Microporella sargassophilia, Jain & Gordon & Huang & Kuklinski & Liow, 2022
|
publication ID |
https://doi.org/ 10.26107/RBZ-2022-0011 |
|
publication LSID |
lsid:zoobank.org:pub:A251050A-4FDA-41DD-A10F-891E92497D03 |
|
persistent identifier |
https://treatment.plazi.org/id/15C5ED5A-30DB-4B67-A890-5F447CA2C342 |
|
taxon LSID |
lsid:zoobank.org:act:15C5ED5A-30DB-4B67-A890-5F447CA2C342 |
|
treatment provided by |
Felipe |
|
scientific name |
Microporella sargassophilia |
| status |
sp. nov. |
Microporella sargassophilia , new species
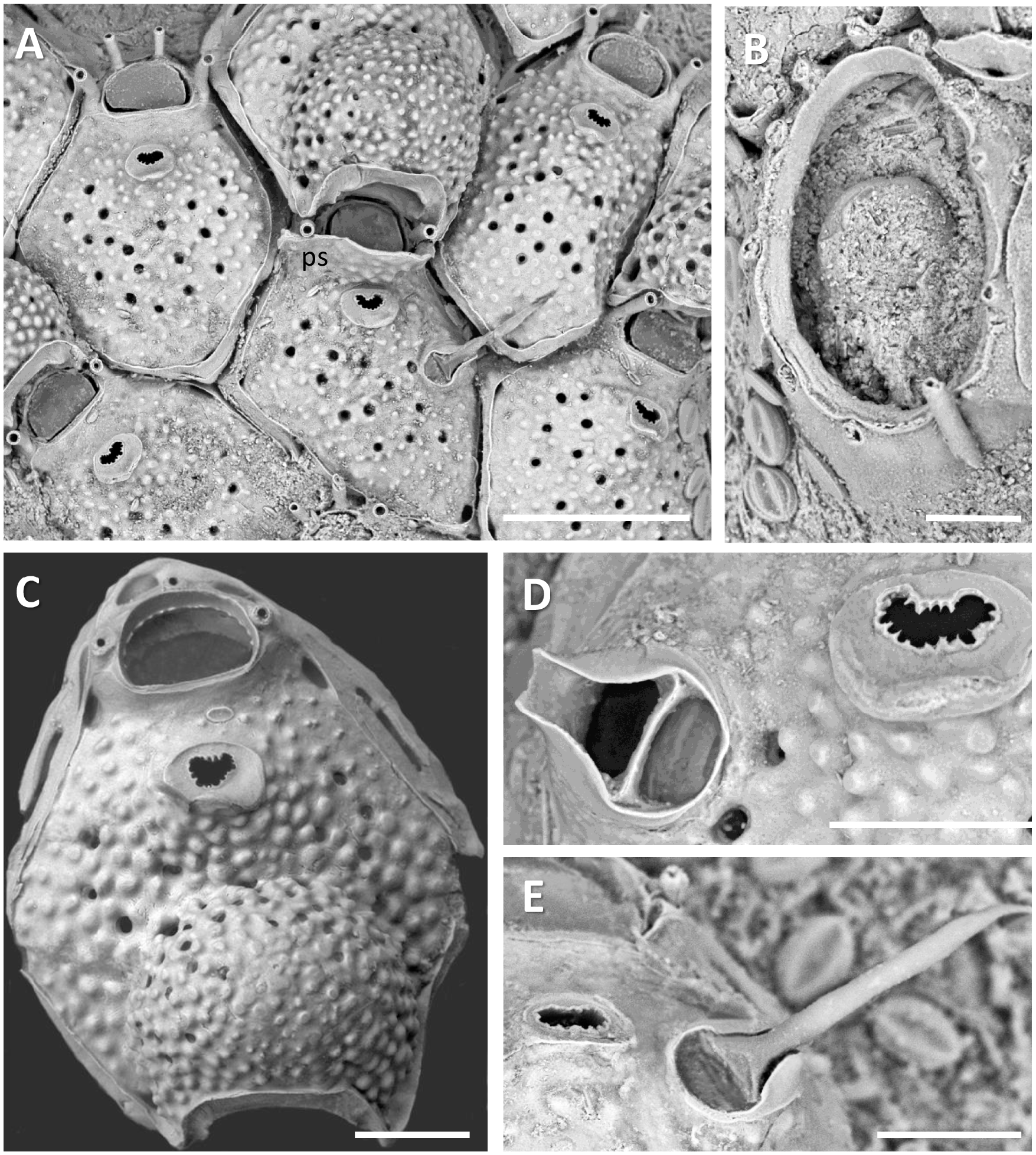
( Fig. 6A‒E View Fig )
Material examined. Holotype: ZRC.BRY.0924 (Zoological Reference Collection, Lee Kong Chian Natural History Museum; collected as SG2019 No. 128; sequenced as BLEED No. 1525), Pulau Tekukor (1.2299°N, 103.8376°E), Singapore, 1 m, coll. S.S. Jain & P. Kuklinski, 6 May 2019. GoogleMaps
Etymology. Named for the macroalgal host, Sargassum ilicifolium (see Yip et al., 2018), on which the species was found.
Diagnosis. Colony encrusting on Sargassum laminae, unilaminar, tiny. Zooidal frontal shield nodular and sparsely pseudoporous, pores simple; thin gymnocystal margin around each zooid. Orifice with 3‒5 (mostly three) jointed oral spines; proximal rim smooth, inner distal margin minutely denticulated. Ascopore non-reticulate, crescentic, denticulate, set within small raised gymnocystal area. Avicularia single, sparse, not found on every zooid, set some distance from ascopore. Ovicell surface like that of frontal shield but with few or no pseudopores apically; proximofrontal margin of ooecium with smooth raised band of calcification; some zooids with broad bridge of calcification embracing orifice distal to ascopore, the tip of an oral spine sometimes showing on each side inside its rim. Ancestrula tatiform with 11 spines.
Description. Colony encrusting, unilaminar, multiserial, tiny, up to only about 3 mm diameter, found only on the laminae of Sargassum ilicifolium . Colony colour whitish-transparent. Zooids subhexagonal, widest about midlength, interzooidal boundaries in furrows with thin, slight edges of gymnocyst visible in places [ZL 286‒407 (350), n = 20; ZW 192‒446 (275), n = 20]. Frontal shield pustulose, very sparsely pseudoporous (12‒18 pores in zone of astogenetic repetition), pores simple. Orifice transversely D-shaped with straight to slightly concave proximal margin; proximal smooth and non-denticulated, but inner face of distal oral arch weakly denticulate [OrL 43‒61 (53), n = 20; OrW 64‒100 (81), n = 20]. Typically three basally articulated oral spines, 4‒5 near colony origin, joints not dark. Ascopore transversely bean-shaped and weakly denticulate, set within the distal half of a slightly raised, smooth, shallow gymnocystal area; area between ascopore and proximal margin of orifice relatively smooth with a few tiny pustules. Avicularia sparse, single, not on every zooid, produced from an areolar-septular pore, not a pore-chamber; with complete crossbar, rostrum triangular overall with concave sides, the distal tip broadly rounded, open, channel-like. Mandible setiform, stilettolike with expanded triangular base [AvL 52‒69 (61), n = 7; AvW 43‒54 (48), n = 7; AvML 118‒147 (128), n = 7]; open mandible not reaching to opposite margin of zooid. Ovicell conspicuous, globular, ooecial surface pustulose like the frontal shield with a few peripheral pseudopores; strongly personate, but not in all zooids, with a broad bridge of calcification embracing the orifice distal to the ascopore, the tip of an oral spine showing on each side inside its rim in some zooids [OvL 168‒216 (190), n = 9; OvW 188‒215 (199), n = 9]. Embryos reddish. Interzooidal communications comprising well-developed basal pore-chambers, 1‒2 on each lateral wall and 2‒3 small such chambers below and around the orifice. Ancestrula tatiform, with elongate-oval opesia bordered by 11 spines; gymnocyst smooth (AnL 262‒280 (271), n = 2; AnW 180‒228 (204), n = 2). Two daughter zooids produced, each in a distolateral position.
Remarks. We closely examined three fertile colonies at 1.88‒2.00 mm maximum length. The largest of the three had 31 zooids (8 frontally broken), of which 11 were ovicellate and three bore an avicularium. A colony of 26 zooids had seven that were ovicellate and four bore an avicularium. The third colony had 25 zooids, of which seven bore ovicells and four had an avicularium. Thus c. 26‒28% of the zooids in a sexually reproducing colony are actively producing larvae.
Tilbrook (2006) noted that relatively few Microporella species had been described with personate ovicells, mentioning three of them— Microporella personata ( Busk, 1854) from the Falkland ( Malvinas) Islands, Microporella orientalis Harmer, 1957 from Indonesia, and Microporella harmeri Hayward, 1988 from Mauritius. Microporella orientalis differs from M. sargassophilia , new species, in developing extensive sheet-like colonies on hard substrata, that there are many more pseudopores in the frontal shield, and that the personate rim is not associated with spines. Microporella harmeri has large lateral avicularia, numerous pseudopores, and a denticulate proximal orificial rim (see also Harmelin et al., 2011).
There are several other warm-water personate species. Microporella epihalimeda Tilbrook, 2006 from the Solomon Islands forms small colonies on the calcareous green alga Halimeda but differs from M. sargassophilia , new species, in having pustules between the ascopore and orifice and on the proximal face of the personate process as well as no spines associated with the personate process, characters shared with Microporella hawaiiensis Soule, Chaney & Morris, 2003 . In Microporella pontifica Osburn, 1952 from western Mexico and Microporella wrigleyi Soule, Chaney & Morris, 2004 from California, the ascopore is embraced by the personate process. This is not the case in Microporella lepueana Soule, Chaney & Morris, 2004 from American Samoa, but this species has a denticulate oral rim and often paired avicularia. Harmelin et al. (2011) described six personate species, three of them new, from the tropical Indian Ocean. Of these, the closest in morphology to M. sargassophilia is Microporella genisii ( Audouin, 1826) from the Red Sea and eastern Mediterranean. It occurs on the marine angiosperm Cymodocea as well as hard substrata and has a pair of spines inside the corners of the personate peristome. It differs from M. sargassophilia in having up to six oral spines in proximal parts of the colony, that the ascopore is closer to the orifice and the area between is pustulose, and that the orifice has slight denticulation of the proximal rim but not the distal rim. The only other warm-water personate species is Microporella clypeiformis Liu in Liu, Yin & Ma, 2001 from China. It differs, inter alia, in having only a narrow peristomial bridge.
In the description, it has been noted that the avicularium in M. sargassophilia , new species, is budded from an areolarseptular pore, in contrast to those Microporella species in which the avicularian cystid extends to the substratum, replacing a basal pore-chamber. Hastings (1963) made this distinction, regarding the latter type as effectively interzooidal. Overall, apart from skeletal characters, M. sargassophilia is distinctive among personate species for its very small colony size and algal substratum. Colonies are fragile, in that zooids are not strongly interconnected and easily separate when bleached for scanning electron microscopy. Zooid surfaces are generally consistently fouled by diatoms.
Distribution. The species is so far known only from Singapore.
| ZRC |
Zoological Reference Collection, National University of Singapore |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.