LAMPRIDIFORMES GOODRICH, 1909
|
publication ID |
https://doi.org/ 10.1111/zoj.12166 |
|
persistent identifier |
https://treatment.plazi.org/id/BD3787FE-5370-FFC7-FEE1-FE44FD4AFDA9 |
|
treatment provided by |
Marcus |
|
scientific name |
LAMPRIDIFORMES GOODRICH, 1909 |
| status |
|
Synonyms: Allotriognathi Regan, 1907 ; Lampriformes Goodrich, 1909 .
Remark: A debate exists on whether the order should be named ‘Lampriformes’ or ‘Lampridiformes’. ‘Lampriformes’ has been used several times following the recommendations of Steyskal (1980), for example in Olney (1984), Grande et al. (2013), and most notably in Nelson’s widely used handbook Fishes of the World ( Nelson, 2006), as well as in the online database FishBase ( Froese & Pauly, 2014; http://www.fishbase.org); however, Patterson, in an appendix to Olney et al. (1993), advocated the use of ‘Lampridiformes’. This orthography has been followed by the vast majority of recent phylogenetic papers, including the review of teleost classification by Wiley & Johnson (2010) and the DeepFin - EToL classification (Betancur-R. et al., 2013b). Therefore, we chose to maintain current usage and use the name ‘Lampridiformes’.
Included taxa: See reviews in Olney et al., 1993; Roberts, 2012.
Olney et al. (1993) proposed four osteological synapomorphies for Lampridiformes . All of these were retrieved here (one has an ambiguous optimization and another is not a discrete character state in our matrix), and we propose additional ones, for a total of 14 synapomorphies (four cannot be unambiguously assigned to the clade because of missing data in the fossil sister groups).
1. Ascending process of the premaxilla equal or longer than the alveolar process (character 2, 0 → 1).
An elongate ascending process is a lampridiform synapomorphy according to Olney et al. (1993: 148).
2. Loss of the supramaxillae (character 3, 0 → 2).
It is also the case in modern paracanthopterygians and in myctophids, by convergence.
3. Loss of the anterior palatine process (character 4, 0 → 1).
This lampridiform synapomorphy, according to Olney et al. (1993: 147), is here optimized as ambiguous, because the character state data are missing for the immediate fossil outgroups in our matrix.
4. Loss of the ascending process on the lachrymal (character 8, 1 → 0).
This is a reversal of Lampridomorpha character number one.
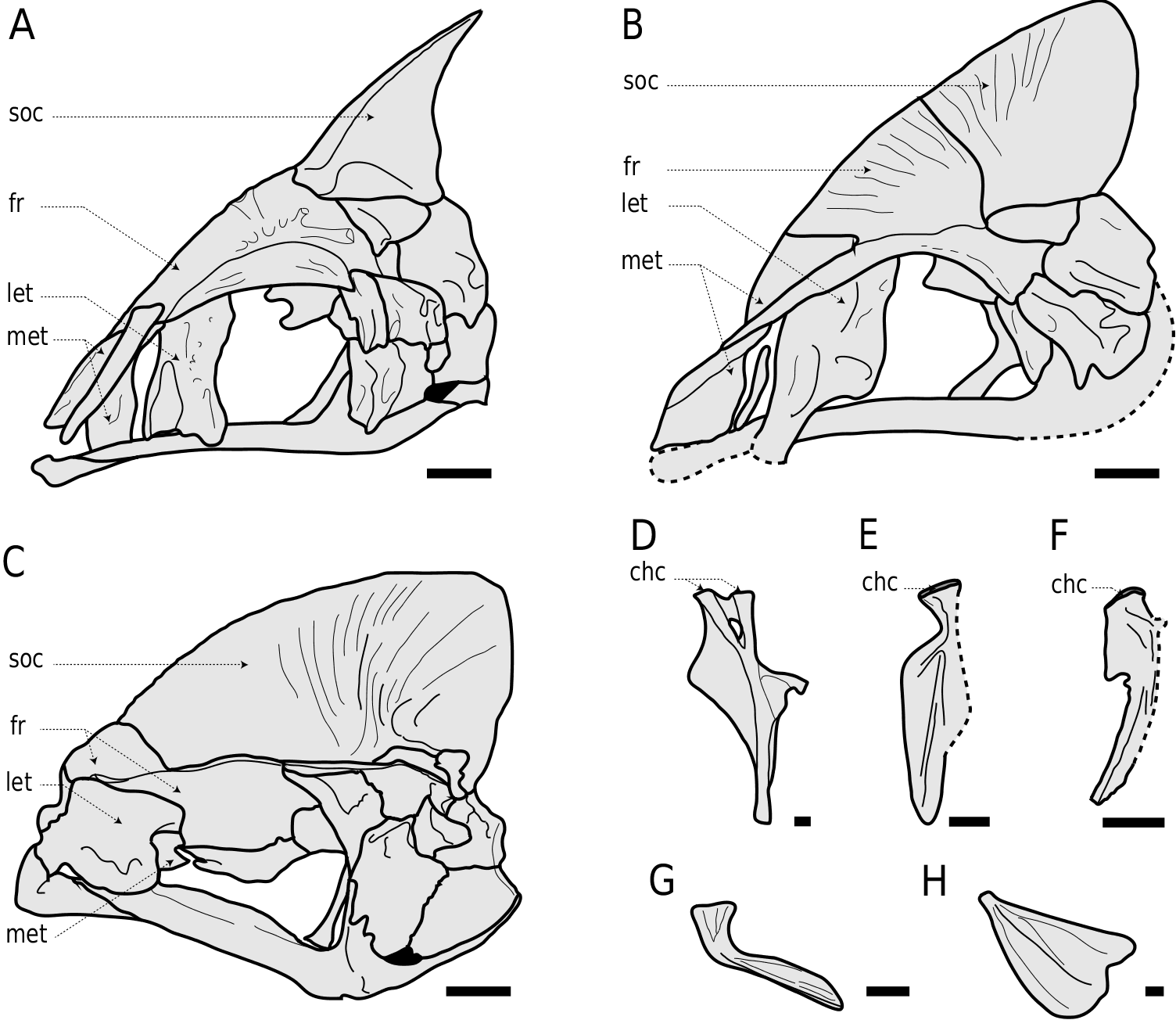
5. ‘Vault’ or ‘cradle’ on the frontal, accommodating the premaxilla and the rostral cartilage (character 12, 0 → 1).
This state is part of Olney et al.’s (1993: 148) synapomorphy number 3.
6. Seven or fewer branchiostegal rays (character 28, 1 → 2).
This state is convergent with paracanthopterygians and Polymixia .
7. Urohyal expanded by a large ventral lamina ( Fig. 1H View Figure 1 ; character 29, 0 → 1).
The optimization is ambiguous for this state because of missing data in fossils. A large ventral lamina on the urohyal is also present in many deepbodied euacanthopterygians not included in the analysis, like menids and some carangids.
8. Epineurals lost on the postabdominal vertebrae (character 36, 0 → 1).
This state is convergent with extant paracanthopterygians.
9. Loss of the epipleurals (character 37, 0 → 1).
An independent loss occurred in Euacanthopterygii + Paracanthopterygii and in † Pycnosteroides .
10. Only two supraneurals (character 38, 0 → 2).
All lampridiforms included in our analysis have one supraneural at most (hence the character state presented here); however, Velifer hypselopterus Bleeker, 1879 (not included) is the only extant species with two supraneurals ( Fig. 2B), suggesting that the reduction to two supraneurals is the lampridiform synapomorphy. Such a reduction is convergent with paracanthopterygians and Myripristis .
11. First dorsal pterygiophore inserting anterior to the first neural spine ( Fig. 2B; character 40, 1 → 2).
This character state is also observed (by convergence) in the † Pharmacichthys + † Pycnosteroides clade (with a reversion in † Pharmacichthys venenifer ) and in euacanthopterygians not includ- ed in the analysis, such as echeneids, pleuronectoids, pataecids, and coryphaenids.
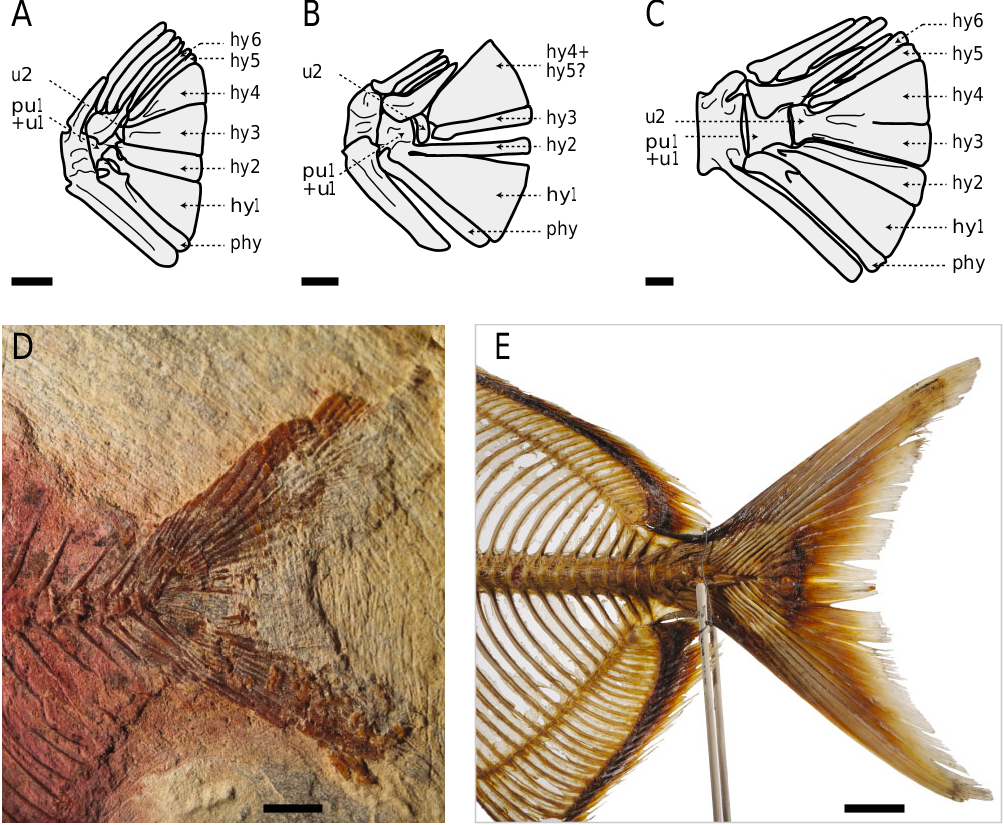
12. Upper hypurals fused together ( Fig. 3B, C View Figure 3 ; character 52, 0 → 1).
The character can also be considered a synapomorphy of a potential † Aspesaipichthys + Lampridiformes clade (recovered in several parsimonious trees), convergent with modern paracanthopterygians.
13. Upper hypurals fused to the second ural centrum ( Fig. 3C View Figure 3 ; character 53, 0 → 1).
Although it could also be interpreted as convergent in lampridids and veliferids, we follow Wiley & Johnson (2010), who favoured this character state as a lampridiform synapomorphy, as already noted by Patterson (1968). There is a reversal in taeniosomes, which have greatly modified caudal skeletons, presumably associated with a reduction of the caudal fin. The state is also convergent with extant paracanthopterygians.
14. Postcleithra fused together (character 61, 0 → 1). This state is convergent with extant paracanthopterygians.
Our results are consistent with previous results regarding relationships within Lampridiformes (e.g. Olney et al., 1993; Wiley et al., 1998): veliferids are sister to a clade composed by lampridids and taeniosomes.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.