Acalypha, L.
|
publication ID |
https://doi.org/ 10.5252/adansonia2023v45a26 |
|
DOI |
https://doi.org/10.5281/zenodo.10671400 |
|
persistent identifier |
https://treatment.plazi.org/id/C00D879E-FFA1-5335-2342-FCAC88FFF84D |
|
treatment provided by |
Plazi |
|
scientific name |
Acalypha |
| status |
|
TAXONOMIC HISTORY OF ACALYPHA View in CoL OF WIOR
The first species of Acalypha from WIOR, A. filiformis Poir. and A. venosa Poir. , both from Mauritius, were described by Poiret (1804). Willdenow (1805) soon added another species from Mauritius, A. integrifolia Willd. Bojer’s Hortus Mauritianus ( Bojer 1837) , which included the first catalogue of the native species of Mauritius, listed eight Acalypha species, two of them newly named ( A. lantanaefolia Bojer and A. tomentosa Bojer ), but unfortunately Bojer’s designations are both nomina nuda.
One of the 19th century botanists who contributed the most to the study of Acalypha in WIOR was Henri Baillon. He initially listed two species from Madagascar ( A. reticulata (Poir.) Müll.Arg. and A. salviifolia Baill. ) and three from the Mascarene Islands ( A. marginata (Poir.) Spreng. , A. colorata (Poir.) Spreng. , and A. arborea Comm. in Poir.) ( Baillon 1858). Soon thereafter, he listed nine species from Madagascar ( A. acuminata Vahl ex Baill. , A. emirnensis Baill. , A. goudotiana Baill. , A. gracilipes Baill. , A. pervilleana Baill. , A. rottleroides Baill. , A. salviifolia , A. spachiana Baill. , and A. urophylla Boivin ex Baill. ), four species from the Comoros ( A. chibomboa Baill. , A. codonocalyx Baill. , A. ovalifolia Baill. , and A. richardiana Baill. ), and three species from the Mascarene Islands ( A. arborea , A. commersoniana Baill. , and A. marginata ); 11 of these species were newly described ( Baillon, 1861). Much later, Baillon’s (1891) contribution to Histoire naturelle des Plantes volume III ( Atlas ) of Grandidier’s Histoire physique, naturelle et politique de Madagascar ( Baillon 1891) included excellent plates, made by the illustrator André Revillon d’Apreval, of ten Acalypha species, five of them new to science ( A. diminuta Baill. , A. humblotiana Baill. , A. leptomyura Baill. , A. madreporica Baill. , and A. polynema Baill. ). It should be noted that only plates were published in this work, with no accompanying descriptions or additional information, but under the International Code of Nomenclature ( Turland et al. 2018), these are sufficient to validate Baillon’s new names. Finally, Baillon (1892, 1895a, 1895b) listed 32 Acalypha species from Madagascar, of which five were described for the first time ( A. bakeriana Baill. , A. hildebrandtii Baill. , A. meiodonta Baill. , A. leonii Baill. , and A. vulneraria Baill. ); he also provided descriptions of some of the species illustrated in the 1891 atlas.
The treatment of Acalypha in de Candolle’s Prodromus ( Müller Argoviensis 1865, 1866) included 11 Acalypha species from WIOR, six from Madagascar ( A. emirnensis , A. reticulata , A. rottleroides , A. spachiana Baill. , A. spiciflora Burm. f. and , as a novelty, A. fasciculata Müll.Arg. ), three from the Comoros ( A. chibomboa , A. codonocalyx , and A. richardiana ), and two from the Mascarene Islands ( A. commersoniana and A. marginata ). Müller Argoviensis (1882) described one more species from Madagascar, A. buchenavii Müll.Arg.
Other 19 th century authors also treated Acalypha from the WIOR. Baker (1877) listed five Acalypha species from Mauritius and the Seychelles and provided an identification key. Baker also described four new species from Madagascar ( A. baronii Baker , A. hologyna Baker , A. lyallii Baker , and A. radula Baker ) ( Baker 1883, 1884). Strangely, Baron (1889) included only two Acalypha species ( A. radula and A. urophylla ) from Madagascar. Pax (1894) described four new species from Madagascar and the Comoros ( A. juliflora Pax , A. comorensis Pax , A. urophylla Pax , and A. squarrosa Pax ). Cordemoy (1895) wrote the first flora of La Réunion, in which five Acalypha species were included ( A. indica L. , A. poiretii Spreng. , A. reticulata , A. colorata , and A. marginata ).
Early in the 20th century, Palacký (1907) compiled a list of 44 previously described Acalypha species from Madagascar. Voeltzkow (1917) included seven Acalypha species from the Comoros. Hemsley (1919), in his flora of Aldabra and nearby islands, included two species of Acalypha endemic to Seychelles ( A. claoxyloides Hutch. and A. fryeri Hutch. ), both previously described by Hutchinson (1918). The last worldwide treatment of the genus, in Engler’s Das Pflanzenreich ( Pax & Hoffmann, 1924), included 31 Acalypha species from WIOR, only one of which was newly described ( A. madagascariensis Pax & K.Hoffm. ).
Leandri (1942) made an important contribution to our understanding of Acalypha in WIOR. In a thorough taxonomic review of Acalypha in Madagasar, he treated 22 species and 14 varieties, of which eight species were newly described ( A. andringitrensis Leandri , A. boinensis Leandri , A. decaryana Leandri , A. gagnepainii Leandri , A. humbertii Leandri , A. lepidopagensis Leandri , A. linearifolia Leandri , and A. perrieri Leandri ). Leandri (1942) also proposed many new synonyms and provided illustrations and an identification key. Shortly after, he cited seven Acalypha species collected by Lam and Meeuse ( Leandri, 1948).
Coode (1978, 1979, 1982) reviewed Acalypha for the Mascarene Islands. He included five species, three of them introduced, and proposed some new subspecies and varieties. Robertson (1989) recognized five Acalypha species from the Seychelles. Several thematic works on ethnobotany, conservation, etc. (e.g.: Heckel 1903; Jenkins 1987, 1990; Goodman 1996; Schatz 2001; Seebaluck et al. 2015; Seebaluck-Sandorama, 2018), cited Acalypha species from WIOR, although they do not provide relevant taxonomic information.
Due to the insufficient and outdated state of knowledge of Acalypha in this region, we started a monographic study of Acalypha species from WIOR in 2017, as a part of the PhD dissertation of the first author ( Montero Muñoz 2021). The first step was a nomenclatural review of the genus in this region ( Montero Muñoz et al. 2018a). That was followed by the publication of 12 new species from the region ( Montero Muñoz et al. 2018b, 2020a, b, 2022).
MATERIAL AND METHODS
The present work is based mainly on the study of 2 312 herbarium specimens of Acalypha stored in the following herbaria: A, B, BM, BREM, BRNU, C, CAN, CAS, E, FR, G, GB, GDC, GH, JE, K, L, LD, LE, LMU, M, MA, MAO, MAU, MAUAM, MO, MPU, NY, P, PRE, S, TAN, TEF,TUB, UPS, US, W, and WAG (acronyms according to Thiers 2022). They correspond to 1 646 collections from WIOR. Of them, 1 117 collections (1 597 specimens) are from Madagascar, 91 (140 specimens) from the Comoros, 356 (463 specimens) from the Mascarene Islands, 81 (111 specimens) from Seychelles, and one (one specimen) from the Scattered Islands. We have also reviewed numerous collections from continental Africa and other regions of the Paleotropics to improve our understanding of the diversity and evolution of the genus. Most of the specimens were studied in situ, either in the different herbaria or through loans.We also consulted digitized collections accessible through JSTOR Global Plants (https://plants.jstor.org) and other virtual herbaria, especially to review type collections.
The morphological terms used follow those proposed by Harris & Harris (2001) and Ellis et al. (2009).
The micromorphological analysis of the epidermal surface was carried out using scanning electron microscopy (SEM). For this purpose, we selected 52 specimens from different herbaria (BREM, COL, G, GH, K, MO, P, SEL, U, and W) corresponding to 30 native and five introduced species of our study area (Appendix I). For the analysis of the foliar surface, we used a scalpel to extract two approximately 3 mm 2 fragments from each specimen. We also examined bracts and flowers using a fragment or the entire organ. The samples were placed on SEM-specific slides and then metallized with a 15 nm gold layer, using a Quorum Q150T-S metallizer. For the observation and analysis of the samples, we used a HITACHI S-3000N electron microscope with X-ray detector (INCAx-sight, Oxford Instruments), which enables energy dispersive X-ray (EDX) analysis.
The information obtained was incorporated into databases (nomenclature, collections, bibliography, morphology, distribution and habitat, images, etc.) using Microsoft Access 2016 © (Redmond, Washington, United States). These databases are integrated and managed through an online information system named Acalypha Taxonomic Information System ( Cardiel et al. 2022b). This website aims to collect all the taxonomic and biogeographic information of Acalypha worldwide in open access, and it is integrated into global biodiversity networks through GBIF. The system allows detailed consultation of all the studied specimens and the available images of them; it also generates distribution maps.
Most collections studied lacked geographical coordinates (81.7%) so we georeferenced them using GeoLocate v.3.22 ( Ríos & Bart 2010) and Google Earth. For the older collections (from the 18 th and 19 th centuries) we use the “Gazetteer to Malagasy Botanical Collecting Localities” from the Missouri Botanical Garden ( Schatz & Lescot 2003), which includes many old names for localities in Madagascar. When localities were ambiguous or imprecise, we assigned generic country or island coordinates. Distribution maps were prepared using QGIS Desktop 3.2.2. (QGIS Development Team 2018). The layers of the administrative boundaries of all the archipelagos of our study area were obtained from DIVA-GIS 7.5 ( Hijmans et al. 2012; https://www.diva-gis.org/), and the altitude layers from SRTM Tile Grabber ( Watkins 2020; https://dwtkns.com/srtm/).
Preliminary conservation assessments are based on the IUCN Red List Categories and Criteria ( IUCN 2017). Area of occupancy (AOO) and extent of occurrence (EOO) were calculated with GeoCAT, a geospatial conservation assessment tool ( Bachman et al. 2011; http://geocat.kew.org/), using a 2 × 2 km grid cell size as recommended by IUCN (2012, 2017).
The nomenclature is based primarily on our previous work ( Montero Muñoz et al. 2018a). The genus description is based on the WIOR species. The accepted species are arranged first by subgenera, and then alphabetically arranged followed by their author and place of publication. We then list, as appropriate, the basionym, homotypic synonyms in chronological order, and heterotypic synonyms in chronological order, following the same format as for the accepted name. Types are cited for all valid taxa, indicating the type locality, date of collection, collector, collection number, and herbarium/a where they are deposited, including the herbarium barcode if available. Missing information is indicated as s.loc. (without locality), n.d. (undated), and s.n. (without number). If the locality information is ambiguous or imprecise, we indicate it in quotation marks as it appears in the protologue. We provide the bibliographic sources for illustrations of the species, or the number of the figure included in this work. Descriptions are based on the studied specimens and follow the same sequence. When necessary, we use round brackets (parentheses) to indicate uncommon variation in morphological ranges and square brackets to indicate rare variation. The etymology of the specific epithet of each accepted name is also provided.
For each species, we report its general geographic distribution and its distribution in WIOR. For Madagascar, we also indicate the provinces for which there are collection records; for the other archipelagos, we report the island/s where the species occurs. We also describe the habitat, according to the vegetation typology of Gautier et al. (2018), and the altitudinal range. This information is based exclusively on the collections studied.
Preliminary conservation assessments of each species are provided, indicating the IUCN category, the justification, and the criteria and sub-criteria followed.We cite chronologically the publications (with page numbers) in which the species are cited for WIOR; after that, we report the total number of collections examined, identifying each one with the primary collector’s name and number; we also cite the herbarium barcode (when available). For full information about each collection, readers can consult the Acalypha Taxonomic Information System website (http://www.acalypha.es/secc/specimens.asp, using the information provided in the material examined section to search for the collection. Finally, in the notes section, we indicate any additional taxonomic or nomenclatural clarification, or other additional relevant information.
DESCRIPTIVE MORPHOLOGY
Habit
Most native Acalypha species from WIOR are shrubs or small trees to six meters high. We only found two herbs or subshrubs: A. leandrii I.Montero & Cardiel and A. rabesahalana I.Montero & Cardiel. Deciduous species predominate; only A. andringitrensis , A. berryi I.Montero, Cardiel & G.A.Levin , sp. nov., A. chibomboa , A. leonii , A. linearifolia , A. radula , and probably A. leandrii and A. vulneraria , are evergreen species. For ten species ( A. crateriana (Coode) I.Montero & Cardiel , comb. nov., A. filiformis , A. gracilipes , A. integrifolia , A. isaloensis I.Montero & Cardiel , A. magistri I.Montero & Cardiel , A. marginata , A. pervilleana , A. richardiana , and A. urophylla Boivin ex Baill. ) we do not have enough information to know if they are deciduous or evergreen.
Branching is usually lax, with thin and slender branches, sometimes divaricate, and with many lenticels. Some species have sprawling branches, such as Acalypha gracilipes , A. leandrii , and A. urophylla . All species are unarmed except A. baretiae I.Montero & Cardiel , whose old branches become thorny. Some species have brachyblasts covered by stipules and with a tuft of leaves at the apex; these appear in A. baretiae , A. diminuta , and A. decaryana . The presence of brachyblasts has not been described in Acalypha before Montero Muñoz et al. (2018b).
Indument
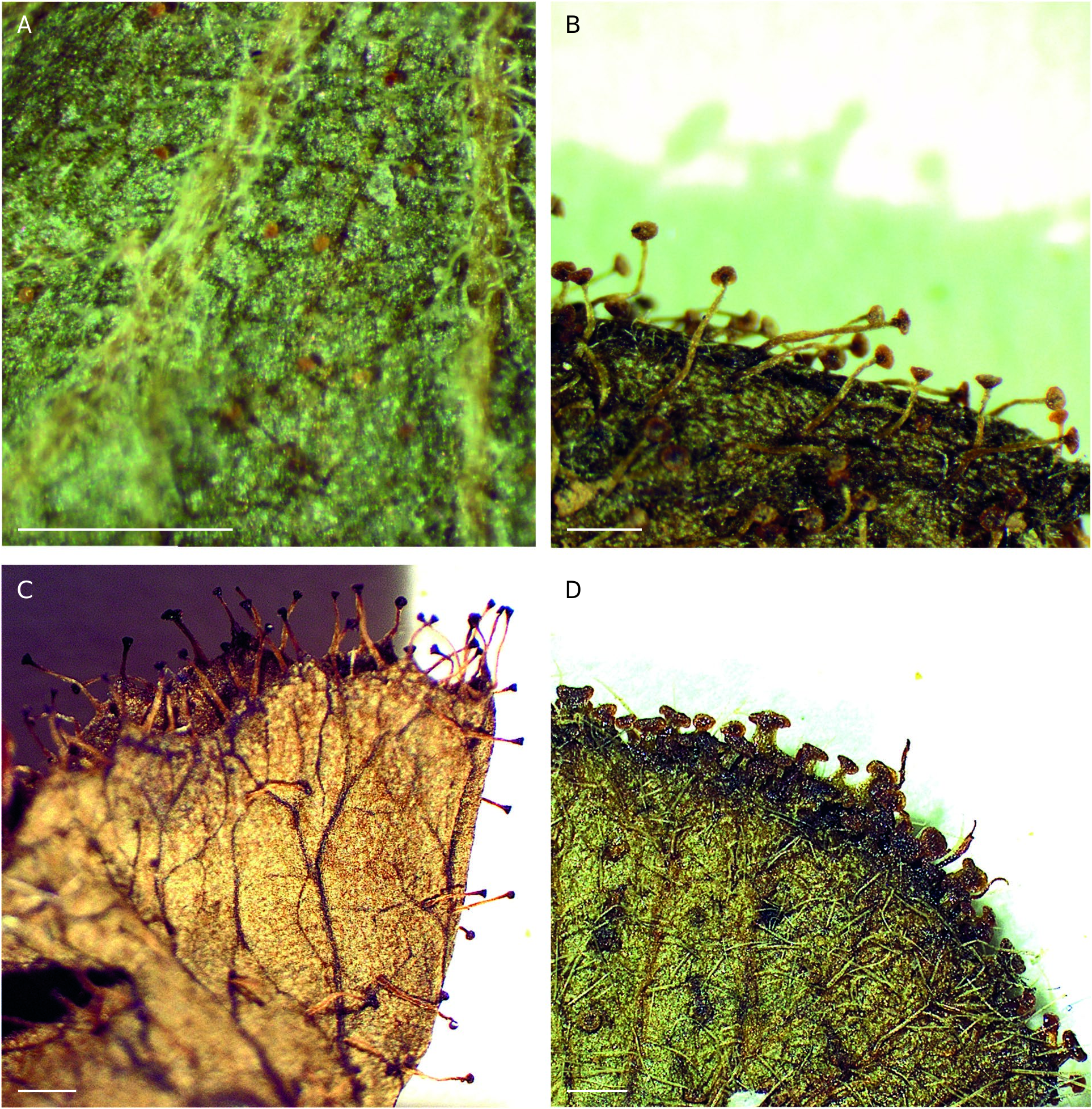
Most Acalypha species present one or, more frequently, several types of trichomes of variable density distributed through most of the plant; only A. gracilipes is glabrous. Simple trichomes predominate, but glandular trichomes are also frequent; stellate or fasciculate trichomes appear only in A. linearifolia and A. radula ( Fig. 2C, D View FIG ). Simple trichomes ( Fig. 2A, B View FIG ) vary in length and thickness, and can be appressed or erect, straight or curved, and antrorse or retrorse. We also observed another type of simple trichome that is thin and vermiform, which we refer to as arachnoid. This type of trichome gives rise to a whitish, woolly-looking indument, and is found in A. baretiae , A. burmanii I.Montero & Cardiel , A. chibomboa , A. claoxyloides , A. diminuta , A. mayottensis I.Montero & Cardiel , A. medibracteata Radcl. -Sm. & Govaerts, A. menavody (Leandri) I.Montero & Cardiel , and A. perrieri .
Glandular trichomes are topped by a flat or rounded head and can be stalked or sessile. Stalked glandular trichomes reach up to 1.5 mm long in Acalypha andringitrensis , A. leandrii , A. radula , A. rottleroides , A. spachiana , and A. vulneraria , or up to 0.5 mm long in A. ankaranensis I.Montero & Cardiel , A. cardielii I.Montero & G.A.Levin , A. levinii I.Montero & Cardiel , A. pervilleana , A. perrieri , and A. urophylla ( Figs 2H View FIG ; 3 View FIG ). Sessile glandular trichomes are usually flattened or sometimes rounded, resinous or amber-colored; they appear in A. chibomboa , A. claoxyloides , A. decaryana , A. diminuta , A. emirnensis , A. medibracteata , and A. lepidopagensis ( Figs 2 View FIG E-G; 4).
Axillary buds
A characteristic of numerous Acalypha species in WIOR is the presence of buds protected by perules. The perules are two or four scales that partially or completely cover the buds. The scales can be membranous and valvate or slightly imbricated, or coriaceous and then overlapping to form a continuous cover on the bud. Perules are especially conspicuous in deciduous species ( Fig. 5 View FIG ) and are rarely found in species outside WIOR (e.g., A. gillmanii Radcl. -Sm.; Radcliffe-Smith 1975).
Leaves
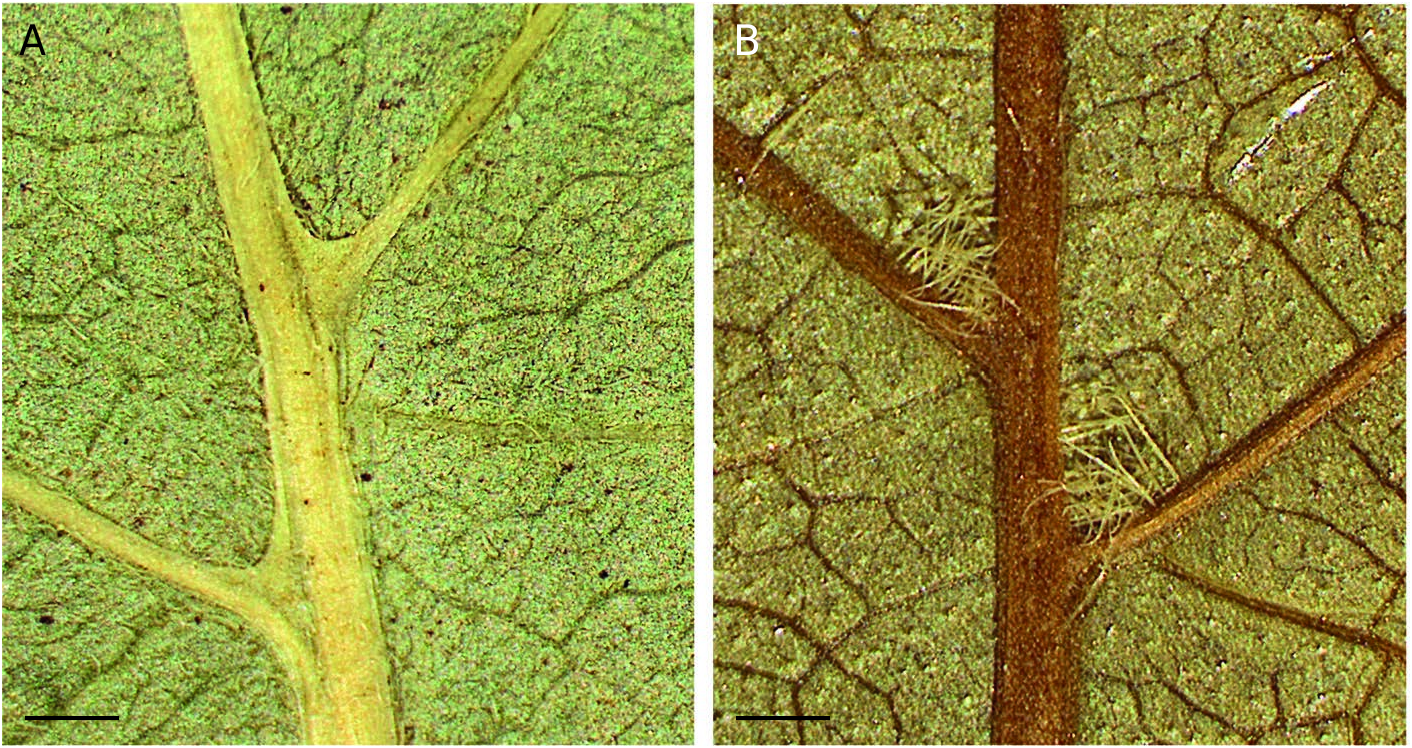
Leaves are simple, alternate, and petiolate; only Acalypha linearifolia has subsessile leaves, with petioles up to 2 mm long. Leaf blades can vary from linear ( A. linearifolia ) to broadly ovate-lanceolate. They are unlobed except in A. gillespieae G.A.Levin & I.Montero , which is one of the few Acalypha species that has lobed leaves. The base varies from cuneate to cordate, the apex from rounded to caudate, and the margin varies from entire to serrate, and sometimes is revolute, reddish or discolored, and/or slightly callous. The texture is usually membranous or chartaceous; only A. linearifolia and A. integrifolia have coriaceous leaf blades. The venation usually is actinodromous, but is pinnate in some species. Several species have domatia in the axils of the secondary veins. They can be membranous, forming a small pocket in each axil, as in A. isaloensis ( Fig. 6A View FIG ), or can be a dense tuft of hairs ( Fig. 6B View FIG ). Pocket-shaped domatia had not been described in Acalypha prior to our work.
Stipules and stipels
All species produce stipules; these usually are deciduous, so can be observed only on young branches. The shape and size of the stipules vary among species, so they are good diagnostic characters. Their size varies from 1 mm long in Acalypha linearifolia , up to 13 mm in A. andringitrensis , and their shape varies from filiform to triangular-lanceolate. Sometimes they have scarious ( A. leandrii , A. radula , and A. tremula I.Montero & Cardiel ) or greenish margins ( A. integrifolia ).
Many Acalypha species bear stipels, which are two or more minute and inconspicuous appendages at the base of the leaf blade. We found them in 17 species of WIOR. They can be glandular and tiny ( A. berryi sp. nov., A. boinensis , A. burmanii , A. cardielii , A. claoxyloides , A. emirnensis , A. lamiana (Leandri) I.Montero & Cardiel , A. medibracteata , A. menavody , A. perrieri , A. richardiana , A. spachiana , A. urophylla , and A. vulneraria ), filiform ( A. ankaranensis , A. levinii ), triangular ( A. leandrii ), or linear-lanceolate ( A. leptomyura ).
Inflorescences
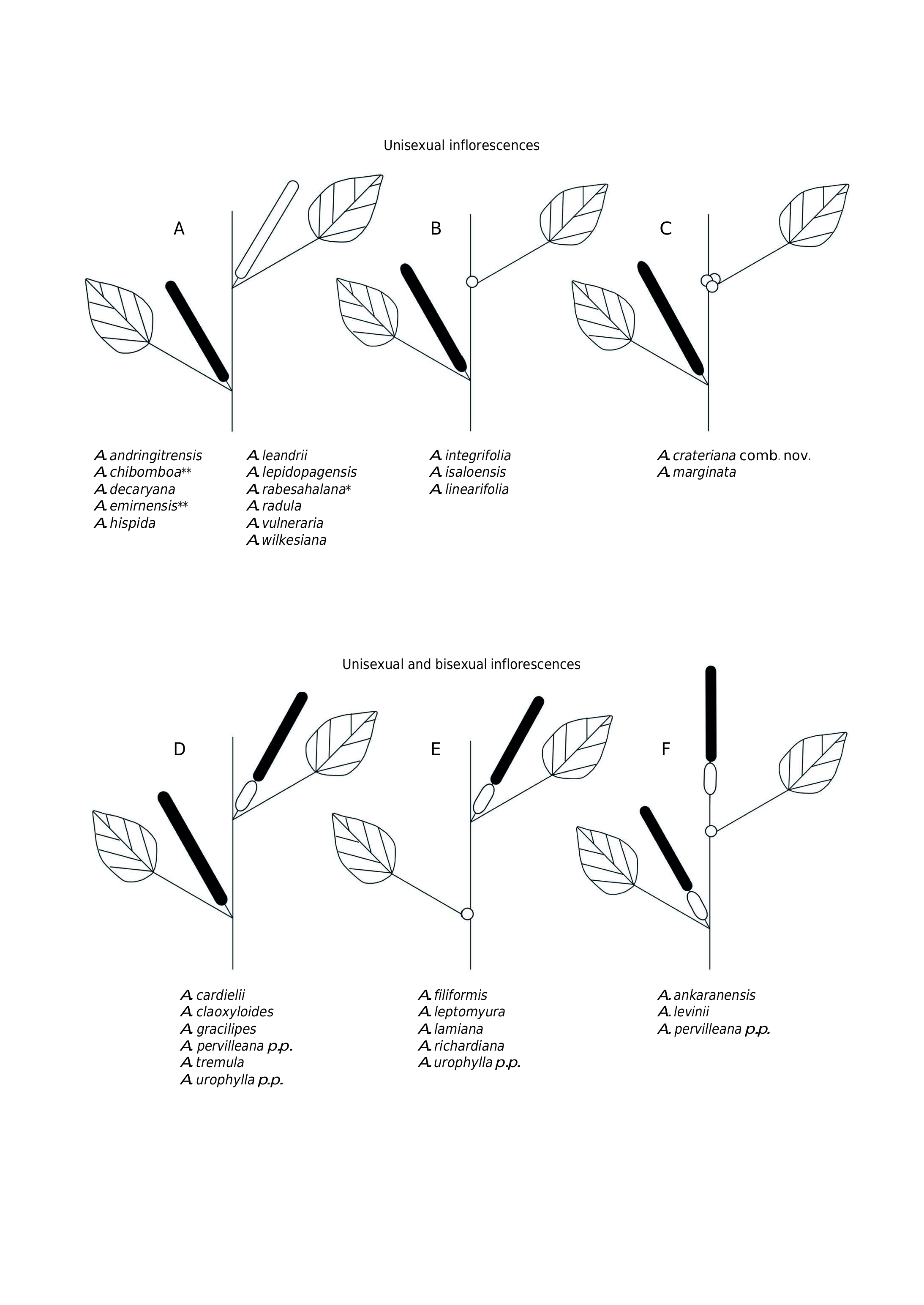
Most of the species are monoecious. Only Acalypha leandrii appears strictly dioecious. Other species, such as A. andringitrensis , A. chibomboa , A. filiformis , A. integrifolia , A. marginata , and A. radula , have a clear tendency to dioecy, with most specimens we studied having inflorescences of only one sex, but some specimens having both male and female inflorescences on the same branch. Inflorescences can be unisexual or bisexual, and usually are axillary, rarely terminal or sub-terminal. They usually are spiciform, rarely racemose, paniculate or glomerulate. Solitary female flowers sometimes are present.
Male inflorescences are always axillary (in other regions they can be terminal) and densely flowered. They are spiciform thyrses with short-pedicellate flowers grouped in small cymes (flower glomeruli) along the rachis.Male inflorescences can be pedunculate or subsessile and vary from 1.4 cm long in Acalypha linearifolia to 12 cm long in A. chibomboa ( Fig. 7 View FIG A-D).
The female inflorescences are axillary (subterminal only in Acalypha rabesahalana ), usually spiciform, and densely to laxly flowered. They can be pedunculate or sessile, and their lengths vary from 3 cm, in A. decaryana and A. rabesahalana , to 13 cm in A. vulneraria ( Fig. 7A View FIG ). In some species the female inflorescences are reduced to a single bract with one (rarely to three) flowers; this bract can be long-pedunculate ( A. filiformis ), subsessile ( A. linearifolia ), or sessile ( A. ankaranensis ,
A. berryi sp. nov., A. integrifolia , A. isaloensis , A. lamiana , A. leptomyura , A. levinii , A. pervilleana , A. richardiana , and A. urophylla ) ( Fig. 7B, E, F View FIG ).
Acalypha marginata and A. crateriana comb.nov. have a unique female inflorescence structure, consisting of a single glomerulus or compact cyme of 3-4 sessile or subsessile flowers ( Fig. 7C View FIG ).
Bisexual inflorescences usually are axillary, except in Acalypha ankaranensis , A.diminuta , A.levinii , A. pervilleana , and A.spachiBisexual inflorescences ana, which can have terminal inflorescences ( Figs 7F View FIG ; 8C View FIG ). They usually are androgynous (female proximally and male distally), or rarely (only A. baretiae ; Fig. 8B View FIG ) gynecandrous (male proximally and female distally). They vary in length from 1 cm in A. gillespieae to 11 cm in A. filiformis and A. chibomboa .
Most androgynous inflorescences display one of two patterns of sexuality. In one, the inflorescence is mostly female and the male segment is very short and inconspicuous (less 1.5 cm long); sometimes it is deciduous, so the inflorescence can appear to be female late in development ( Fig. 8C, D View FIG ). An inflorescence that follows the other pattern has a long (usually more than 2 cm), conspicuous, and persistent male segment, with the female segment much shorter, with one or more bracts. A few species do not conform to either pattern. In A. chibomboa , the male and female segments are of about equal length, and A. gillespieae and A. nusbaumeri I.Montero & Cardiel have very short androgynous inflorescences (up to 1.5 cm long), with a persistent male segment to 1 cm long ( Fig. 8E View FIG ). Species that have androgynous inflorescences often also have male inflorescences and/or solitary female bracts.
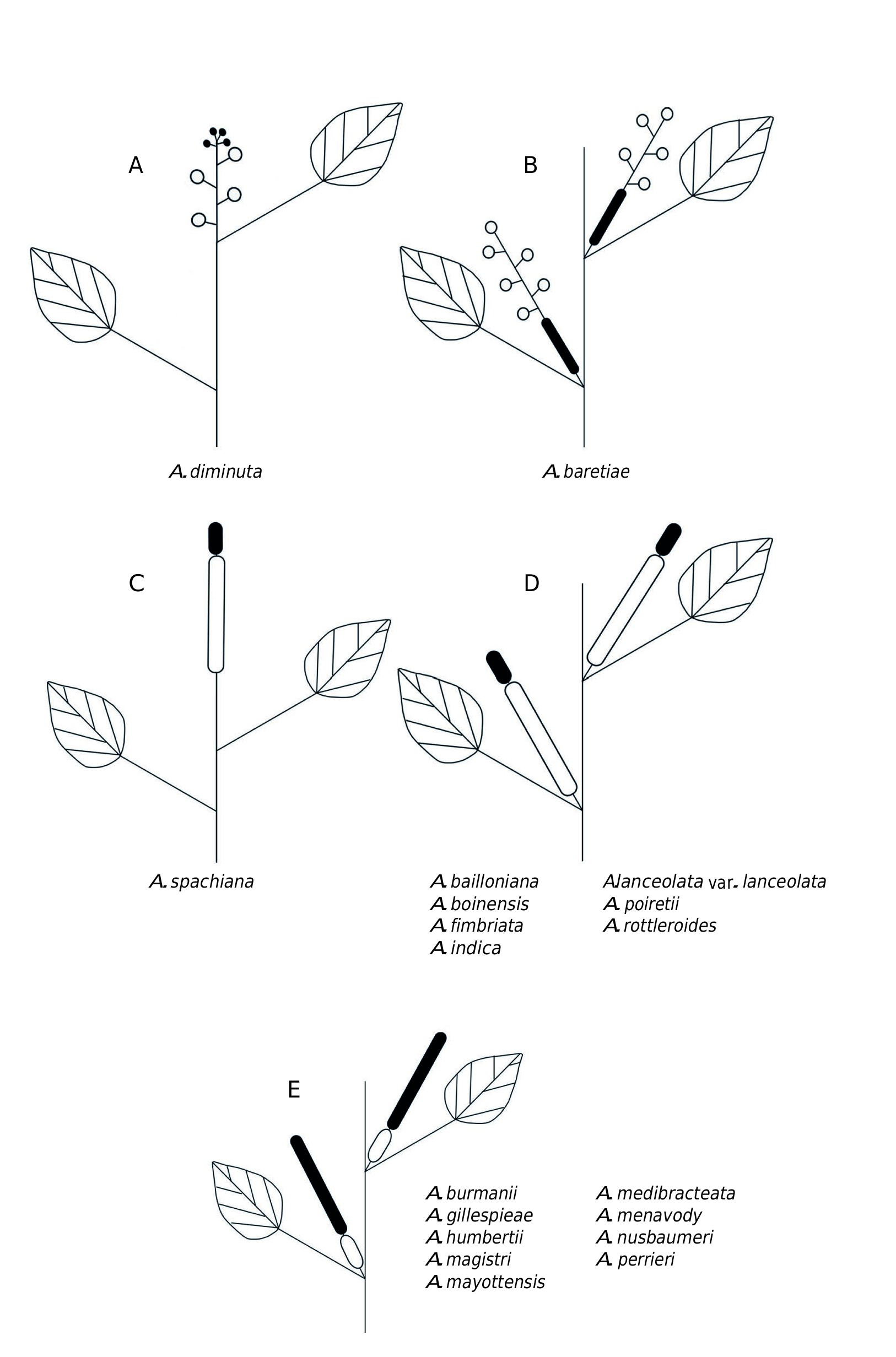
Acalypha baretiae and A. diminuta are the only species with racemose inflorescences and pedicellate female flowers. Acalypha baretiae is unique among WIOR Acalypha in having gynecandrous inflorescences. Acalypha diminuta has androgynous inflorescences with 1-7 pedicellate female flowers proximally and a short terminal racemose to subumbelliform cluster of male flowers ( Fig. 8A View FIG ).
The position and sexuality of the inflorescences in Acalypha has been frequently used as diagnostic character for groups of species and forms the basis for the infrageneric classification of subg. Acalypha . Although inflorescences position and sexuality have limited phylogenetic utility worldwide ( Sagun et al. 2010; Levin et al. 2022), these characters are relatively stable in the WIOR species, which can be grouped using the models shown in Figs 7 View FIG and 8 View FIG .
Bracts
Bracts of the male inflorescences, or of the male segment of bisexual inflorescences, are usually inconspicuous (up to 1 mm long), although longer in Acalypha rottleroides (to 1.5 mm long) and A. boinensis and A. emirnensis (to 2 mm long). They are usually triangular, although they can vary from ovate-lanceolate to orbicular, and in A. andringitrensis they are spatulate. In A. leonii and A. filiformis they can be slightly fleshy.
Bracts of the female inflorescences, or of the female segment of bisexual inflorescences, are usually foliaceous, growing markedly as the fruit matures. The mature sizes of these accrescent bracts range from 2 mm long in Acalypha nusbaumeri to 21 mm in A. mayottensis . Only A. baretiae , A. crateriana comb. nov., A. diminuta , A. hispida Burm.f. , and A. marginata have small, non-accrescent female bracts. The margin of the mature bracts can be entire, subentire, crenate, or dentate, and is reddish or discolorous in a few species.Although outside WIOR the bracts can be deeply divided, among the species native to WIOR they are mostly no more than shallowly toothed; only A. rabesahalana has bracts with teeth up to 1/3 of the bract length. In several species, two small appendages up to 2 mm long are borne at the base of the bract. A detailed developmental study is needed to determine the nature of these structures. Based on their position, they might be homologous with stipules, the interpretation adopted by Sagun et al. (2010), who referred to them as bract stipules. Alternatively, they might be bracteoles, an interpretation supported by their complete absence in many species and which we follow here. They usually are linear-lanceolate to triangular-lanceolate, but are glandular in A. claoxyloides . We observed bracteoles in A. andringitrensis , A. ankaranensis , A. berryi sp. nov., A. boinensis , A. claoxyloides , A. decaryana , A. emirnensis , A. humbertii , A. lepidopagensis , A. nusbaumeri , A. rabesahalana , A. radula , and A. vulneraria .
Female bracts usually subtend a single flower, or rarely two or three. The size, shape, and indument of mature female bracts are characteristic of each species and therefore good diagnostic characters.
Flowers
Acalypha has small unisexual, apetalous, and discless flowers. Male flowers are minute, pedicellate or rarely subsessile, with four valvate sepals and eight stamens with two elongated thecae that become vermiform at anthesis. Most specimens bear only closed flowers (probably mostly mature or nearly mature buds). These flowers are very similar in all species, with slight variation only in the indument and the presence or absence of papillae. Regular female flowers usually are sessile, being pedicellate only in A. baretiae and A. diminuta . The calyx usually has three sepals, sometimes four in A. chibomboa and A. mayottensis , and five in A. baretiae and A. diminuta ; the sepals are distinct or connate basally. The ovary usually is trilocular (bilocular only in A. baretiae ) with one ovule per locule. Its surface sculpturing, which can be smooth, papillose, or echinate, and indument are highly variable and usually different in each species. Styles are usually three (two in A. baretiae ), bright red, distinct or slightly connate at the base, and divided into numerous slender segments, giving them a feathery appearance. In some species, the rachis is thickened and may bear papillae and/or simple trichomes.
Allomorphic female flowers. Some Acalypha species produce a second type of female flower, which was called “allomorphic” by Radcliffe-Smith (1973), who first described them in detail. In WIOR species, they are solitary, ebracteate, and axillary or borne at the inflorescence apex. These flowers produce viable seeds identical to those found in regular flowers. In WIOR species, they usually are borne on a filiform pedicel up to 2 cm long, and have 3-4 sepals, usually a single carpel (two in A. spachiana ), and a basal style (two in A. spachiana ). Their capsules are indehiscent (schizocarps with two indehiscent mericarps in A. spachiana ), usually with the distal part fimbriate or with long, sharp papillae. The very rare allomorphic flowers of A. emirnensis are exceptional. They are subsessile at the apex of the staminate inflorescences and have three carpels and three apical styles; we have not seen mature capsules. In the genus as a whole, they are more frequent on herbaceous species, but in WIOR they appear mainly on shrubs ( A. decaryana , A. emirnensis , A. integrifolia , A. lamiana , A. pervilleana , A. radula , A. richardiana , A. spachiana , A. levinii , and A. cardielii ); they are also present on the herbaceous A. rabesahalana .
Fruits and seeds
The fruit formed from the normal female flowers is a dehiscent capsule, to 2-3.5 mm in diameter, reaching 6 mm in diameter in Acalypha burmanii . The surface is smooth, papillose, or echinate, sometimes with projections up to 3.5 mm long, as in A. tremula . The seeds are usually pyriform, sometimes subglobose or globose, with a very reduced caruncle. The surface is foveolate, but the sculpturing can be so fine that the seed appears almost smooth. The seeds are very similar in all the WIOR species region.
Epidermal crystals
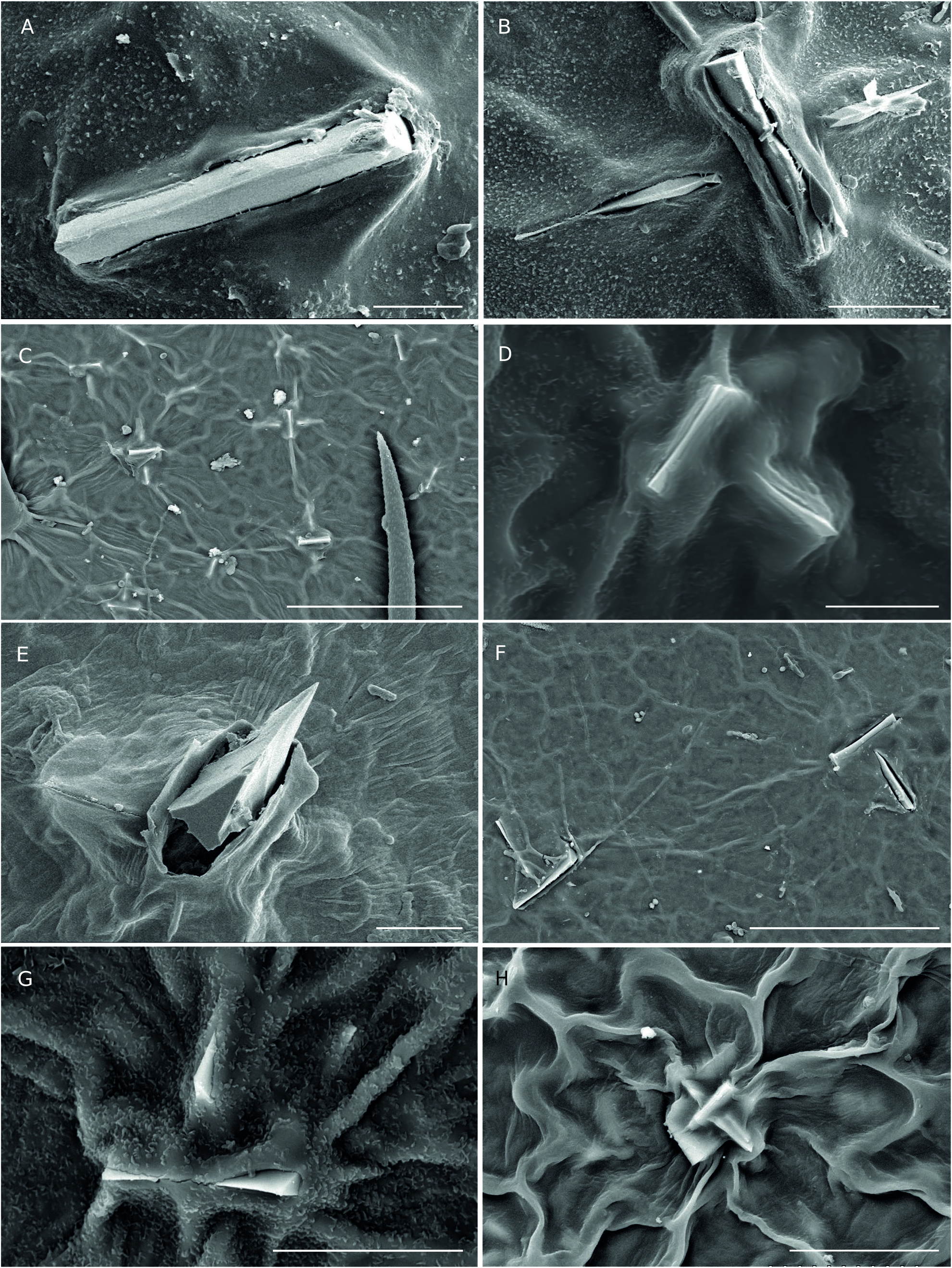
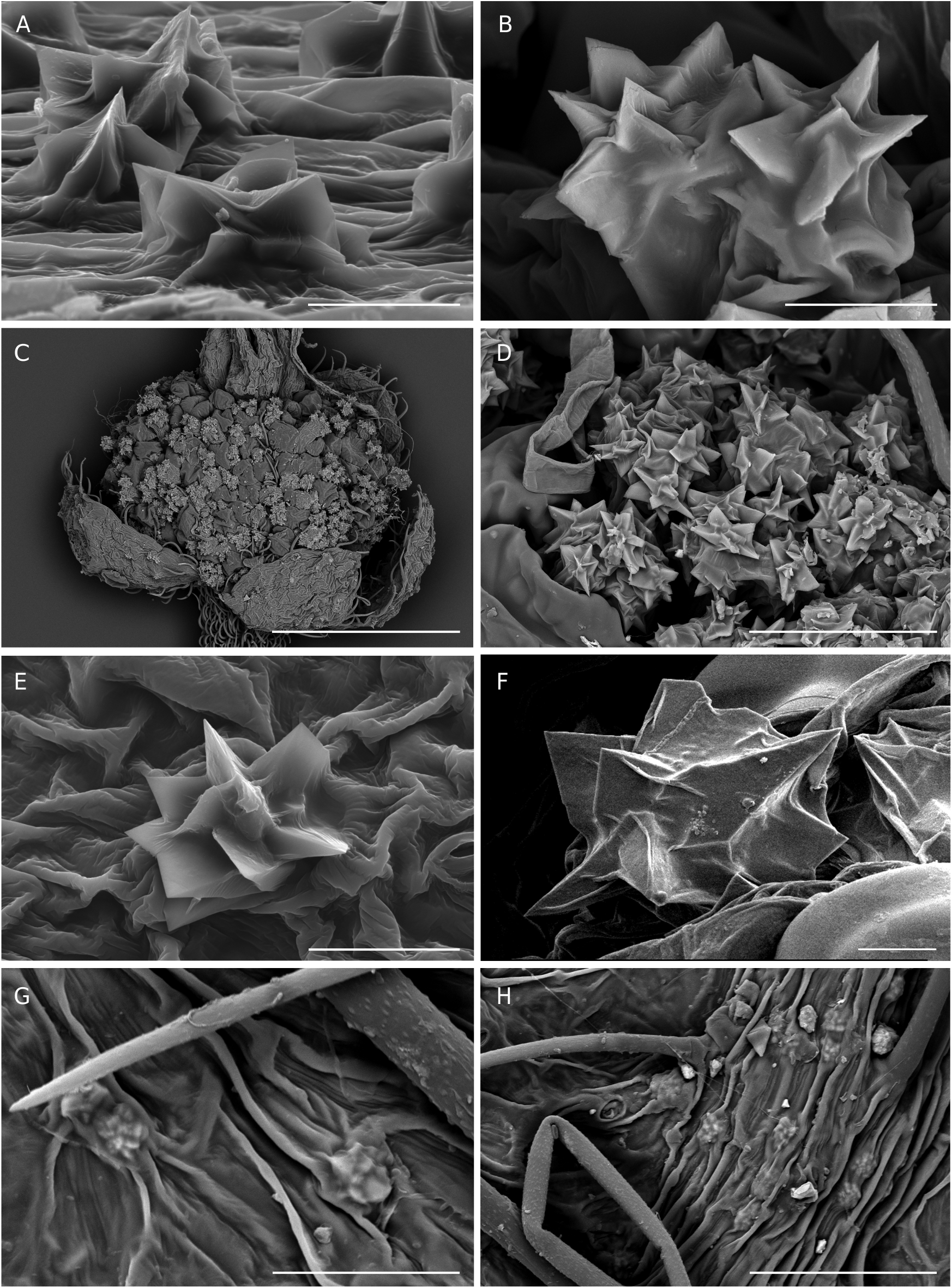
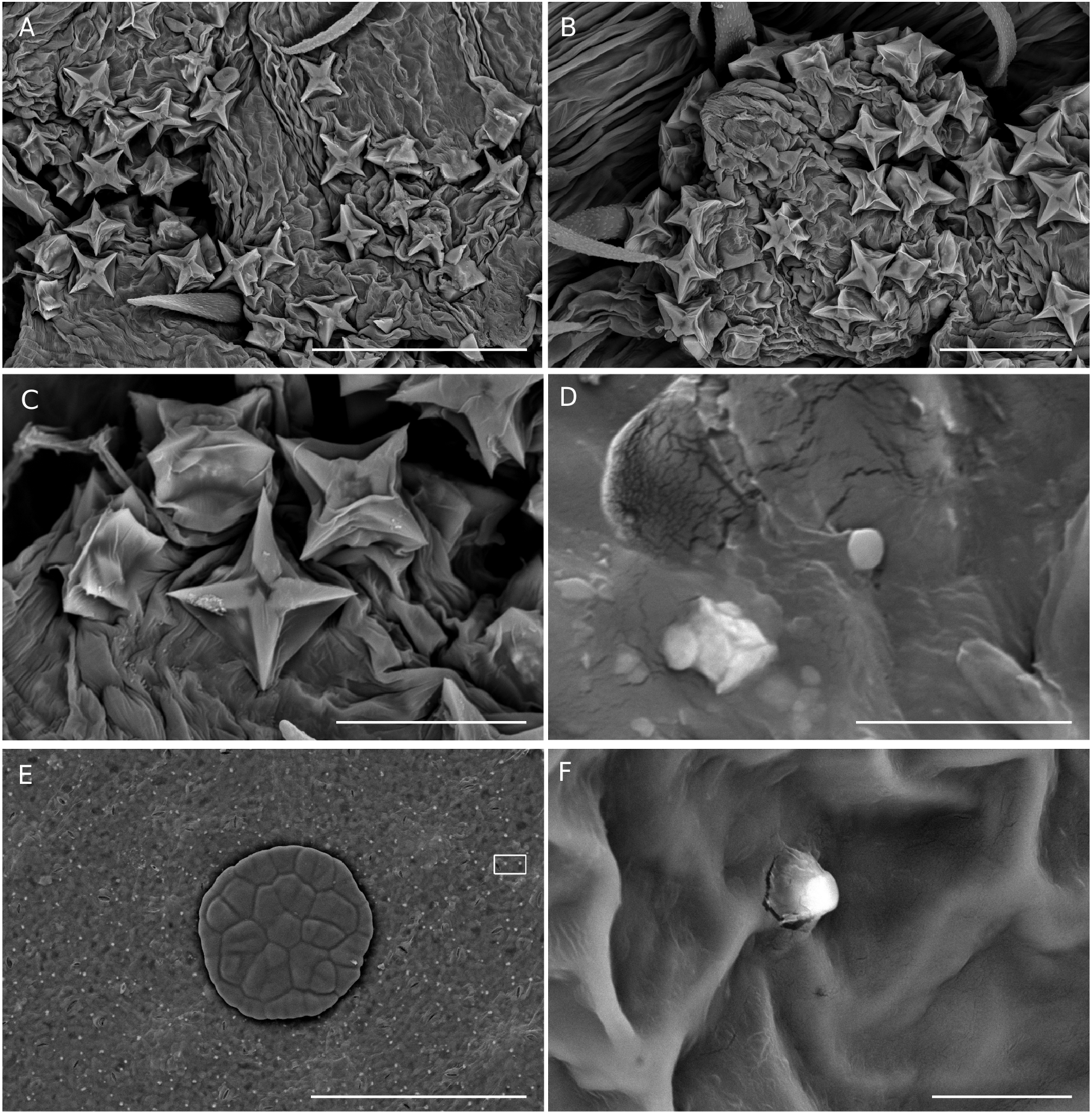
Epidermal crystals in Acalypha were recently studied by Cardiel et al. (2020), who identified them as a new taxonomic trait of the genus. In that study, four types of crystals were recognized: prismatic crystals, styloids, stellate crystals, and druses. In the WIOR species, we found three types (prismatic, stellate, and druses) in 30 of the 34 analyzed species ( Table 1); they appear mainly on the upper and lower leaf surfaces, and often also on the bracts and flowers. Prismatic crystals ( Fig. 9 View FIG ) can appear solitary or grouped, and we frequently observed two overlapping perpendicular crystals. These crystals appear on both the upper and lower leaf surfaces of most of the species studied, and on the female bracts of A. mayottensis . Druses can appear solitary or grouped ( Fig. 10 View FIG ). We have found them in nine species, on upper and lower leaf surfaces, bracts, and flowers. Stellate crystals ( Fig. 11 View FIG A-C) are very infrequent, appearing mainly in some South American species ( Cardiel et al. 2020). We have not found them in native species from WIOR, but we have observed them in the male flowers of A. fimbriata Schumach. & Thonn. , a species native to continental Africa and introduced in Madagascar. Due to insufficient sampling thus far, the systematic importance of crystals across Acalypha and among WIOR species requires further evaluation. In addition to the crystals described by Cardiel et al. (2020), we have recently identified another crystal form found on the leaf epidermis, which we have provisionally named “granules”. They have a rounded and smooth appearance and are c. 5 µm in diameter ( Fig. 11 View FIG D-F). We have found them only on the foliar surface of three native species, A. claoxyloides , A. emirnensis , and A. mayottensis ( Table 1). We still do not have conclusive results regarding their nature and composition.
Epicuticular waxes
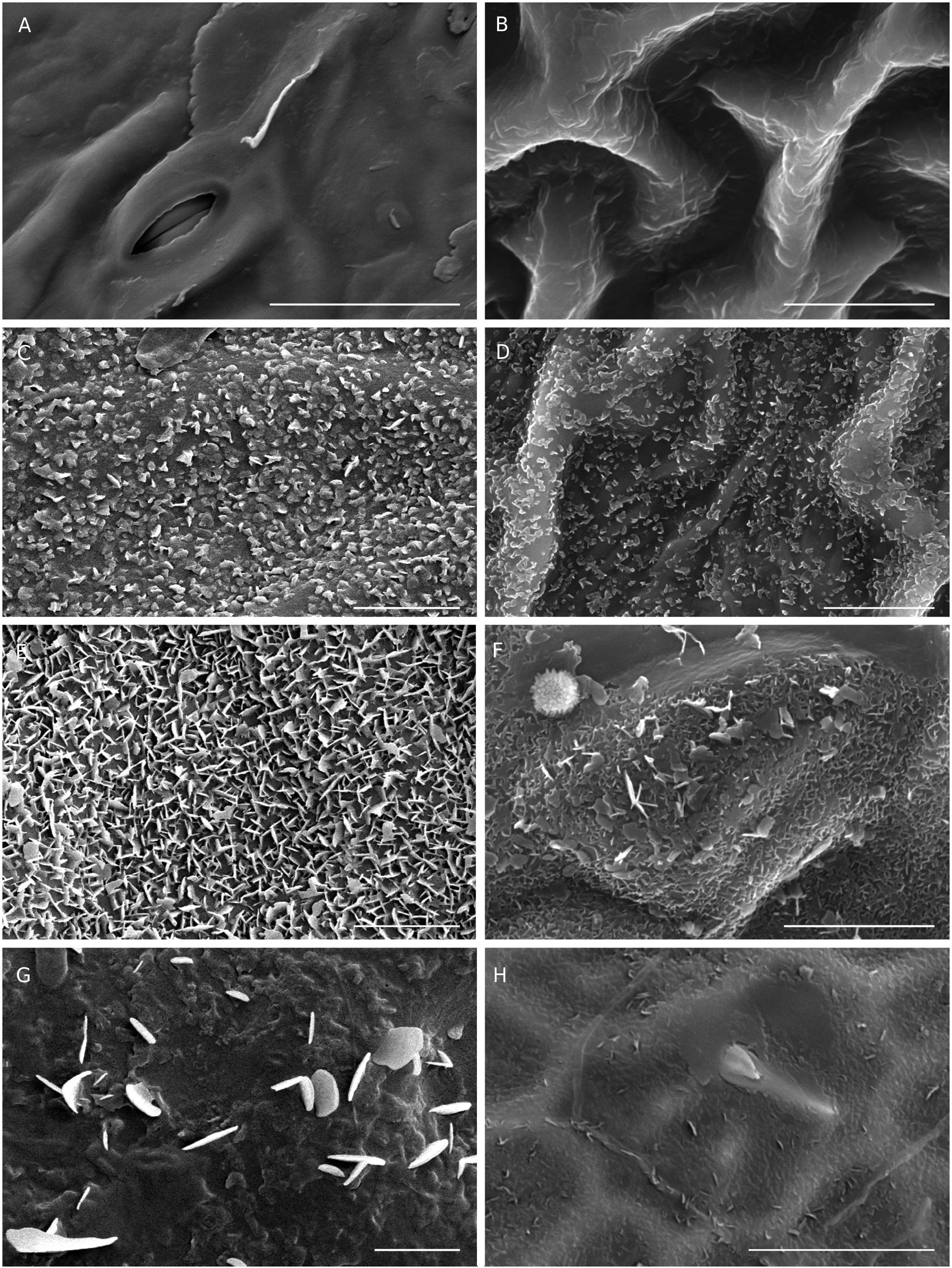
The leaf surface of most Acalypha species studied is covered by epicuticular waxes of different shapes and densities. Following the classification of Barthlott et al. (1998), we found two basic types of waxes: layers and crystalloids ( Table 2). Layers are continuous and homogeneous sheets that cover the entire surface; they vary in thicknesses and can appear folded or fractured ( Fig. 12A, B View FIG ). Crystalloids form a discontinuous cover, are grouped or solitary, have different shapes and sizes, and can appear together with other types of waxes ( Barthlott et al. 1998). In the WIOR species we have found three types of crystalloids: granules, platelets, and plates. Granules are crystalloids with irregular shapes, but usually isodiametric and rounded ( Fig. 12C, D View FIG ). Platelets and plates are flat, plate-shaped crystalloids; they are the most common crystalloids in plants ( Barthlott et al. 1998). Platelets have irregular margins ( Fig. 12E, F View FIG ), whereas plates are polygonal and have entire margins ( Fig. 12G, H View FIG ). We do not yet know what, if any, taxonomic significance epicuticular waxes have in Acalypha .
| U |
Nationaal Herbarium Nederland |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.