Jacforus, Ng & Clark, 2003
|
publication ID |
https://doi.org/10.5281/zenodo.5393704 |
|
persistent identifier |
https://treatment.plazi.org/id/C52B5713-CB01-1610-FCFE-0FCB80A7381A |
|
treatment provided by |
Marcus |
|
scientific name |
Jacforus |
| status |
gen. nov. |
Genus Jacforus View in CoL n. gen.
TYPE SPECIES. — Cycloxanthops cavatus Rathbun, 1907 , by present designation.
ETYMOLOGY. — The genus name is named for Professor Jacques Forest. Gender masculine.
DIAGNOSIS. — Carapace quadrate, regions well defined, with deep grooves separating most regions; entire dorsal surface covered with very small granules. Front weakly produced, gently deflexed, appearing almost entire from dorsal view, inner supraorbital tooth weak, rounded; orbits sloping posteriorly. External orbital tooth low, with distinct granulated crest joining rest of anterolateral margin; anterolateral margin strongly arcuate, with four low but prominent lobes, first smallest. Posterolateral margin distincly shorter than anterolateral margin, deeply concave. Third maxilliped and anteromedian margin deeply concave and the anteroexternal angle prominently auriculiform. Chelipeds short, stout; outer surfaces covered with very small granules, deeply excavated with numerous uneven depressions, appears eroded; fingers shorter than palm, tips sharp; larger (usually right) chela with large gently curved basal cutting tooth on cutting edge of dactylus. Ambulatory legs short, dorsal margins of merus, carpus and propodus cristate. Lateral margins of fused male abdominal segments 3-5 entire, continuous; telson semicircular, lateral margins gently convex, tip rounded. G1 distal part with only short, stout spines and short simple setae, without long setae or processes.
REMARKS
The taxonomy of the type species of Jacforus n. gen., Cycloxanthops cavatus , has an interesting history, and clearly reflects the uncertainties regarding its generic placement. It was originally assigned to Cycloxanthops Rathbun, 1897 . This species was subsequently described twice from the Pacific, under separate genera and species by Edmondson: Euxanthus minutus Edmondson, 1925 , and Megametope sulcatus Edmondson, 1931 .
Guinot (1962b: 8, 9) noted that C. cavatus was closer to species in Medaeus Dana, 1851 , particularly M. noelensis Ward, 1934 , with regards to the form of the carapace, anterolateral margin, and eroded surfaces of the chelipeds. Further, Edmondson (1962), in discussing C. cavatus , citing a letter by Jacques Forest, commented that the genus Cycloxanthops should perhaps be split. This genus was formally split by Guinot (1968) into Cycloxanthops Rathbun, 1897 s.s., and a new genus, Neoxanthops . However, she did not place C. cavatus in either of these taxa. Takeda & Miyake (1968) also commented on the similarity between C. cavatus and M. noelensis , however, they noted that there were basic differences in the form of the anterolateral margin and structure of the G1 between Cycloxanthops [ s.l.] and Paramedaeus where M. noelensis had been classified. Serène (1968) provisionally referred C. cavatus to Neoxanthops , a decision accepted by Sakai (1976). Serène (1984: 211), however, later indicated his uncertainty concerning the taxonomic position of this species in commenting that N. cavatus was problematical at the genus level and consequently it was provisionally assigned near to Neoxanthops . Serène (1984: 212) further highlighted this issue by identifying the species as “Aff. Neoxanthops cavatus ( Rathbun, 1907) ”.
Ng (1993: 706), in describing a new genus, Cranaothus , from New Caledonia, briefly observed that Neoxanthops cavatus is not a typi- cal member of Neoxanthops and should probably be transferred to a new genus. It differs markedly from the type species of the genus, N. lineatus A. Milne Edwards, 1867 , in many aspects.
The placement of C. cavatus in Neoxanthops is clearly unsatisfactory. Direct comparisons of C. cavatus with N. lineatus show the following differences which are significant and these include: the anterolateral margin gradually becomes lower and gently curves below the orbits, ending just below the suborbital region, and does not meet the external orbital angle or supraorbital margin; the frontal margin is not distinctly produced beyond the internal angle of the supraorbital margin; the anterolateral margin is not distinctly cristiform; the surface is more domed, distinctly sculptured and appears eroded, and not gently convex and completely smooth; and the fingers of the chelipeds are very short and not pigmented black.
The similarity between Jacforus cavatus n. comb. and Medaeus noelensis has already been noted by Guinot (1962b), Takeda & Miyake (1968) and Serène (1984), but the affinities of the two species are actually superficial. These species resemble each other in having an eroded carapace (although more so in J. cavatus n. comb.), a short, non-produced front, and the anterolateral margin gradually curving to the suborbital region and not joining the external orbital angle and supraorbital margin. There are, however, several marked differences between J. cavatus n. comb. and M. noelensis which suggest that they are not congeneric, viz. the carapace proportions are quite different, with J. cavatus n. comb. being a more squarish animal; the carapace is more convex with higher ridges and deeper grooves in J. cavatus n. comb.; the anterolateral margin of J. cavatus n. comb. is much longer than the posterolateral margin (margins subequal in length in M. noelensis ); the posterolateral margin of J. cavatus n. comb. is strongly concave (straight or gently convex in M. noelensis ); the cheliped fingers are proportionately shorter in J. cavatus n. comb.; thoracic sternites 1-3 are completely fused without trace of any sutures (suture between sternites 2 and 3 are still distinct in M. noelensis ; and the G1 is much stouter in J. cavatus n. comb. (see Serène 1984: fig. 128), the subdistal part without any trace of the distinctive long plumose setae characteristic of M. noelensis (see Serène 1984: fig. 51).
Although some of the above listed characters are probably important only at the specific level, features like the proportions of the antero- and posterolateral margins, structure of the anterior sternal plastron and form of the G1 strongly suggest that two distinct genera should be recognised (see also discussion for Danielea n. gen., where M. noelensis is now assigned).
Like Danielea View in CoL n. gen., Jacforus View in CoL n. gen. has the special cutting (peeling) tooth present on the right chela and probably functions in the same manner as calappids ( Ng & Tan 1984, 1985).
Jacforus View in CoL n. gen. can be separated from Marratha View in CoL n. gen. by the shapes of their carapaces as well as the form and armature of the antero- and posterolateral margins, the proportionally narrower anterior thoracic sternum, the relatively shorter and stouter third maxilliped with the anteromedian margin of the merus deeply concave and the anteroexternal angle prominently auriculiform (vs squarish merus), and the G1 lacking long plumose setae.
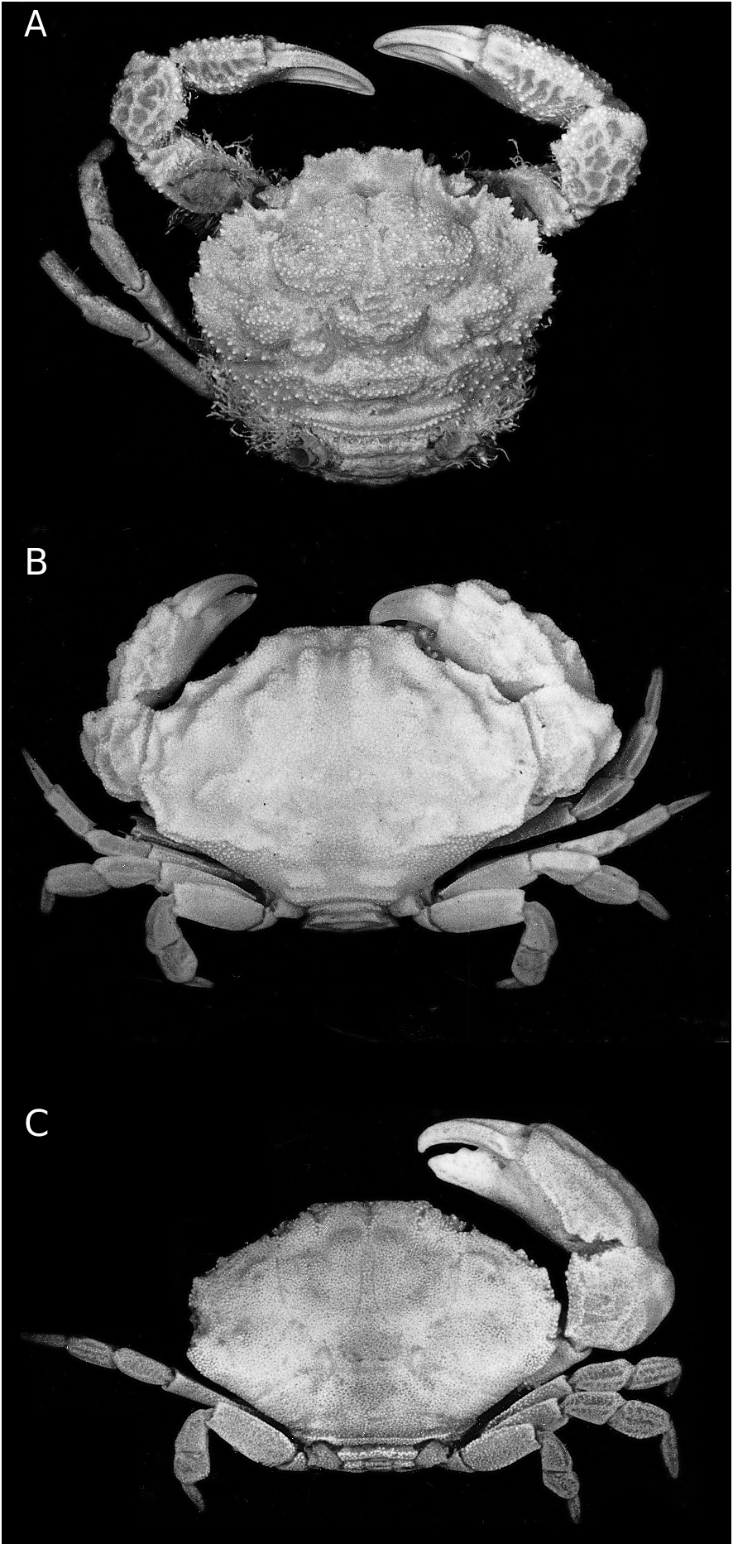
Jacforus cavatus ( Rathbun, 1907) View in CoL n. comb. ( Figs 1B View FIG ; 3 View FIG )
Cycloxanthops cavatus Rathbun, 1907: 41 View in CoL , pl. 5 fig. 8, pl. 6 fig. 3-3a. — Balss 1938: 43. — Guinot- Dumortier 1960: 155 (footnote). — Edmondson 1962: 233, fig. 5c. — Takeda & Miyake 1968: 2, fig. 1, pl. 1 fig. B. — Ng 1993: 706.
Euxanthus minutus Edmondson, 1925: 46 View in CoL , fig. 8a-d, pl. 3B.
Megametope sulcatus Edmondson, 1931: 11 View in CoL , fig. 3e-f, pl. 4A.
Cycloxanthops cavata – Ward 1932: 244.
Cycloxanthops View in CoL (?) cavatus View in CoL – Guinot 1962b: 8, figs 9, 10.
Neoxanthops View in CoL ? cavatus View in CoL – Serène 1968: 78.
Neoxanthops cavatus View in CoL – Sakai 1976: 437, fig. 230b.
Aff. Neoxanthops cavatus View in CoL – Serène 1984: 212, fig. 128, pl. 29F.
MATERIAL EXAMINED. — Australia. NW Island, Capricorn Group, Queensland, coll. and det. M. Ward, 1 cl 5.7 mm, cw 8.3 mm ( NHM 1931.4.14.17).
Kenya. Mombassa, 4°14.1’S, 38°36.5’E, intertidal, 28.II.1971, coll. A. J. Bruce, 1 ( MNHN MP-B 7942).
Hawaii. Makena, Maui, among dead coral near shore, 1926, coll. J. K. Skinner, 1 cl 5.2 mm, cw 7.1 mm ( BPBM-S 2588) ( holotype of Megametope sulcatus Edmondson, 1931 ). — Oahu, 7.VII.1952, 1
cl 2.8 mm, cw 3.4 mm ( BPBM-S 5824). — Kawela Bay, Oahu, 25-28. VI.1934, 1 cl 4.3 mm, cw 6.1 mm. — Napoopoo, I.1987, 1 cl 4.4, cw 6.2 mm ( BPBM-S 510412); 1 cl 4.5 mm, cl 6.6 mm ( BPBM-S 5140411). — Kahala, Oahu, II.1930, 1 badly damaged specimen ( BPBM-S 3590). — Kawela Bay, Oahu, 15-17.VII.1935, coll. C. H. Edmondson, 1 cl 4.2 mm, cw 6.0 mm ( ZRC ex BPBM-S 4053); 10.VII.1937, 1 cl 5.0 mm, cw 7.0 mm ( BPBM-S 4346). — Kahala, Oahu, 1931, coll. C. H. Edmondson, 1 (damaged) cl 4.0 mm, cw 6.4 mm ( BPBM-S 3434).
Christmas Island. South of Hawaii, Whipp Poor Will Expedition, 1924, 2 cl 2.5 and 3.1 mm, cw 3.9 and 4.7 mm respectively; 1 cl 3.9 mm, cw 6.5 mm ( BPBM-S 2316).
Wake Island. Tanager Expedition, 1923, 1
cl 3.5 mm, cw 4.5 mm ( BPBM-S 1808 ) ( holotype of Euxanthus minutus Edmondson, 1925 ) ; 2 cl 1.3 and 2.0 mm, cw 2.5 and 3.3 mm respectively (BPBM- S 1809).
DISTRIBUTION. — Mombasa, Kenya ( Serène 1984); NW Island, Capricorn Group, Queensland, Australia ( Ward 1932); Ishigaki-jima, Okinawa-jima, Japan ( Takeda & Miyake 1968); Ryukyus, Japan ( Sakai 1976); Fakarava Island, Paumotus ( Rathbun 1907); Aranuka and Apamama, Gilbert Island ( Balss 1938); North West and Gilbert islands ( Guinot 1962b), Wake, Hawaii ( Edmondson 1925); Maui, Hawaii ( Edmondson 1931); Christmas, Washington, Wake, Maui and Oahu Islands, Hawaii ( Edmondson 1962).
DESCRIPTION
Carapace quadrate, wider than broad, appears rounded due to strongly arcuate margins; entire dorsal surface covered with very small granules; regions well defined, with deep groove separating most regions; 1F, 2F, 1M recognisable, sometimes appearing almost confluent with 2M; 2M prominent, longitudinal median groove prominent to shallow; lateral regions (1-4L) relatively prominent; 2P narrow but distinct. Front weakly produced, gently deflexed, appearing almost entire from dorsal view; from frontal view, front clearly separated into two broad, truncate lobes, separated by shallow V-shaped cleft; inner supraorbital tooth low, rounded, distinctly posterior to front; orbits distinctly sloping posteriorly; separated from external orbital tooth by shallow groove. External orbital tooth low, rounded, joined to rest of anterolateral margin by distinct granulated crest. Anterolateral margin strongly arcuate, with four low but prominent lobes; first lobe smallest; second and third lobes broadest, from dorsal view, second lobe joins first as an incomplete crest which ends above the first lobe, forming prominent, deep depression between them; fourth lobe directed obliquely posteriorly. Posterolateral margin distinctly shorter than anterolateral margin, deeply concave. Posterior carapace margin very short, shorter than frontal margin, distinctly granulated. Subhepatic region with prominent excavation, but not interrupting coalition of anterolateral margin with external orbital tooth. Suborbital and sub-branchial regions covered with prominent granules. Infraorbital margin granulated, short, entire. Eyes short, eyestalk granulated, with two or three relatively larger granules at dorsal junction of cornea, appears beaded. Basal antennal segment stout, rectangular, closing orbital hiatus, with relatively short flagellum entering orbit. Antennules folding laterally; fossa relatively large. Endostomial ridges not discernible. Third maxilliped relatively short, surface finely granular; merus auriculiform at anteroexternal angle, anteromedi- an margin deeply concave, inner margin serrulate; ischium with very shallow, almost undiscernible oblique median sulcus; exopod relatively broad, not reaching anterior edge of merus, with well-developed flagellum.
Chelipeds short, stout; all outer surfaces densely covered with very small granules, deeply excavat- ed with numerous uneven depressions, appears eroded to varying degrees. Merus short, with short submedian tooth on dorsal margin. Carpus rounded, inner angle with one prominent round- ed tooth and one lobe anterior to it; outer angle with one low tooth and one lobe posterior to it. Chela very short, stout; inner surface finely granular; fingers distinctly shorter than palm, tips sharp; palm with prominent uneven dorsal crest, with weaker crest on inner subdorsal surface; outer surface with three low crests, median one most prominent, subdorsal crest most uneven, may appear weakly dentiform, most ventral crest weakest, gradually extending to base of pollex which has additional short weak crest. Major (usually right) chela with two or three teeth and pronounced molariform basal cutting tooth on cutting edge of dactylus; cutting edge of pollex with two or three teeth; dorsal margin of dactylus bicarinate. Minor (usually left) chela with fingers more slender, cutting edges blade-like, with three or four denticles each. Ambulatory legs short, surfaces finely granular, almost glabrous, unarmed; dorsal margins of merus, carpus and propodus cristate, entire, with weak submedian longitudinal carina.
Thoracic sternum relatively narrow, entire surface covered with small granules; suture between sternites 1 and 2 poorly demarcated, suture between sternites 3 and 4 more pronounced laterally, medium part somewhat shallower, sutures between sternites 4 and 5, 5 and 6, and 6 and 7 incomplete; abdomen reaching to imaginary line joining posterior bases of chelipeds. Gonopore coxal, opening below abdominal segment 3.
Abdomen with segments 3-5 completely fused, sutures separating segments not discernible, lateral margins entire, without clefts; segments 1-3 trapezoidal, segment 6 squarish, lateral margins almost straight and parallel or gently sinose telson semicircular, lateral margins convex, tip rounded; surfaces of all segments finely granular.
G1 relatively short, stout, proximal part relatively stouter, distal part gently bent outwards, gently tapering to rounded tip; lateral margins lined with short spines or simple setae, without trace of long plumose setae, long spines or other processes. G2 short, slender, distal part with subpetaloid process.
REMARKS
Cycloxanthops cavatus Rathbun, 1907 was described from one male specimen (cl 4.7 mm, cw 6.6 mm, USNM 32848) from “Fakarava Island, Paumotus” (= Tuamotu, Polynesia). Euxanthus minutus Edmondson, 1925 was described on the basis of only one female holotype specimen from Wake Island in the Pacific. There are two other female specimens in the BPBM (No. 1809) apparently collected at the same time as the holotype but they were not mentioned, and as such, are not paratypes. Megametope sulcatus Edmondson, 1931 was described from a single female from near Maui, Hawaii. Edmondson (1931: 11) gave the measurements of the specimen as cl 6.0 mm and cw 8.0 mm but our measurements are 5.2 and 7.1 mm respectively. We have no doubt this is the holotype as it agrees with his figures. All three names are without doubt synonyms. While we have not examined the type of Cycloxanthops cavatus , her description and excellent figures leave no doubt about its identity. In comparing the types of Euxanthus minutus , and Megametope sulcatus (all females), we find no major differences between them, other than those of E. minutus are much larger than those of M. sulcatus , and the grooves on the chelae somewhat deeper.
There is some variation in the strength of the granules on the carapace, which in some specimens appear to be somewhat larger. In a few specimens, the carapace may be encrusted with calcareous deposits which makes the carapace appear rougher and more eroded. In larger specimens, the regions on the carapace appear more prominent, with the grooves delineating them somewhat deeper; and the longitudinal grooves on the chelae are also slightly deeper. These differences, however, are not significant. The strength of the anterolateral teeth does not vary much with size.
Takeda & Miyake (1968) first figured the G1 of this interesting species on the basis of a male from southern Japan. The G1s of the present male specimens from Hawaii agree with their figures in all major aspects.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Jacforus
| Ng, Peter K. L. & Clark, Paul F. 2003 |
Neoxanthops cavatus
| SAKAI T. 1976: 437 |
Neoxanthops
| SERENE R. 1968: 78 |
Cycloxanthops
| GUINOT D. 1962: 8 |
Cycloxanthops cavata
| WARD M. 1932: 244 |
Megametope sulcatus
| EDMONDSON C. H. 1931: 11 |
Euxanthus minutus
| EDMONDSON C. H. 1925: 46 |
Cycloxanthops cavatus
| NG P. K. L. 1993: 706 |
| TAKEDA M. & MIYAKE S. 1968: 2 |
| EDMONDSON C. H. 1962: 233 |
| DUMORTIER D. 1960: 155 |
| BALSS H. 1938: 43 |
| RATHBUN M. J. 1907: 41 |