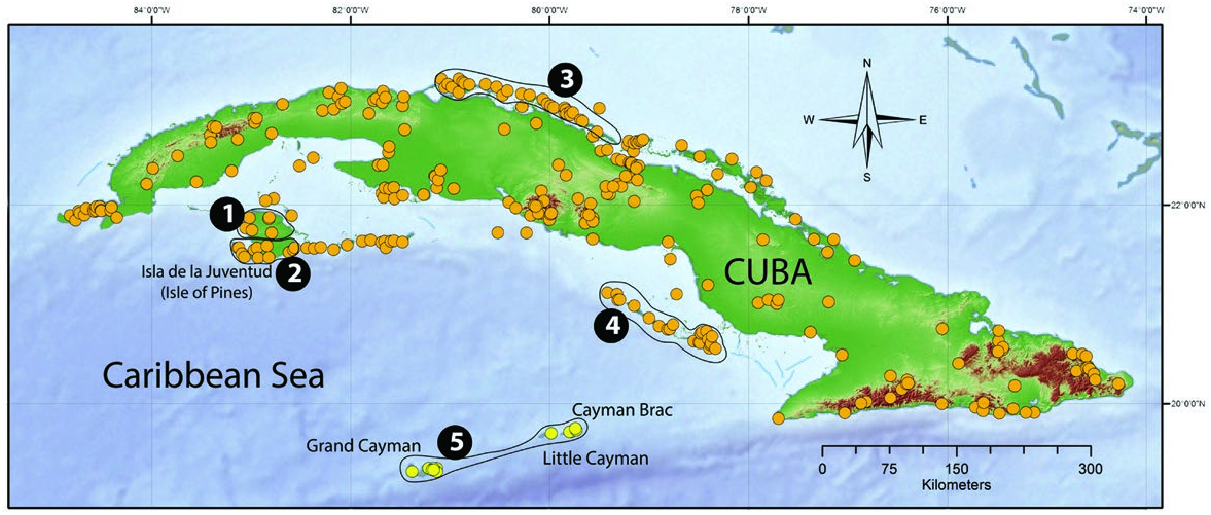
Capromys pilorides currently occurs only in the Cuban archipelago where it is widely distributed, occurring at elevations ranging from sea level to about 1,200 m in eastern Cuba . It is known from more than 190 localities on mainland Cuba and Isla de la Juventud (former Isla de Pinos), as well as on more than 120 outlying keys ( Fig. 5 View Fig ; Silva Taboada et al. 2007; Borroto-Páez 2011a).
Mean external measurements (mm or kg; ± SD; parenthetical n; sexes combined) of C. pilorides doceleguas from Archipiélago de la Reina were: total length, 692.0 ± 37.2 (3); tail length, 237.7 ± 23.1 (3); ear length, 35.0 ± 4.3 (3); length of hind foot, 88.0 ± 0.0 (3); body mass, 2.133 ± 0.4163 (3— Silva Taboada et al. 2007). Mean external measurements (mm or kg; ± SD; parenthetical n; sexes combined) of C. pilorides gundlachianus from Archipiélago de Sabana (Cayo Juan Clarito) were: body length, 481.8 ± 35.1 (90); tail length, 234.8 ± 17.9 (90); ear length, 38.7 ± 3.8 (90); length of hind foot, 92.8 ± 5.7 (90), body mass, 3.6123 ± 0.9531 (90— Berovides Álvarez et al. 1990c). Additional relative measurements and corporal indexes may be found in Berovides Álvarez et al. (1990a) and Fuente and Berovides Álvarez (2015a).
FOSSIL RECORD
Late Quaternary fossil and subfossil remains of Capromys pilorides are extensive on the Cuban archipelago and Cayman Islands. At least 67 paleontological and archeological sites dispersed throughout the Cuban main island contain abundant fossil and subfossil remains of this species ( Silva Taboada et al. 2007). Many of these fossil remains have been found associated with now-extinct species of megalonychid sloths, Antillean shrews ( Nesophontes and Solenodon ), bats, echimyids ( Boromys ), and other capromyid rodents ( Arredondo de la Mata 1970; Varona and Arredondo 1979; Silva Taboada et al. 2007). Fossils of C. pilorides have been reported from cave deposits in Grand Cayman, Cayman Brac, and Little Cayman; on the Cayman Islands the now-extinct taxa of Nesophontes , Geocapromys , and Crocodylus rhombifer have been frequently recovered with C. pilorides in fossil deposits ( Morgan 1994; Morgan et al. 2019). The extinction date of the Cayman Brac Capromys population was estimated as 1700 (95% confidence interval: 1632–1774— Morgan et al. 2019).
Capromys pilorides is known from a midden in the Dominican Republic and it has been associated with transportation by people ( Rímoli 1974; Olson 1982). Complete nasals, mandibles, and upper incisors, which do not differ from the corresponding parts of Cuban skulls of C. pilorides , were found in a kitchen midden at a depth of about 3 feet ( Miller 1929). The source of the skeletal elements were animals brought to the cave as food, either by indigenous people or early European sailors ( Miller 1929; Olson 1982).
FORM AND FUNCTION
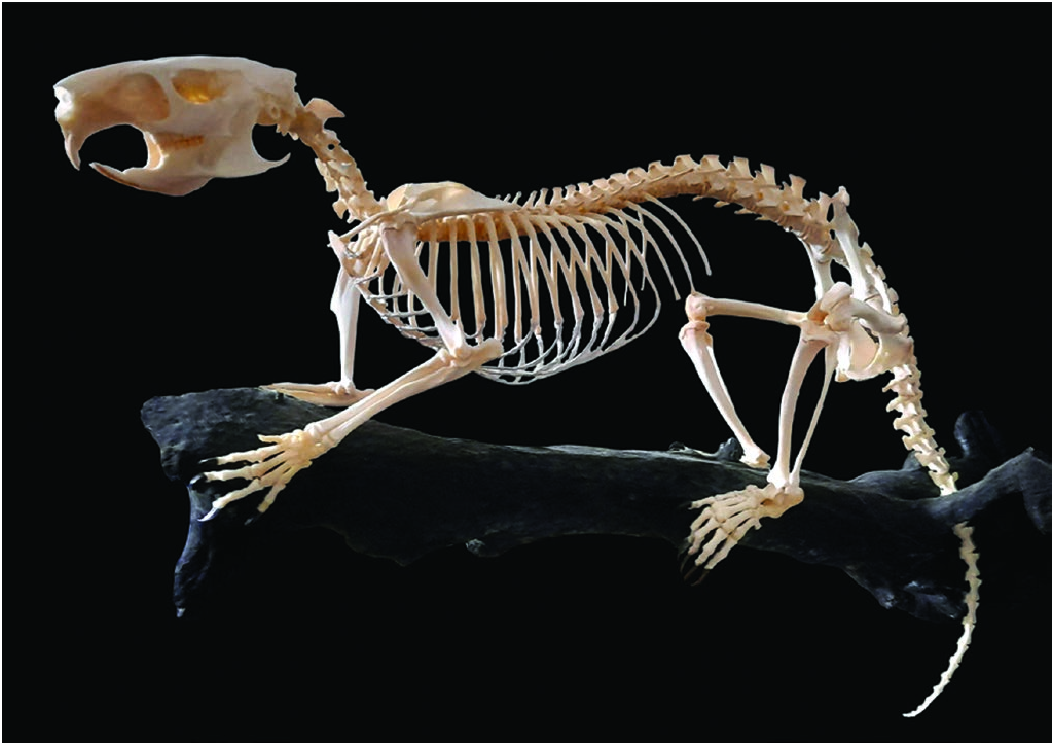
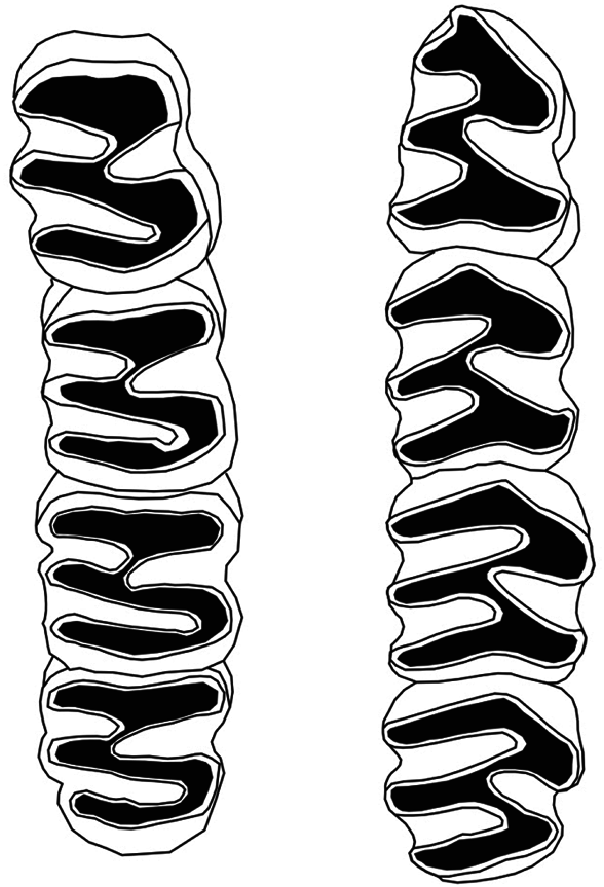
Form.— The dental formula of Capromys pilorides is i1/1, c0/0, p1/1, m3/3, total 20 ( Silva Taboada et al. 2007). The incisors are long and curved, implanted frontally with little space between them; incisor pigment is generally of similar intensity in both jaws and the coloration varies from white to yellow ( Granados 1973). The pigment intensity increases with age ( Abreu and Manójina 1989). Populations of C. pilorides that inhabit small keys have a higher frequency of white incisors compared to those associated with forest habitats on the mainland that have a higher frequency of yellow pigment ( Berovides Álvarez 2003). Vertebral formula of C. pilorides is 7 C, 16 T, 7 L, 4 S, 21–27 Ca, total 55–61 ( Allen 1918; Borroto-Páez 2002); the sternum is short and there are 17 pairs of ribs, 9 sternal and 8 asternal ( Fig. 6 View Fig ). The upper and lower molariform teeth have two labial and one lingual reentrant enamel folds (flexids); on the upper teeth the mesoflexid is much longer than the paraflexid and on the lower teeth the hipoflexid and metaflexid are superposed ( Fig. 7 View Fig ). The enamel reentrant folds lie at a 90° angle to the body axis ( Woods and Howland 1979; Silva Taboada et al. 2007).
Mean cranial capacity (cm 3; ± SD) for 37 individuals was 11.97 ± 1.0, and the encephalization quotient was 0.48 which is lower compared to other capromyid rodents ( Borroto-Páez 2012). Populations that inhabit small keys have higher fluctuating asymmetry of the skull compared to those of Cuba and Isla de la Juventud ( Pérez and Mancina 2020). Capromys pilorides exhibits a buttressed sacrum that consists of four or five fused vertebrae. It has dorsal projections of the cranial and caudal articular processes on the sacral vertebrae that are well-developed and appear to provide extensive facets for muscular attachment ( Hermanson and Woods 2012). The hyoid consists of a central body, formed by the basihyal, and an anterior and posterior cornu. It is a bowed rod with a ventrally projecting apex and two ends projecting dorsolaterally. There are pronounced dorsal “shoulders” at the point where the ceratohyal articulates ( Woods and Howland 1979).
The erect penis is about 100 mm long; its mucosa is covered with spines. The inguinal testes sit in a rudimentary scrotum throughout the year ( Angulo and Álvarez 1948). The baculum has an elongated mid-shaft with sides more or less parallel, without constriction or subapical stretching at the distal end, it has two small nodules well marked at the basal end. It is dorsoventrally flattened at the mid-shaft, with a convex dorsal area and a ventral concave area ( Varona 2012; Casinos et al. 2020). Mean baculum measurements (mm; ± SD; parenthetical range) of 57 individuals were: total length, 16.6 ± 2.0 (13.0–21.5); extreme basal width, 3.1 ± 0.4 (2.3–4.3); maximum basal width, 4.1 ± 0.6 (3.1–5.5); extreme apical width, 3.0 ± 0.5 (1.9–4.1); maximum apical width, 3.4 ± 0.6 (2.0–4.8); basal height, 1.3 ± 0.2 (0.9–1.9); apical height, 0.9 ± 0.1 (0.6–1.3— Ramos García and Borroto-Páez 2012). The clitoris has a conical shape, it is 25 mm long and occludes the vaginal opening. The vagina is closed in immature individuals, opening at puberty. The ovaries are situated near the external edge of the kidneys. There are four nipples situated in pairs on both sides of the body, the anterior pair is thoracic, and the posterior abdominal. The thoracic nipples are located at the midpoint between the dorsal and ventral midlines ( Angulo 1946).
The esophagus is narrow, the stomach has an oblong shape and the small intestines are about 5 m long ( Owen 1832). There is a well-developed cecum about 450 mm long by 60 mm wide ( Angulo 1946). The liver is a singular structure subdivided into five compacted lobules, the functional role of these hepatic lobes are unknown ( Borroto-Páez and Woods 2012). The pancreas consists of two parts, the more compact part extends behind the stomach and the other part is thin and ramified in the duodenal mesentery. The kidneys have a simple form and structure with a single papilla and small pelvis. The lungs are divided into three lobes and the thyroid gland is lobulated and proportionally large ( Owen 1832; Angulo 1946).
In the head region, the masseter superficialis and temporalis muscles are small, whereas the masseter posterior muscle is substantial. The muscle masseter medialis is covered by a broad aponeurotic sheet. The origin of the muscle pterygoideus externus is lateral to the maxillary toothrow because of the restricted pterygoid plate. ( Woods 1972; Woods and Howland 1979). In the abdomen, the placement of the rectus abdominis muscle results in the decussation and interlacing of its bundles at their pubic insertion ( Angulo and Álvarez 1948).
Function.— Capromys pilorides chews in a propalinal manner, characterized by caudal to rostral motion of the mandibular molar toothrow relative to the maxillary molar toothrow, a movement that is approximately parallel to the long axis of the body. It is related to reentrant enamel folds oriented nearly 90° relative to the sagittal axis of the body ( Woods and McKeen 1989; Hermanson and Woods 2012).
Externally the hands have only four fingers. The thumb is rudimentary and has no individual movements. Capromys pilorides can adopt a bipedal position for feeding using the tail and hind feet in support of body. The forefeet are used in feeding and defense when attacked ( Angulo 1946).
Capromys pilorides has a basal rate of metabolism of 0.227 cm 3 O 2 g−1 h−1 and thermal conductance of 0.019 cm 3 O 2 g−1 h−1 °C− 1. This value of basal rate is 64% of that expected for mammals of the same body mass. Among caviomorphs, this has been associated with a folivorous diet and an island distribution where the resources may be limited ( McNab 1978; Arends and McNab 2001). Similar to other capromyids the water requirements of C. pilorides are low. The urine of C. pilorides has a distinctive scent. The kidneys have long loops of Henle allowing production of highly concentrated urine ( Johnson et al. 1975). Populations that inhabit areas where freshwater is limited, such as coastal areas and mangroves, tend to have higher relative medullary thickness compared with those of forest habitats ( Berovides Álvarez et al. 1990c; Sánchez et al. 1992; Borroto-Páez and Mancina 2006; Fuente and Berovides Álvarez 2013).
Based on morphological variation in mandible size and shape among populations of C. pilorides , two extreme shape morphotypes associated with the general robustness of the mandible can be observed: (1) populations that inhabit small keys possess a short coronoid process, long condylar process, and narrow horizontal ramus; (2) populations of mainland Cuba have long coronoid and angular processes, a short and robust condylar process, and a broad horizontal ramus. Similarity in mandible shape is not correlated with minimal geographic distance between populations. Mandible shape variation probably reflects adaptation to exploiting different resources available in different habitats (e.g., more omnivorous diet associated with terrestrial habitats; phytophagous diet associated with mangroves— Mancina et al. 2019).
ONTOGENY AND REPRODUCTION
The young of Capromys pilorides are precocial, born with fur, open eyes, erect ears, and the ability to move around ( Taylor 1970 [not seen, cited in Silva Taboada et al. 2007]; Manójina et al. 1987a). Mean body mass at birth (g; parenthetical range, n) for females and males, respectively, was: 218 (90–515, 13) and 227 (150–310, 12); mean total lengths at birth (mm; parenthetical range, n) for females and males, respectively, were 283 (245–352, 9) and 302 (268–350, 9— Taylor 1970 [not seen, cited in Silva Taboada et al. 2007]; Manójina et al. 1987a, 1987b; Silva Taboada et al. 2007; Linares et al. 2014). There is a male bias in the number of embryos in the wild and in litter composition in captivity ( Manójina and Abreu 1987).
Males reach sexual maturity between 7 and 10 months of age and females at about 10 months ( Johnson et al. 1975). In western Cuba , males are reproductively active, based on descended testes, after reaching 3 kg of body mass or 700 mm of total length ( Manójina and Abreu 1987; González Grau et al. 1990). However, in other regions, males of shorter body lengths have been found with scrotal testes ( Smith Canet and Berovides Álvarez 1984b). Capromys pilorides shows spermatogenic activity only with descended testes ( Angulo and Álvarez 1948).
Capromys pilorides breeds throughout the year, although birth peaks have been recorded between April and July ( Smith Canet and Berovides Álvarez 1984b; Frías et al. 1987; Manójina and Abreu 1987; Gutiérrez et al. 1999; Linares et al. 2014; Fabre et al. 2016), probably associated with seasonal weather patterns and food availability. Females are polyestrous with a mean litter size of 2–3 (range 1–6); litters with four or more young have been observed only in captivity ( Bucher 1937; Taylor 1970 [not seen, cited in Silva Taboada et al. 2007]; Smith Canet and Berovides Álvarez 1984b; Frías et al. 1987; Manójina and Abreu 1987; Manójina et al. 1987a; Amaro-Valdés et al. 2019). The estrus cycle occurs at mean intervals of 16.3 days (range 13–19), the gestation period lasts 120–150 days and lactation occurs for 90–180 days ( Taylor 1970 [not seen, cited in Silva Taboada et al. 2007]; Weir 1974; Johnson et al. 1975; Smith Canet and Berovides Álvarez 1984b; Frías et al. 1987; Silva Taboada et al. 2007). Birth is followed by postpartum estrus ( Camacho et al. 1995; Gutiérrez et al. 1999). Parturition has a mean duration of 327 min (range 60–760 min), and interval release ranges 20–270 min with an average of 130 min ( Manójina et al., 1987a; Comas González et al. 1994a; Silva Taboada et al. 2007).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Capromys pilorides ( Say, 1822 )
| Silva, Hansel Caballero & Mancina, Carlos A. 2023 |
Capromys fourniere
| Woods C. A. & Kilpatrick C. W. 2005: 1594 |
Capromys arredondoi
| Varona L. S. 1984: 2 |
Capromys pappus
| Varona L. S. 1984: 2 |
Capromys megas
| Varona L. S. & Arredondo O. 1979: 12 |
Capromys intermedius
| Arredondo de la Mata O. 1958: 48 |
Procapromys geayi
| Chapman F. 1901: 322 |
Capromys geayi
| Pousargues E. de 1899: 152 |
Capromys
| Waterhouse G. R. 1848: 150 |
Capromys pilorides
| Waterhouse G. R. 1848: 287 |
Capromys
| Waterhouse G. R. 1848: 287 |
Capromys Furnieri Desmarest, 1822b:43
| Desmarest A. G. 1822: 43 |