Sparganothoides, Lambert and Powell, 1986
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2150.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/BD2F87FB-FFDC-F468-FF70-FD80FB96FC04 |
|
treatment provided by |
Felipe |
|
scientific name |
Sparganothoides |
| status |
|
Biology of Sparganothoides View in CoL
Sparganothoides is primarily Neotropical, with its greatest species richness in the mountains of Mexico and Central America. With few exceptions, species of Sparganothoides occur between 1,000 and 2,800 m, in mixed hardwood and oak-pine habitats, both arid and mesic. As is typical for other Neotropical Tortricinae and microlepidoptera generally, collection records are fragmentary, and knowledge of Sparganothoides biology is scant. There are no published records of larval biology, and we found only one specimen ( S. lugens from Vera Cruz) that had been reared from a larval collection (i.e., “misc. foliage”). The following summary of life history information is derived from collection records of adults and from laboratory rearings of larvae from eggs by Powell.
Rearing Data
Gravid females of sparganothine moths usually deposit eggs on smooth surfaces in the absence of any plant stimulus, which is typical of generalist feeding Tortricinae . A summary of our rearing trials is given in Appendix 3. All together, 103 Sparganothoides females of 10 species representing 5 of the 6 species groups were confined during 1972–1999; 73 females deposited eggs, although those of several females were infertile or failed to develop completely owing to desiccation. Progeny of 23 females, representing 8 species of 4 species groups, were reared to pupation. Failure in other lots was due most often to deterioration of the diet, developing mold or bacterial growth, or to desiccation when not monitored closely, especially when larvae became quiescent during overwintering.
Seasonal Life History
Flight periods of the three most frequently collected species in America north of Mexico are well documented, each with more than 40 collection records. Sparganothoides lentiginosana , the only species that occurs at low elevations, is widespread in the southeastern U.S. Adults have been collected in every month except October and December, indicating a multivoltine pattern, and larvae from eggs deposited in May (Arkansas) and September (Virginia) grew to maturity and metamorphosed without diapause. In the Southwest, S. hydeana and S. machimiana have been recorded from June to September, which typically is late in the dry season through the height of the summer wet season. Hence, there may be one annual generation in these species, although there is one anomalous April record for S. machimiana among 70+ collections.
Most Sparganothoides in Mexico and Central America are poorly documented, with 21 of 29 species known from 5 or fewer records, only 3 species from more than 10 collection dates, and none from more than 20. Virtually all Mexican records are restricted to May to September, when lepidopterists primarily devote their sampling effort. Many of the species occur at high elevations, and winter temperature ranges may preclude activity during October through early spring. Conversely, in Costa Rica, where the parataxonomist program operated by the Instituto Nacional de Biodiversidad has enabled year around sampling, the better known species are recorded during extended seasons: e.g., S. ocrisana in every month except April and September; S. torusana in March, October, and December; and S. morata , which occurs in Panama and Venezuela, in January–March, June, and October–November.
Based on lab rearing, it appears that diapause does not occur at any stage. Larvae of summer-fall flying species in Mexico and Arizona sometimes pupated and eclosed in October–December (e.g., S. hydeana , S. machimiana , S. canities , S. ocrisana , S. arcuatana , S. cornutana ), but often, partially grown sibs became quiescent through winter, which is the dry season. They are capable of waiting several months in dry conditions before resuming feeding and maturing. Thus a univoltine pattern may be typical for many species in northern montane habitats, while multivoltine life cycles appear to be characteristic of Sparganothoides of lowland southeastern U.S. and southern Central America and Venezuela.
Adult Behavior
Adults are nocturnal, and both sexes of all species for which we have firsthand information are attracted to lights, even during cold nights (8–10°C) in high montane habitats (e.g., Sparganothoides hydeana at 2,750 –2,800 + m in New Mexico and Durango, Mexico). Adults of a few species have been flushed and netted from oaks ( Quercus ) or manzanita ( Arctostaphylos ) during the daytime in Arizona and Mexico, but these same species frequently are recorded at lights. Specimens of a few species, such as S. castanea and S. vinolenta , were taken in the 19 th century, before availability of electric lights in remote areas, but it is unlikely that any species is diurnal. Oviposition in the lab occurred only nocturnally. We did not attempt lab mating trials, and no mated pairs were observed in the field.
Females deposit the eggs in round to oval, imbricate patches, as is characteristic of sparganothine moths. We recorded oviposition only by females taken in the field, so we do not know if any prior oviposition bouts occurred. In contrast to many Archipini , which deposit a large patch of eggs (50–150 or more), followed by several successively smaller ones in subsequent nights, females of captive Sparganothoides produced only moderate to small masses (generally 5 to 30 eggs; = 13.3 eggs/patch); only 6 of more than 75 masses counted had more than 30 eggs, none more than 50. A larger patch often was followed by one or more smaller ones but not consistently fewer eggs per successive bout. A total of 80 eggs in 3 masses by a female of S. licrosana was the maximum we observed. Females produced a colorless or rarely slightly milky colored colleterial solution (n = 1 of 28 female S. machimiana ; 1 of 13 S. arcuatana ) that exceeded the perimeter of the patch narrowly, typically by 0.5–1.0 times the width of an egg.
Egg Development
Eggs of Sparganothoides are flattened, slightly convex and usually not as strongly overlapped within patches as those of many other sparganothine and archipine species. They are slightly oval in outline and range from 0.60 X 0.90 mm in S. prolesana to 0.85 X 1.20 and 0.90 X 1.25 mm in larger species such as S. machimiana . When newly laid, the eggs are peach- or orange-colored to rust or dark orange-tan, although the single female of S. umbrosana that we observed produced cream-colored eggs. As embryonic development proceeds, a translucent embryo is visible by 24–48 hours, and the colored yolk is restricted peripherally. Later the stemmatal patches, then the head capsule structure are visible preceding eclosion. Incubation time is temperature-related and probably requires no more than 7–10 days in field conditions. Usually we delayed egg maturation by storage in a camp cooler or air conditioned lab, and most eclosed between 11–23 days. Eggs of S. machimiana held in a cabin without temperature control in Arizona in July incubated in 7–8 days (72G3). Infertile eggs are deposited singly or in irregular patches, and the yolk appears granular by 48 hours.
Larval Foods
Most species of the related genera Sparganothis and Platynota are generalist plant feeders, often on low growing herbaceous vegetation, and 30 years ago similar larval biologies were assumed to be characteristic of North American species of Sparganothoides , which were then assigned to Sparganothis . However, absence of any larval collection records for the two widespread and sometimes abundant species in the southeastern and southwestern U.S. ( S. lentiginosana and S. machimiana ) casts doubt on that hypothesis. We collected adults of S. hydeana and S. machimiana from trees ( Quercus , especially Q. hypoleucoides ) and shrubs ( Arctostaphylos ) in Arizona and Mexico on several occasions but failed to find larvae among several kinds of foliage feeding microlepidoptera on those hosts. We perceive a curious parallel between Sparganothoides and the genus Anopina ( Tortricinae : Euliini ), which also has widespread U.S. species for which larvae have not been discovered in the field, occurs in similar montane elevations throughout Mexico, and whose larvae prefer dead leaves and are capable of waiting long periods in dry conditions when food is unavailable. Brown and Powell (2000) postulate that larvae of Anopina are ground-dwellers that feed on fallen leaves, a habit that is typical of numerous Epitymbiini (Tortricinae) in Australia ( Common 1990). We do not have as convincing laboratory evidence for Sparganothoides as Anopina exhibited, but a ground dwelling larval biology is suggested.
Newly eclosed larvae of all Sparganothoides we obtained accepted synthetic diet, an indication that they are generalists, based on rearing numerous species of Archipini and Sparganothini (Powell unpublished data). Specialists, such as many Tortricini , Euliini (Tortricidae) , and Ethmiinae (Gelechioidea) ignore synthetic diet and starve. The primary goal in our rearing efforts was confirmation of the association of the sexes, so we provided only synthetic diet in most instances because larvae feeding on it grew faster than on deteriorating leaves, and there were fewer problems with mold and disease.
Our early efforts to feed first instars of S. machimiana dry season foliage of several trees and shrubs in Arizona failed, and subsequently we found that leaves are eaten by larvae of Sparganothoides only when soft, whether green or old and brown. We found that newly hatched, 3-day old, and 20-day old larvae of S. hydeana did not eat green leaves of Prunus (Rosaceae) (76J1, J12), while those offered only synthetic diet survived to pupation, as did others offered both diet and Quercus lobata (Fagaceae) (86H20, H23). First instar larvae of S. machimiana (86H7) given fresh leaves of Quercus or Amorpha (Fabaceae) in the absence of diet refused Amorpha and starved, while others fed on Quercus leaves in all lots (n= 5); they were provided with additional leaves after 15, 30, 45, and 60 days with survivorship diminishing. Overwintering survivors (when green leaves of Quercus were not available) waited through periods of dry diet, and eventually metamorphosed. Second instar larvae of S. ocrisana (92C64), a species that had been reared successfully on synthetic diet (90G14), were transferred to Prunus after 30 days; they adapted to it poorly and grew very slowly; later (70+ days) they ate the brown but not dry leaves, but none survived to pupation.
Larvae of S. arcuatana (87H7) ate older, brown leaves but not green ones. First instars were given diet and Q. lobata , then Q. agrifolia after 24–27 days. The larvae grew slowly, those feeding on Quercus the slowest, but by 62–64 days all the leaves were well skeletonized. Of three individuals that pupated, two survived on diet, one on Quercus .
In a second trial with S. arcuatana (87H48), Quercus and Prunus were added to diet at 10 days. There was no feeding on the Prunus , and only a little by young instars on Quercus , which was replenished at 35 days. By 100–105 days, however, third instars had skeletonized and heavily webbed Q. lobata , forming shelters in the old, blackened leaves away from the diet; these larvae did not survive winter after Q. agrifolia was provided.
Larvae of S. licrosana grew more successfully on old oak foliage than on diet. Four groups of 15 first instars were given diet or Q. lobata in separate vials; 10 of 15 and 0 of 15 survived on oak after 25 days, and after 125 days, only 2 larvae survived to the last instar on diet, while 10 apparently fully grown larvae had eaten almost completely the old, brown oak leaves in vials dense with silk. They were transferred to diet for the winter, waited through periods of desiccation, and pupated in April.
Larval Development
Under the laboratory circumstances we provided, most larvae of Sparganothoides grew slowly compared to other Tortricidae reared on synthetic diet or to plant-feeding Lepidoptera generally, which usually mature within 30–50 days, rarely as few as 18–20 days. Sparganothoides lentiginosana pupated after 35 days in October–November (97J2, n = 1) but required 57–80 days in June–August (91E78, n = 6). All other species were reared from eggs deposited in late July to September, and larval growth varied between and within species, some proceeding to pupation, others becoming quiescent over winter. Growth of S. cornutana was relatively rapid, 34–49 days to pupation, whereas some individuals of several broods of S. hydeana , S. machimiana , and S. ocrisana matured in 45–65 days, with some sibs waiting over winter. Those of S. arcuatana and S. licrosana grew even more slowly, requiring 85 to 130 days to pupation, with adults emerging in December to February.
Longevity among larvae that overwintered was greatly variable, sometimes unsuccessful, and certainly modified by the lab conditions compared to field behavior. Holdover larvae of S. machimiana pupated in January and March to July (n = 14), approximately 140 to 300 days following egg hatch, and one larva remained alive about 335 days but failed to metamorphose. Adults of S. licrosana emerged from pupae formed in April, after 228 to 250 days larval life.
Pupation
Pupation occurs in a thin, tubular cocoon, within or near the last larval shelter, in the lab constructed between leaves or between leaves or diet fragments and sides of the container. Pupal development proceeded without diapause or lengthy quiescence in all species we observed. Development time was slower than many Tortricinae , ranging 11 to 20 days, usually 12–15.
Larval Morphology
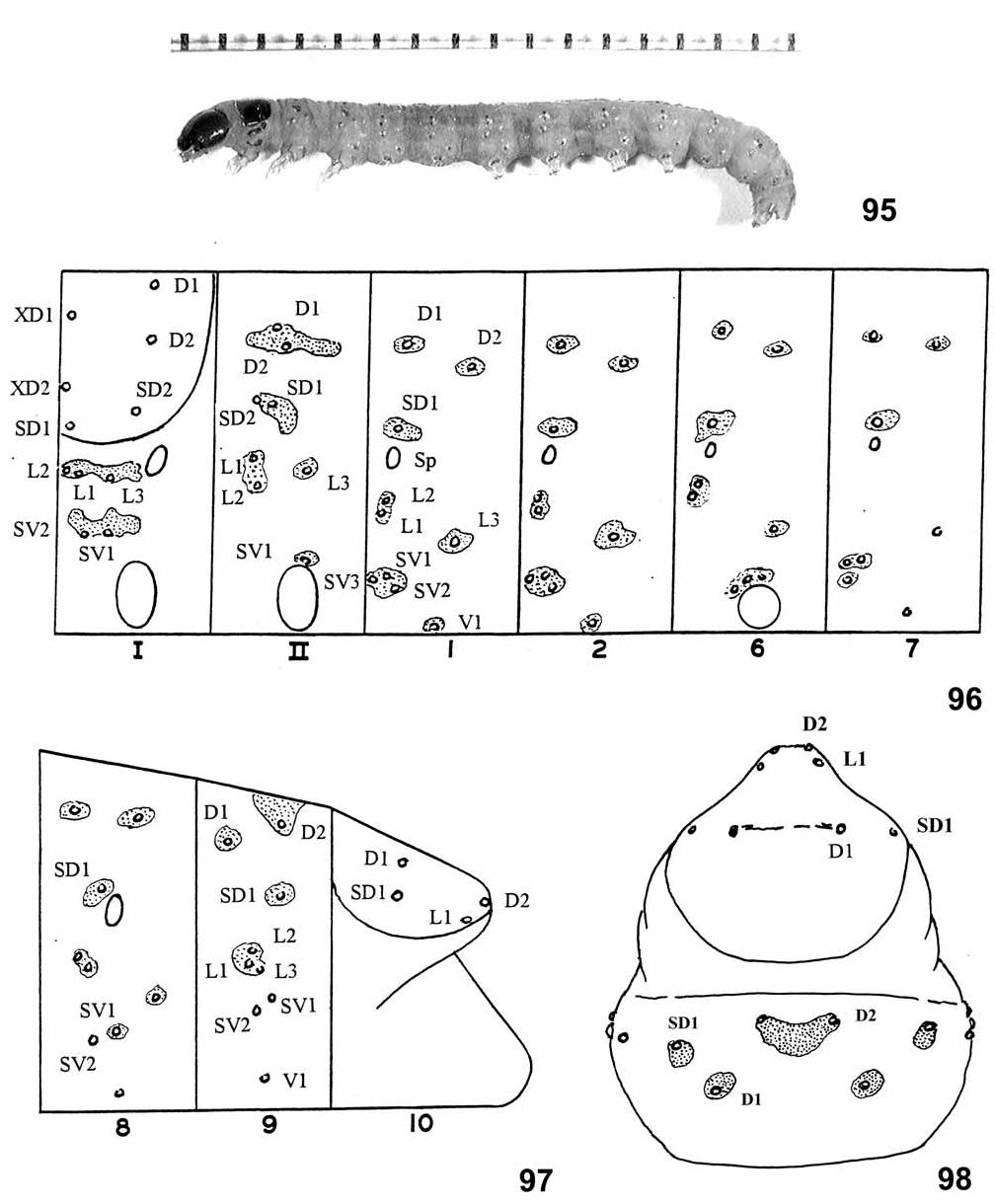
We examined preserved late instar larvae of eight species (Appendix 3). External morphology is extremely similar among species of Sparganothis and Platynota ( MacKay 1962) , so it was not surprising that we found only subtle differences among Sparganothoides species.
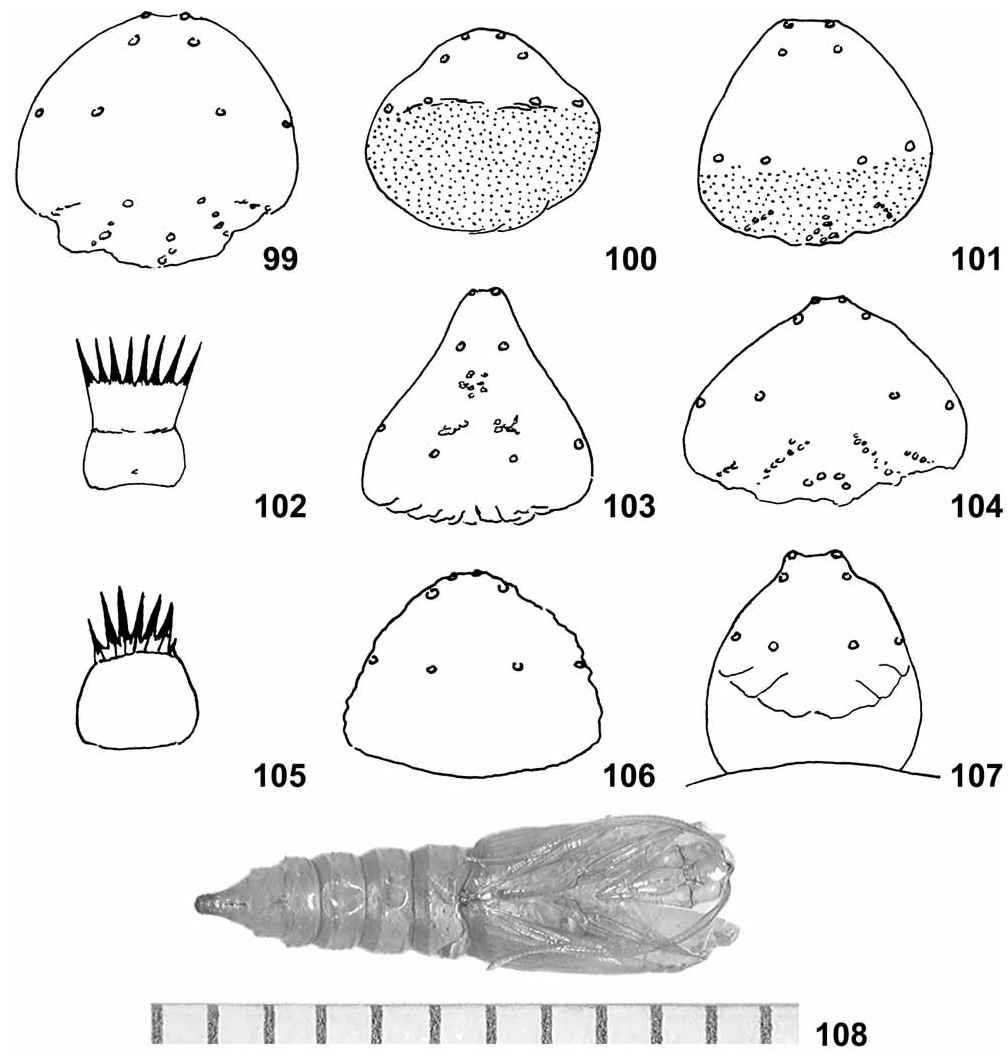
Diagnosis. Based on 8 species representing 4 species groups, Sparganothoides larvae differ from those of related genera, Sparganothis and Cenopis (12 species described by MacKay 1962, and S. senecionana , S. tunicana , S. violaceana, Powell unpublished data), and from Platynota (4 species described by MacKay, and P. exasperatana , P. larreana , P. wenzelana , and several unidentified Neotropical species, Powell unpublished) as follows. Body color in Sparganothoides generally gray to tan, thoracic and anal shields dark brown to pale amber, without darker margins; pinacula pale brown to tan, darker on thorax, correlated with sclerotization of shield, paler but still visible on abdomen, with integumental spicules not obviously darker than pinacula; D and SD thoracic pinacula elongated. Seta XD2 on prothorax situated at anterior edge of shield, closer to SD1 than to SD2. Microsetae SD2 on abdomen represented by setal bases on A1 and rarely A2–3 or absent from all abdominal segments. Anal comb somewhat elongate with base as long or longer than tines, which usually number 7–8 and are deeply sclerotized, black, and fine pointed ( Figs. 102, 105 View FIGURES 99–108 ), except in the Hydeana Group.
By contrast, in Sparganothis , Cenopis , and Platynota the body usually is bright to dark green with the dorsum darker, where minute, dark spinules densely cover the integument, and the faintly tan pinacula are distinctly visible as pale spots on the dark D and SD and often L areas (two or three species of each genus lack the darker dorsal spicular tinge so the pinacula are not obviously differentiated). Seta XD2 usually about equidistant from SD1 and SD2. Thoracic shield pale tan, usually with the ventral and posterior margins broadly darker. SD1 microsetae present on A1–8 or reduced to a trace on posterior segments, on the same pinaculum as SD. Anal comb shorter, base about as long as tines, which are less finely spinelike, blunt-tipped, 5–8 (usually 5–6), as in the Hydeana Group of Sparganothoides .
Description. General: Body color translucent pale yellow to pale orange in first instar, becoming tan to gray when feeding on synthetic diet, grayish green to reddish brown when feeding on green or brown leaves. Pinacula slightly raised, brown to tan, intensity of sclerotization of those of thorax correlated with thoracic shield; abdominal pinacula paler but still visible, spinules of surrounding integument not visibly darker; no other integumental markings ( Fig. 95 View FIGURES 95–98 ). Head: Black or dark brown in early instars, becoming paler in late instars, brown to orange-brown or tan in final instar; an irregular, dark stemmatal patch and a well defined genal bar extending anteriorly from postgenal junction, more conspicuous in species having paler head capsule. Adfrontal sutures extending to vertical angle. Seta A2 about equidistant from A1 and A3; P1 closer to P2 than to Adf2, closer to Adf2 than to F1. Stemmata prominent, similar in size, I and sometimes III slightly larger; II about equidistant between I and III. Thorax: Shield dark brown to tan in final instar, varying between species, often fading to paler anterodorsally, without darker markings. Seta SD2 closer to SD1 than to XD2, which is located at the anterior margin of the shield; L1, 2, and 3 on the same pinaculum and situated in nearly a straight line; pinacula of L group and D, SD groups of T1 and T2 elongated; SV group on T1 and T2 unisetose. Spiracle weakly oval, nearly circular. Abdomen: SV group on A1, 2, 7, 8, 9 with 3:3:3:2:2 setae (plesiomorphic in Sparganothini ). D2 setae of A9 on a single, triangulate pinaculum. V setae farther apart on A9 than on A8 (usually ca. 3:2). Anal shield paler than thoracic, brown to tan; shape variable among species, broad and tapering to a rounded apex, sometimes weakly constricted, to relatively narrow, strongly tapered to a point, with irregular rows of shallow indentations anteriorly in some species; setae long, L1 ≥ length of anal plate, longer than SD1 ca. 5:4. Proleg and anal crotchets biordinal to partially triordinal, about 55–65 and 30–35, respectively. Anal comb well developed, base rectangular or trapezoidal, longer than wide, sometimes constricted below tines, which are strongly attenuate and spinelike ( Fig. 102, 105 View FIGURES 99–108 ), except in the Hydeana Group, 5–9 variable within and among species.
Species Group Diagnoses. Setal patterns and other morphological features are notoriously variable within species of Tortricidae ( MacKay 1959, 1962; R. L. Brown 1987), and several species we examined were represented by few specimens and/or were sibs of a single family from one female. Hence, the following diagnoses of late instar larvae may be regarded as tentative. There appear to be reliable differences, especially in the anal shield and anal comb.
Hydeana Group: Anal shield relatively broad, not acutely tapered posteriorly or constricted laterally, with anterior bands of shallow pits in S. hydeana and S. canities but not S. machimiana . Anal comb small, base wider than long, tines not black and spinelike, 5 or 6 in S. machimiana and S. canities , 9 in the single S. hydeana examined.
Sparganothoides hydeana ( Mexico, Neuvo Leon, JAP 76J1, n = 1). Preserved (distended) length 27 mm; head capsule width 1.8 mm. Head capsule brown, thoracic shield dark brown fading to pale brown anterodorsally. Thoracic pinacula relatively faint, abdominal pinacula scarcely discernible. No trace of setae SD2 on A1–A8. Anal shield ( Fig. 99 View FIGURES 99–108 ) broad, rounded posteriorly; anterior margin rough, diffuse, with posteriorly and inwardly directed rows of shallow pits. Anal comb short, base length approximately equal to tines.
Sparganothoides machimiana ( USA, Arizona, JAP 86H4, H7, H7.6, H7.7, 91H3, H6, n = 20). Distended length 16–18 mm; head capsule width 1.45–1.55 mm; presumed penultimate instar 12.0– 12.5 mm, head capsule width 1.2–1.3 mm (n = 2). SD2 usually absent, rarely present as setal base on A1 (n = 1), A1 and A2 (n = 1), or A1–3 (n = 1). Anal shield relatively broad ( Fig. 100 View FIGURES 99–108 ), slightly tapered and weakly constricted laterally to a rounded caudal end, no shallow pits or other sculpturing. Anal comb small with 5 or 6 tines, base as long as wide to very reduced, base scarcely evident.
Sparganothoides canities ( Mexico, Durango, JAP 86H27, n = 1). Length 18.5 mm; head capsule width 1.4 mm. Pinacula reduced, pale brown on thorax, D, SD elongate, very small, faint on abdomen. SD2 represented by setal base on A1, absent from A2–8. Anal shield ( Fig. 101 View FIGURES 99–108 ) short, broad, similar in shape to that of S. machimiana , darker brown anterior half, similar sculpturing to that of S. hydeana . Anal comb base wider than long, 5 tines, not spinelike.
Ocrisana Group: Anal shield acutely to weakly taperred posteriorly, slightly to markedly constricted laterally. Anal comb elongate with long, spinelike tines, 8–9.
Sparganothoides ocrisana ( Costa Rica, Monteverde, JAP 90G14, n = 4). Length 17–19 mm; head capsule width 1.45–1.55 mm; presumed penultimate instar length 12–15 mm, head capsule 1.35–1.40 mm (n = 2). Thoracic shield entirely dark brown with a curved row of impressed sculpturing above SD2. Abdominal SD2 represented only by a setal base on A1 (n = 2) or absent from A1–8 (n = 2). One specimen not well distended with integument darkened by spinule density and color. Anal shield ( Fig. 103 View FIGURES 99–108 ) strongly tapered to caudal point, slightly constricted laterally, some weak sculpturing mesally but no anterior pits. Anal comb elongate, base constricted mesally, tines black, spinelike, 8–9.
Sparganothoides arcuatana ( Mexico, Veracruz, JAP 87H7, Mexico, Puebla, JAP 87H70, n = 6). Length 21–23 mm; head capsule width 1.6–1.7 mm; presumed penultimate instar 17–20 mm, head capsule width 1.1 mm (n = 2). Head capsule rust, thoracic shield darker, deep rust brown. Pinacula conspicuous, thoracic elongate, abdominal faint but relatively large. No trace of SD2 setae on A1–8. Anal shield ( Fig. 104 View FIGURES 99–108 ) only faintly colored, mottled tan with well developed rows of shallow pits anteriorly. Anal shield tines very spinelike, 9 ( Fig. 105 View FIGURES 99–108 ).
Sparganothoides licrosana ( Mexico, Sinaloa, JAP 86H31, n = 7). Length 20–22 mm; head capsule width 1.5–1.8 mm; presumed penultimate instar length 17–18 mm, head capsule width 1.25–1.35 mm (n = 3). Thoracic shield dark brown fading to pale brown anterodorsally. Pinacula well developed; no trace of SD2 on A1–8 ( Figs. 95 View FIGURES 95–98 ). Anal shield broad with strong constriction laterally, tapered to a rounded caudal tip. Anal comb with 6–8 widely flared tines, base slightly longer than wide, without constriction.
Lentiginosana Group: This group includes the single species S. lentiginosana .
Sparganothoides lentiginosana ( USA, Arkansas, JAP 91E78, n = 4) ( Figs. 96–98 View FIGURES 95–98 ). Length 11–13 mm, presumed final instar; head capsule width 1.0– 1.1 mm. Head capsule and thoracic shield pale amber to tan. Pinacula faint, scarcely visible, very small on abdomen. No trace of SD2 setae on A1–8. Anal shield ( Fig. 106 View FIGURES 99–108 ) weakly sclerotized, pale tan, lateral edge darker; broad, rounded caudally, no sculpturing. Anal comb absent from 3 of 4 individuals; well developed in the fourth, no medial constriction, 6 tines, spinelike.
Aciculana Group: No recognizable group features shown by the single species examined.
Sparganothoides cornutana ( Mexico, Veracruz, JAP 87H45, n = 2). Length 13.0– 14.5 mm, preseumed final instar; head capsule width 1.0– 1.05 mm. Head capsule dark brown, no black area beneath the stemmata. Pinacula small but distinct, including those on abdomen. No trace of SD2 on A1–8. Anal shield ( Fig. 107 View FIGURES 99–108 ) unicolorous pale brown, much paler than the thoracic shield; constricted preapically, with a blunt caudal end; parallel creases directed inwardly from anterior margin but no pits or other sculpturing. Anal comb with 7–9 spinelike tines, base strongly constricted at middle, similar to S. ocrisana ( Fig. 105 View FIGURES 99–108 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |