HOMOLOIDEA De Haan, 1839
|
publication ID |
https://doi.org/10.5281/zenodo.5397969 |
|
persistent identifier |
https://treatment.plazi.org/id/C5482F17-9020-FFC4-C5F7-FDFCFCEBFA2E |
|
treatment provided by |
Marcus |
|
scientific name |
HOMOLOIDEA De Haan, 1839 |
| status |
|
Superfamily HOMOLOIDEA De Haan, 1839 View in CoL
The superfamily Homoloidea has long been associated with the Dromiacea (De Haan 1839; Bouvier 1896), and different authors continue to subordinate them to the Dromiacea ( Gordon 1950; Glaessner 1969; Hartnoll 1975; Š tevč ić 1981; Bishop 1986; Dawson 2002). The classification of Martin & Davis (2001: 49-51, 112) similarly proposes the alliance of the Homoloidea with the Dromioidea , within the Dromiacea. But morphological and spermatological data support the proposition that Homoloidea should be removed from the Dromiacea and form a separate group ( Drach 1971; Guinot 1978). Our view is to distinguish the two subsections Dromiacea and Homolidea to be included in a high-level group, the section Podotremata ( Guinot et al. 1994; Guinot & Richer de Forges 1995; Guinot & Bouchard 1998). The Homolidea should be considered a main podotreme lineage, apart from the dromiacean lineage ( Guinot & Tavares 2001: 531, fig. 16). Dromiacea and Homolidea are basal in the Podotremeta, opposing the Archaeobrachyura (including the Cymonomidae , Cyclodorippidae , Phyllotymolinidae , and Raninoidea ).
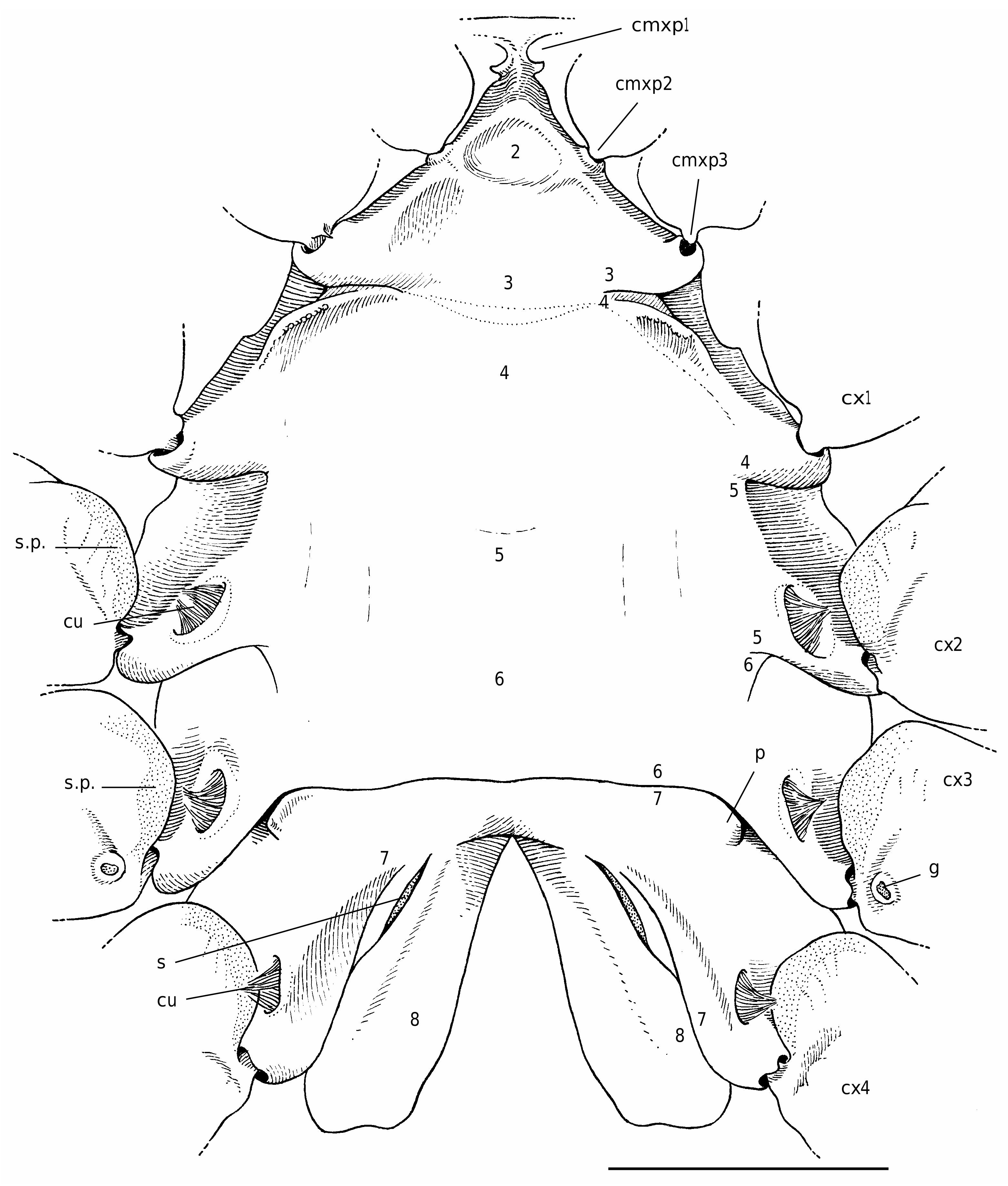
The homoloid thoracic sternum is relatively broad, not much hollowed; in males the sternoabdominal depression is completely covered (at least longitudinally, generally across) by a wide and long abdomen ( Guinot & Bouchard 1998: figs 8, 9). The anterior sternites form a narrow shield between the mxp3; sternites 1-6 are fused together, except laterally where traces of the sutures are still visible near the condylar articulations of the coxae of the pereopods ( Figs 19 View FIG ; 20 View FIG ). In females the posterior part (sternites 7-8) is variously inclined. Sternites 7, which are fused medially, form a wide, low arch that encloses sternite 8. In both sexes, suture 6/7 is always complete, observed as a continuous, horizontal line, and dividing transversally the thoracic sternum into two main parts ( Gordon 1950: 232, figs 13, 16, 18, 20, 21, 22A; Hartnoll 1975: figs 2A, 7A; Guinot & Bouchard 1998: fig. 9C). Internally, this corresponds to a short invagination of the phragma 6/7, leading to the formation of a strong tubular ridge which crosses medially the sternal surface (never present in the Dromiacea); laterally, the usual vertical walls are present. The dissection has shown the very weak port of this ridge 6/7, its weak lumen. Sutures 7/8 are markedly convergent, oblique, and end always apart, approximately at the level of the female gonopores on the P3 coxae, being more or less distinct throughout their course. The suture 7/8 may be not continuous and replaced medially by a weak thickening, to which does not correspond any invagination.
Sternite 8 is folded twice. As in the Dromiacea, there is a dorsal folding but, additionally, sternite 8 doubles up with regard to the median axis of the thorax, which corresponds to a median invagination. Consequently, a longitudinal furrow (median line), visible on the sternal plate of the crab, may divide medially the sternite 8 (along a more or less long distance). Thus, the median line (when present) corresponds to the median junction of the two symmetrical parts of sternite 8. These two parts are in contact, either entirely ( Homola Leach, 1815 , Paromola Wood-Mason in Wood-Mason & Alcock, 1891, Moloha Barnard, 1947 ; Fig. 19 View FIG ) or weakly ( Homolomannia Ihle, 1912 ). When sternite 8 is completely separated into two parts, however, the median line is absent ( Latreillia Roux, 1830 , Eplumula Williams, 1982 ; see Castro et al. 2003), and the abdomen is inserted in the resulting space, that is, the sternoabdominal notch. There is only a very short median line and a deep sterno-abdominal notch in the Poupiniidae ( Fig. 20 View FIG ).
Scholtz & Richter (1995: 304, 316, fig. 1), who included the Brachyura into a new taxon Fractosternalia Scholtz & Richter, 1995 (but the fracosterne has been lost in several groups, including the Brachyura , see Dixon et al. [2003: 957, 969] who established a new group, the Eurysternalia Dixon, Ahyong & Schram, 2003), indicated that homolids and homolodromiids show an incomplete fusion of lateral parts of the last sternites. Sternite 8 may be variously divided in the Homolidea, sometimes at a large extent, while only a short, posterior median line exists in the Homolodromiidae , which never shows a sterno-abdominal notch and thus has an organization similar to that of the Dromiidae .
In the Homolidea, part of the sternum situated around the suture 7/8 is differently modified to form the external part of the spermatheca. Gordon (1950: 232-244, figs 13-22, pl. 1, fig. C, as Thelxiopidae) extensively studied and figured the spermatheca in a number of homoloid genera, and named its different components. Hartnoll (1975: fig. 7) described and figured the spermathecae of Homola barbata (Fabricius, 1793) .
The spermatheca lies in the endosternite 7/8. There is a space between the two sheets of the phragma 7/ 8 in the posterior part, while the rest of the phragma has its vertical sheets joined and calcified. The spermathecal chamber is relatively small, generally with a flexible inner wall and a more rigid external wall (as in the Dromiacea). It communicates directly with the exterior by an aperture surrounded or lined by membranous areas, which varies among the genera and species. The opening is visible as a slit, or it is obscured by membraneous areas.
The skeletal junctions occur by interfingering ( Secretan 1983). In Moloha Barnard, 1947 and Paromola the intertagmal phragma is extended and reaches transverse bridge as in Homola Leach, 1815 ( Secretan 1998: figs 5, 6) ( Fig. 18 View FIG ). A peculiar skeletal arrangement is exhibited by the Poupiniidae (see below).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.