Homalopsis mereljcoxi, Murphy, John C., Voris, Harold K., Traub, Joshua & Cumberbatch, Christina, 2012
|
publication ID |
https://doi.org/10.5281/zenodo.209953 |
|
DOI |
https://doi.org/10.5281/zenodo.6169957 |
|
persistent identifier |
https://treatment.plazi.org/id/C2337B59-FFE9-FFFB-DC91-4753FAD1B276 |
|
treatment provided by |
Plazi |
|
scientific name |
Homalopsis mereljcoxi |
| status |
sp. nov. |
Homalopsis mereljcoxi sp. nov.
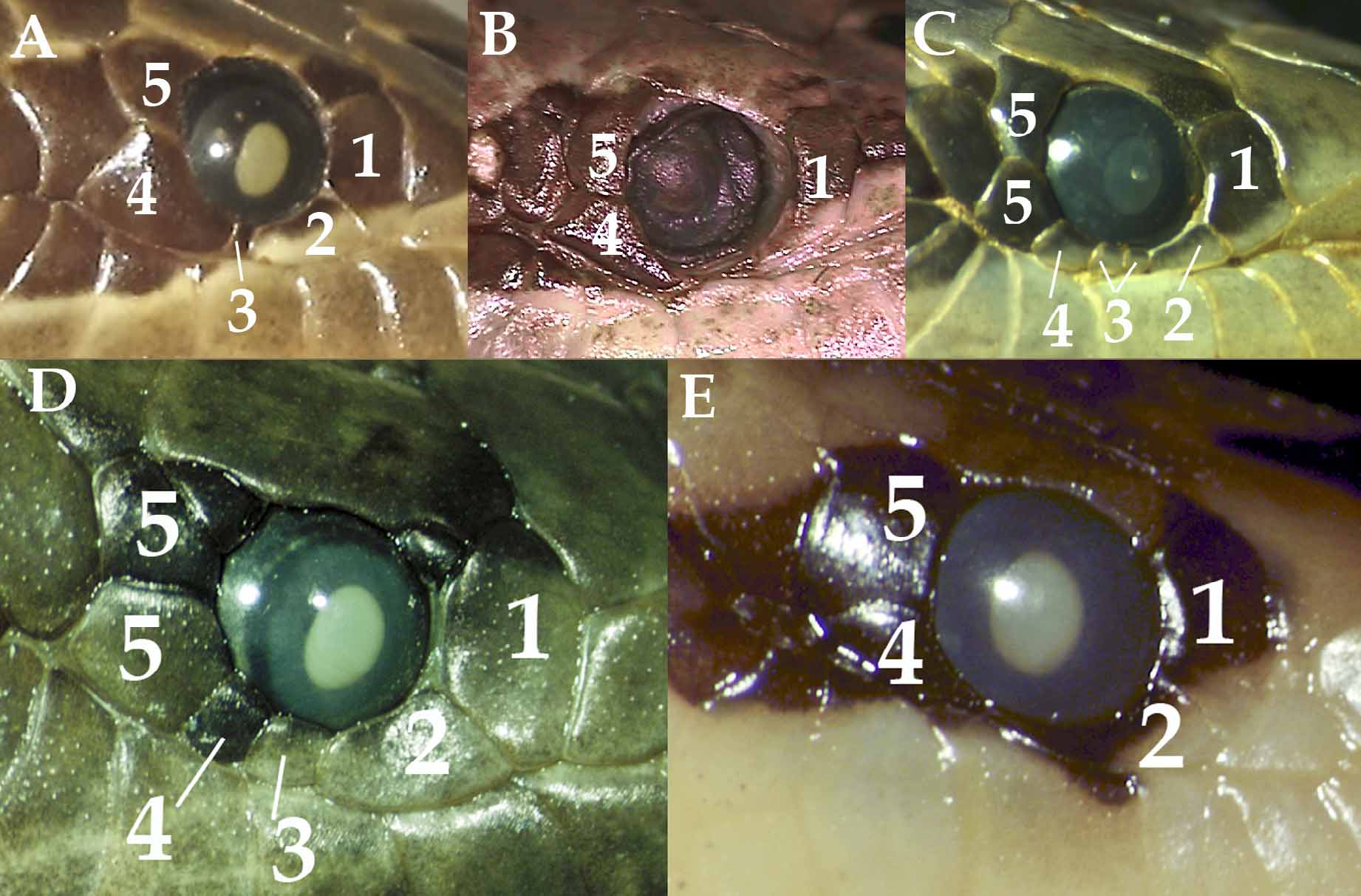
Figs. 1 View FIGURE 1 c, 7, 8b
Homalopsis buccata —Morice 1875: 58; Tirant 1885: 58; Boettger 1888: 139; Boulenger 1890: 374; Sclater 1891: 53; Boulenger 1896: 14; Wall 1903: 94; Volz 1904: 507; Wall 1905: 307; Mocquard 1907: 51; Smith 1914: 101; Gyldenstolpe 1916: 19; Rooji 1917: 186; Mell 1922: 123; Mertens 1929: 6; Bourret 1927: 241; Cochran 1929: 42, 1930: 31; Bourret 1934: 12, 1936: 293; Westermann 1942: 616; Taylor, 1965: 923; Campden-Main 1970: 82; St. Girons & Pfeffer 1971: 551; 1972: 69; Saint Girons 1972: 111; Cox 1991: 61; Nguyen & Ho 1996: 127; Cox et al. 1998: 42; Chan-ard et al. 1999: 169; Pauwels et al. 2000: 142; Stuart et al. 2000: 115; Chanhome et al. 2001: 54; Voris & Murphy 2002: 1625; Stuart 2004: 25; Zhou & Jiang 2005: 3525; Karns et al. 2005: 78; Brooks et al. 2007: 401; Murphy 2007: 193; Nguyen 2007: 149; Nguyen et al. 2007: 350; Brooks et al. 2008: 835 m, 2009: 7, 2010: 2127.
Comment. Photographs of this snake can be found in: Cox (1991: 223, pl 61), Cox et al. (1998: 42), Lim & Lee (1988: 70), Manthey & Grossmann (1997: 356), and Murphy (2007: 194). Field and laboratory studies and observations that relate directly to this species include the following: Brooks et al. (2007, 2008, 2009, 2010), Karns et al. (2005, 2010), Murphy et al. (1999), St. Girons (1972), and Saint Girons & Pfeffer (1971, 1972).
Holotype. A female, FMNH 263756 from Thailand, Nakhon Ratchasima Province, Wang Nam Khieo, Udon Sap (subdistrict), Ban Badan Reservoir ( 14°31’04” N, 101°58’25” E); collected 16 June 2004 by Daryl R. Karns and John C. Murphy.
Referred specimens. CAMBODIA: Kampong Chhnang Prov – FMNH 259306, 259293; Siem Reap Province – FMNH 257267, 259295, 257269–274, 259297–300, 259302-304; THAILAND: Bangkok FMNH 252515-19; UMMZ 65369(4), 65370(2), UMMZ 65371, 65372(2), 96276; Khonkaen Province FMNH 262474-481; Nakhon Ratchasima Province – FMNH 178378, 180108; FMNH 263756, 263763-64, 263770; Phattalung Prov – FMNH 252520–522; Songkhla Province – FMNH 252514, 252525, 252673–674, 252676–678, 252693, 252698, 252705, 257429–430; No specific locality – ANSP 5116–17, CAS 853021; FMNH 579–580, 252514; VIETNAM: Kien Glang FMNH 259088–092; ROM 37941–942. No data – FMNH 11129, 11551.
Etymology. This new species is named in in honor of Merel “Jack” Cox, for his years of dedication to the study of the snakes of Thailand.
Diagnosis. Homalopsis mereljcoxi has a single loreal contacting upper labials 1–4; 40–47 scale rows at midbody, reduced to 30 or more posteriorly; two postocular scales plus a postsubocular; 13 (12–14) upper labials; and ventral counts that are usually greater than 165. Homalopsis buccata has 33–40 dorsal scale rows at midbody, reduced to less than 30 posteriorly; one postocular scale plus a postsubocular scale; and a ventral count that is less than 166. Homalopsis hardwickii has a divided loreal contacting upper labials 1–4; 39 scale rows at midbody reduced to 28 posteriorly; one postocular scale and no presubocular scale; 159 ventrals. Homalopsis nigroventralis has upper labials 1–3 contacting the loreal; 35–39 dorsal scale rows at midbody, reduced to less than 30 posteriorly; 10–12 upper labials; 159–167 ventrals; and a reverse color pattern on the venter (dark olive-gray with white spots). Homalopsis semizonata has a divided or fragmented loreal contacting upper labials 1–4 or 1–5; three prefrontals; one postocular and one postsubocular.
Description of the Holotype. A female, total length 457 mm, tail 112 mm. Rostral broader than tall, slightly visible from above, protrudes over mental scale; nasals very large, semi-divided; internasal divided; prefrontals large, make broad contact with loreal; frontal slightly fragmented, equal in length to parietals; large occipital present on right side only. Supraoculars quadrangular, wider than the frontal; preoculars 1/1; suboculars 3/4; postoculars 2/2; temporal formula 1+2/2+2; upper labials 12/12, 1–4 contact loreal; first horizontally divided upper labial number 7 ( Fig. 8 View FIGURE 8 ). Lower labials 17/19, first 3 contact first pair of chin shields, first divided labial is 10. Dorsal scales keeled, striated, and in 42–44–33 rows; 170 ventrals; 94 divided subcaudals, a few fused. Dorsal pattern of 24 pale colored bands bordered in black, about 1.5 to 2 scale rows wide, separated by dark brown blotches 4 to 5 scale rows wide; the bands go completely across the vertebral line at midbody first three dorsal rows lack the dorsal pattern, and are uniform yellow. Ventral surface of body uniform yellow-cream with dark lateral spot on outer edge of some ventrals; ventral surface of tail mottled with black and cream.
Variation in referred material. The following description is based upon a total of 68 specimens. The rostral is slightly visible from above; the nasals are in contact and divided or semi–divided; the internasal tends to be single in the Thailand and Cambodia populations and divided in the Vietnam populations, and it does not contact the loreal in any population; 2 prefrontals; loreal is elongated and single (with exception) and usually in contact with upper labials 1–4, occasionally 2–4; upper labials number 12–14, 6 usually under orbit, 7 usually the first divided upper labial; frontal longer, or equal to, parietals; temporal formula 1+2. Lower labials 14–19; 3 or 4 pairs chin shields; lower labials 1–3 or 1–4 contact anterior chin shields. Dorsal scales on anterior body 41–45; midbody 40– 49; post body 28–35 (usually 30–35). Ventrals number 160–176 (males 161–176, n=36, x =171.61, SD=3.36; females 160–178, n=36, x =167.97, SD=3.57). Subcaudals number 82–108 (males 82–108, n=33, x =95.79, SD=7.73; females 73–99, n=34, x =86.64, SD=5.49). Males sometimes have spiny keels on the first row of scales near the vent.
Color and pattern. Dark dorsal blotches and an eye stripe; a dorsal pattern of alternating wide dark botches separated by narrow, pale cream blotches, and a ventral surface that is uniform cream with paired spots on the edges of the ventrals make this snake readily recognizable. In alcohol these snakes hold their color remarkably well, and seem to only fade if they have been exposed to light for prolonged periods. The head, the nasals and internasal often have a dark blotch; a v-shaped blotch occurs on the frontal, across the parietals onto the smaller scales behind them. A black or brown mask-like band occurs from the preocular through the eye and extends to the corner of the mouth or the side of the neck where it may fuse with the first dark, dorsal blotch. On the body 20–28 brown transverse blotches 4–6 scale rows wide are separated by paler bands 1–2 scales wide; often these are outlined with black or dark brown pigment. Both dark and pale blotches are absent from the first 3–6 scale rows at midbody. Venter uniform white, cream, or yellow with paired brown or black spots on the outer edge of some ventrals. The underside of the tail is usually mottled. Juvenile patterns consist of strongly contrasting yellow or white bands with dark brown blotches, these lose contrast and pale crossbands become outlined with black with age, some old individuals faded with a more uniform brown-gray pattern. Some populations tend to be darker in color and melanistic.
Size and sexual dimorphism. Fifteen snakes in our sample with SVLs below 400 mm were considered subadults and were not included in the size comparisons. Adult male SVLs ranged from 404 to 914 mm (n=31, x = 654.81, SE=20.61) compared to adult female SVLs that ranged from 400 to 973 mm (n=26, x =626.58, SE=28.43). These differences in SVLs among males and females did not prove significant (F(1,55) = 0.67, P>0.05). However, male tail lengths (n = 29, x = 223.72, SE = 7.02) proved to be significantly longer (F(1,52) = 20.24, P <0.001) than female tail lengths (n = 25, x = 181.24, SE = 6.09). The SDI for this species is -1.045.
Homalopsis mereljcoxi displays strong sexual dimorphism in both the number of ventral scales and subcaudal scales. Males have both significantly more ventrals (males, n = 36, x = 171.61, SE = 0.56; females, n = 36, x = 167.97, SE = 0.60; F(1,70) = 19.83, P <0.001) and more subcaudals (males, n = 33, x = 95.79, SE = 1.35; females, n = 34, x = 86.65, SE = 0.94; F(1,65) = 31.32, P <0.001).
Distribution. Homalopsis mereljcoxi is known from lowland localities in Thailand, Cambodia, and Vietnam. On the western edge of its known range it is found as far north as Bung Cho, Uttaradit Province, Phichai District (~12°N, 104°E), Thailand and as far south as Lake Songkhla (~7°N, 100°E), Thailand. In Vietnam it is found at least as far east as Can Tho Province (~10°N, 105°E) and as far south as Vinh Thuan District Town in Kien Giang Province (~9°N, 105°E). It is present in Cambodia’s Tonle Sap, and it most likely occurs throughout the lower elevations of the Chao Phraya and Mekong drainages. It is possible, but unclear, if this species inhabits Myanmar. Gyi (1970) reports Myanmar Homalopsis with 39–43 middorsal scale rows, Homalopsis semizonata Blyth, 1855 inhabits the area on both side of the Gulf of Martaban and the Ayeyarwady delta and it has 38-43 scale rows which overlap the range of scale rows for H. mereljcoxi .
Natural history. Many authors have discussed the natural history of this species (Saint Girons 1972; Brooks et al. 2007, 2010; Karns et al. 2005, 2010) under the name Homalopsis buccata . The morphology of this species agrees well with the Cambodia specimens described by Saint Girons (1972), however his data included ventral counts of 154–180, a range with some specimens below the lower end of the range observed in our sample. Cambodian specimens with ventral counts lower than 160 (unpublished data) likely belong to one or more cryptic species.
In Thailand, the habitat used by Homalopsis mereljcoxi includes small reservoirs, ditches, ponds, streams, and shallow wetlands ( Karns et al. 2005; 2010). Snakes were primarily obtained from fishers’ gill nets, one specimen was hand collected from a pond at night while it floated near the surface in an ambush posture. Saint Girons (1972) described similar habitats in Cambodia: streams, rivers, irrigation canals, marshes, reservoirs and the banks of lakes and rivers; he also reports it in shallow water at night and describes it resting in burrows or crevices in the bank during the day, noting that specimens move very little while on land. Individuals released into shallow water escaped without hesitation, but placed in a river with steep banks, the snakes immediately sought refuge along the shore.
Karns et al. (2005, 2010) found Homalopsis mereljcoxi made up 3.59% of 668 aquatic snakes collected at seven locations in eastern Thailand; and 4.7% of 786 aquatic snakes from four locations in Thailand’s Central Plain. In a population depletion experiment at Tonle Sap, Brooks et al. (2007) found one specimen per 1718 m 2.
Saint Girons (1972) and Voris & Murphy (2002) reported finding only fish in the gut of Homalopsis mereljcoxi . Karns et al. (2010) found 61.5% of males, and 37.5% of females contained food. One large female H. mereljcoxi ( 86.5 cm SVL, 477.4 g) contained an 82.6 g Tilapia sp. (17.3% of predator’s body weight), but other prey items were relatively much smaller. One male of 8 (12.5%) H. buccata contained two fish (families Cyprinidae and Cichlidae ). Brooks et al. (2009) examined more than 700 specimens from Tonle Sap, Cambodia and found fish from five families ( Anabantidae 57%, Channidae 7.5%, Cyprinidae 2.5%, Mastacembilidae 3%, Osphronemidae 31%). Fish consumed are usually less than 10% of the predator’s mass ( Brooks et al. 2009).
Reproduction of this species in Cambodia was commented upon by Saint Girons (1972), he reported a sexual cycle with spermatogenesis in July, spermatozoa present in August with simultaneous development of the sexual segment of kidney. In February, sperm and the sexual segment were in decline. Females start vitellogenesis in November and mating occurs in December or early January, ovulation takes place in February. Saint Girons found 6 females with 13 to 33 large ovarian follicles or recently ovulated eggs in February. Gestation lasts until May.
Karns et al. (2005) reported a 817 mm SVL, 650 g female with 17 near full term embryos (mean SVL 23.8 mm ±0,14, range = 23.2–25.0; mean mass = 11.3 g ±0.15, range = 10.0–12.3); total embryo mass without yolks was 191.5 g, a relative litter mass (RLM) of 29.0%.
Material examined. CAMBODIA: Kampong Chhnang Prov FMNH 259306, 259293; (floating Vietnamese fishing community, Tonle Sap River, Cambodia, near 12°16'08" N, 104°40'50" E), Siem Reap Province FMNH 257267, 259295, 257269–274 (Siem Reap town, Psa Kroam market, 13°20'46" N, 103°50'53" E), 259297–304 (Siem Reap town, Chong Khneas Port, Tonle Sap Lake). THAILAND: Bangkok UMMZ 65369(4), 65370(2), UMMZ 65371, 65372(2), 96276; FMNH 178378, Nakhon Ratchasima Province 180108 (Amphoe Pak Thong Chai, Sakaerat Exp Sta); Phattalung Prov FMNH 252520–522 (Thale Noi, 7°47'00" N, 100°7'30" E), Songkhla Province FMNH 252673–674, 252676–678, 252693, 252698, 252705, 257429–430 (Ban Tha Hin, 7°23'00" N, 100°25'00" E); No specific locality ANSP 5117, FMNH 579–580, 252514 (Bangkok, Market). VIETNAM: Kien Glang FMNH 259088 (Vinh Thuan District Town, 09°30'48" N, 105°15'32" E) 259089 (An Minh District Town, 09°38'52" N, 105°08'35" E), 259090-94 (U Minh Thuong Nature Reserve, 09°34'38" N, 105°04'00" E, < 10m); Vi Thanh ROM 37941–942 (Phung Hiep vicinity, purchased in local market). No data FMNH 11129, 11551.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Homalopsis mereljcoxi
| Murphy, John C., Voris, Harold K., Traub, Joshua & Cumberbatch, Christina 2012 |
Homalopsis buccata
| Brooks 2008: 835 |
| Brooks 2007: 401 |
| Murphy 2007: 193 |
| Nguyen 2007: 149 |
| Nguyen 2007: 350 |
| Karns 2005: 78 |
| Stuart 2004: 25 |
| Voris 2002: 1625 |
| Chanhome 2001: 54 |
| Pauwels 2000: 142 |
| Stuart 2000: 115 |
| Cox 1998: 42 |
| Nguyen 1996: 127 |
| Cox 1991: 61 |
| Girons 1971: 551 |
| Campden-Main 1970: 82 |
| Taylor 1965: 923 |
| Westermann 1942: 616 |
| Bourret 1934: 12 |
| Mertens 1929: 6 |
| Cochran 1929: 42 |
| Bourret 1927: 241 |
| Mell 1922: 123 |
| Gyldenstolpe 1916: 19 |
| Smith 1914: 101 |
| Mocquard 1907: 51 |
| Wall 1905: 307 |
| Volz 1904: 507 |
| Wall 1903: 94 |
| Boulenger 1896: 14 |
| Sclater 1891: 53 |
| Boulenger 1890: 374 |
| Tirant 1885: 58 |