Bryoconversor tutus, Lörz & Myers & Gordon, 2014
|
publication ID |
https://doi.org/ 10.5852/ejt.2014.72 |
|
publication LSID |
lsid:zoobank.org:pub:94AB5EE1-49F4-4398-B081-C0189747CF23 |
|
DOI |
https://doi.org/10.5281/zenodo.3851666 |
|
persistent identifier |
https://treatment.plazi.org/id/93156279-10B8-4435-A6E0-FF53BFD5F252 |
|
taxon LSID |
lsid:zoobank.org:act:93156279-10B8-4435-A6E0-FF53BFD5F252 |
|
treatment provided by |
Tatiana |
|
scientific name |
Bryoconversor tutus |
| status |
sp. nov. |
Bryoconversor tutus View in CoL sp. nov.
urn:lsid:zoobank.org:act:93156279-10B8-4435-A6E0-FF53BFD5F252
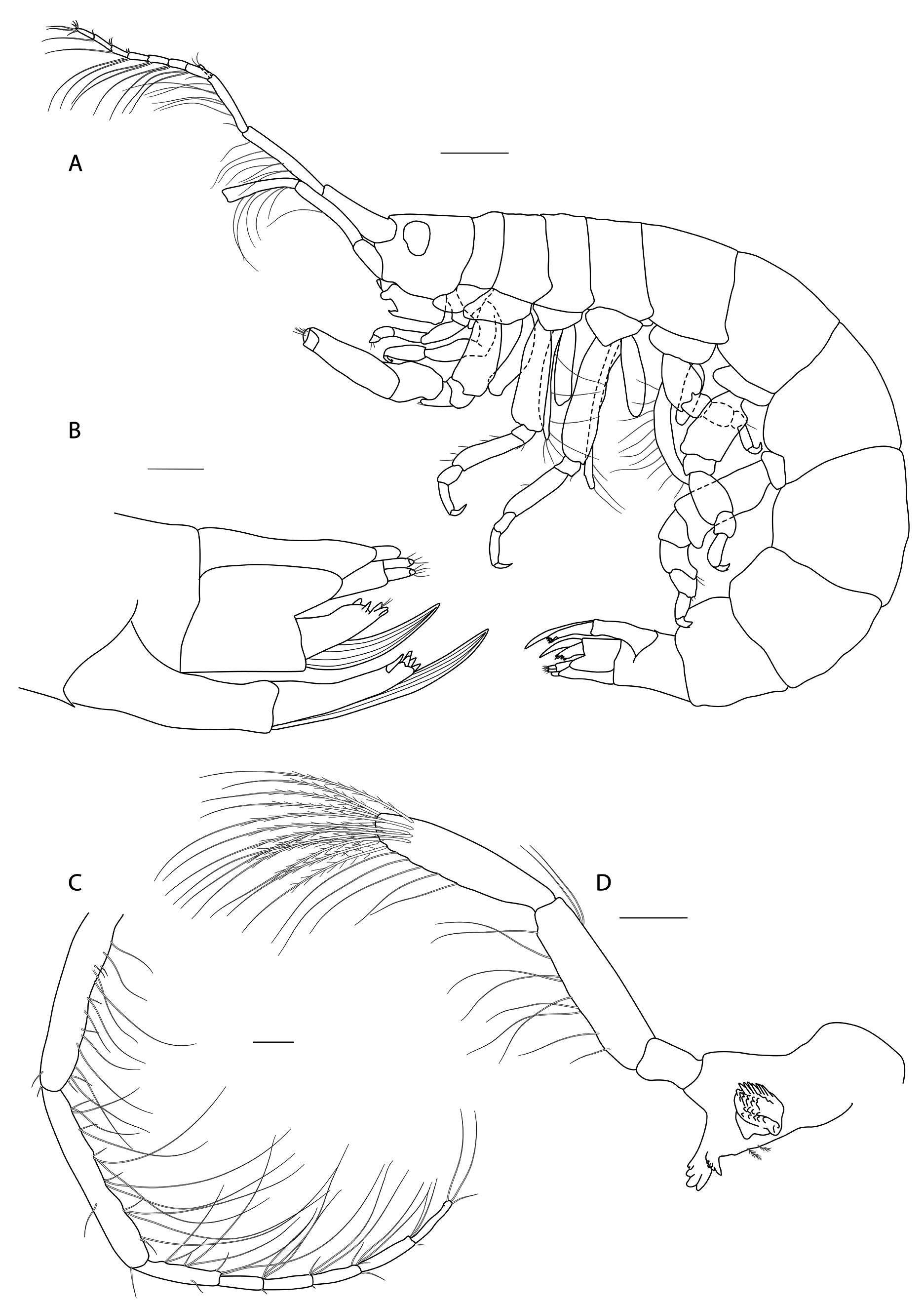
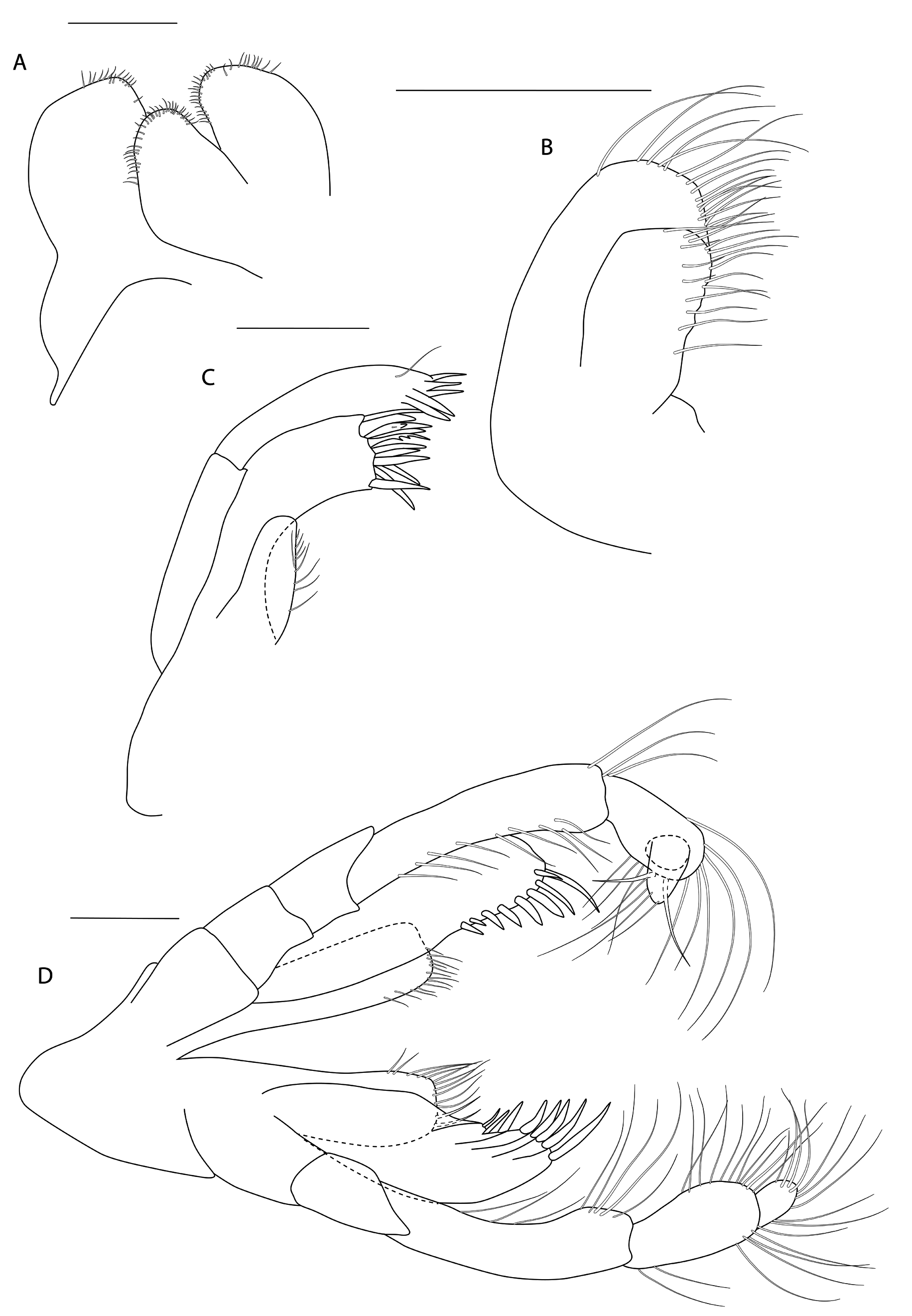
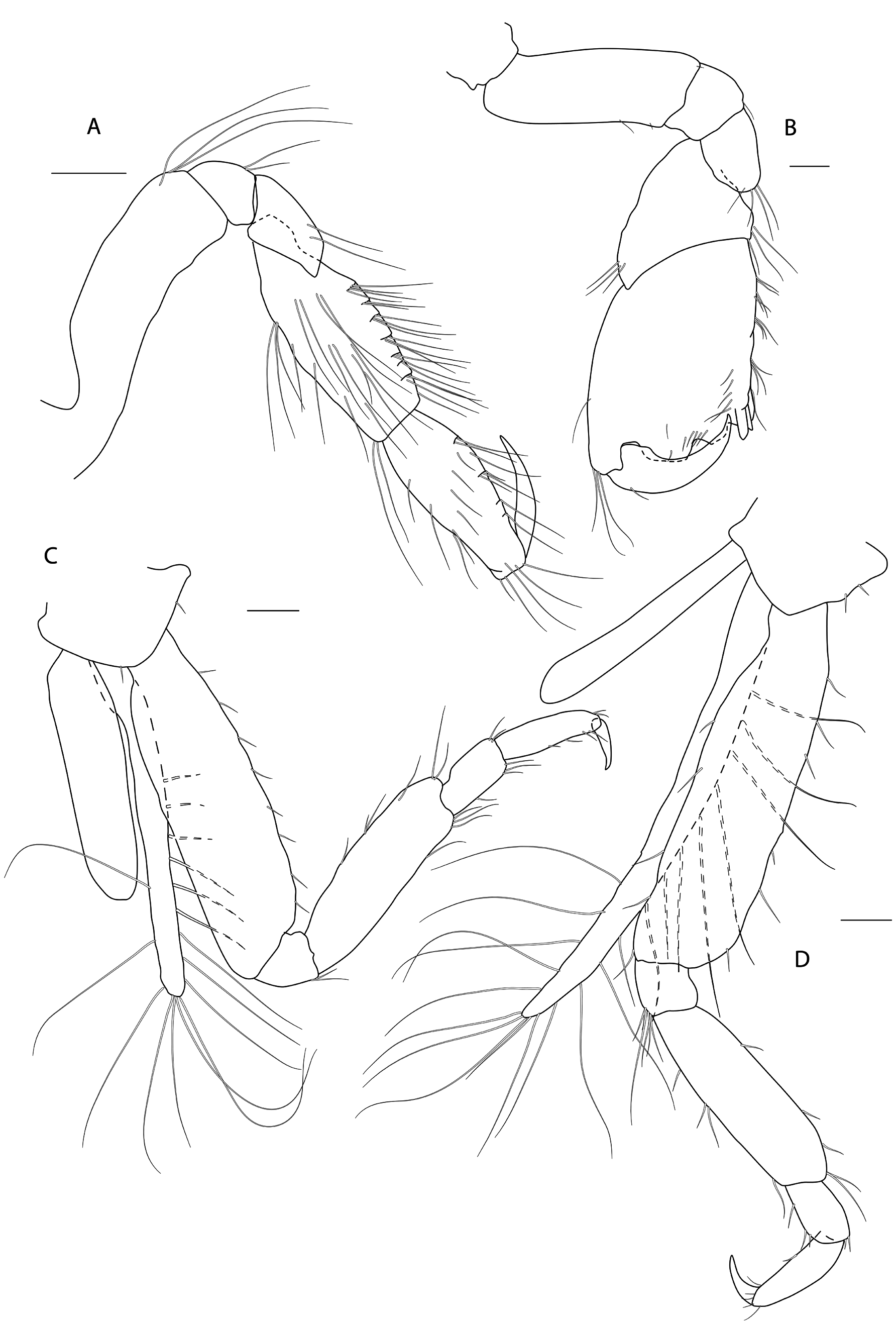
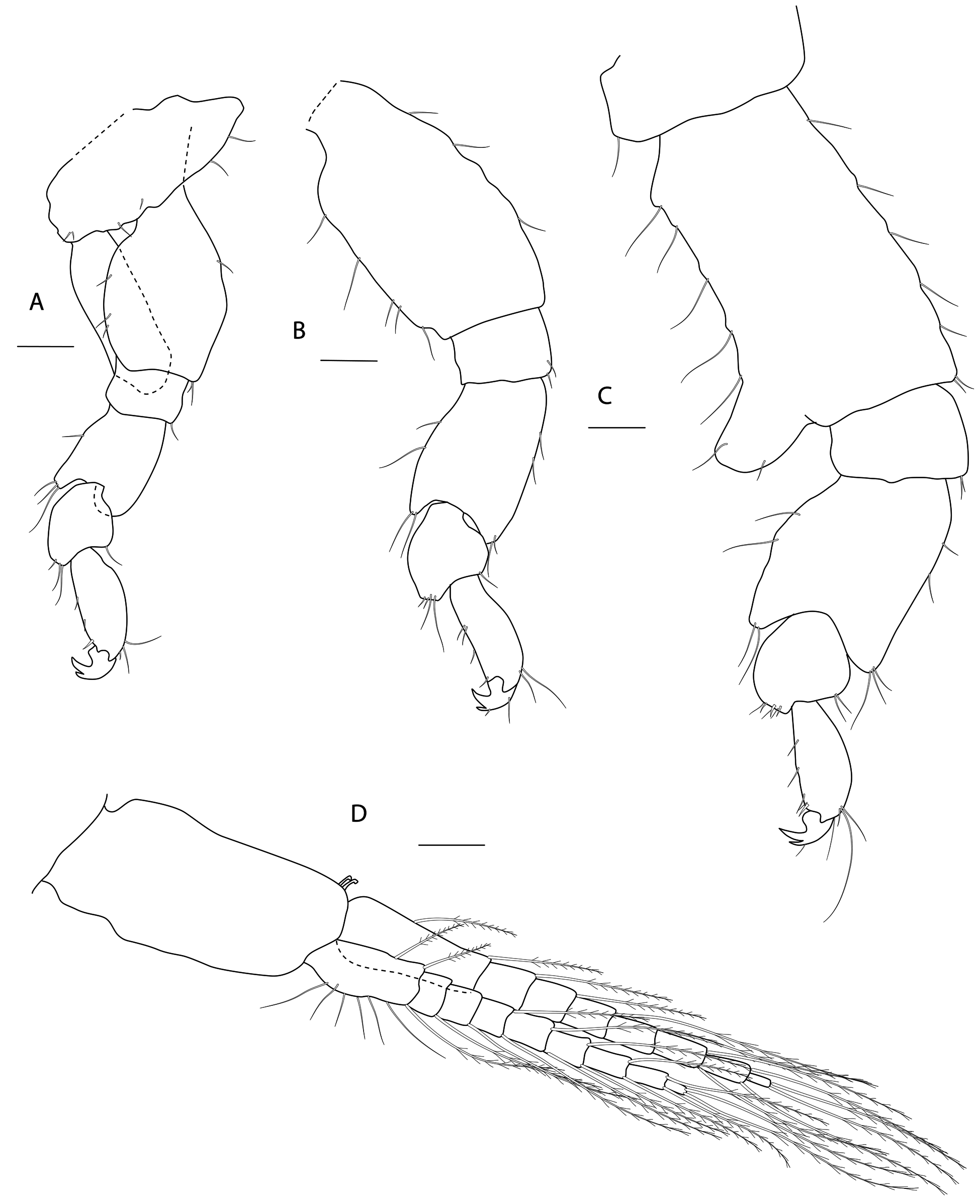
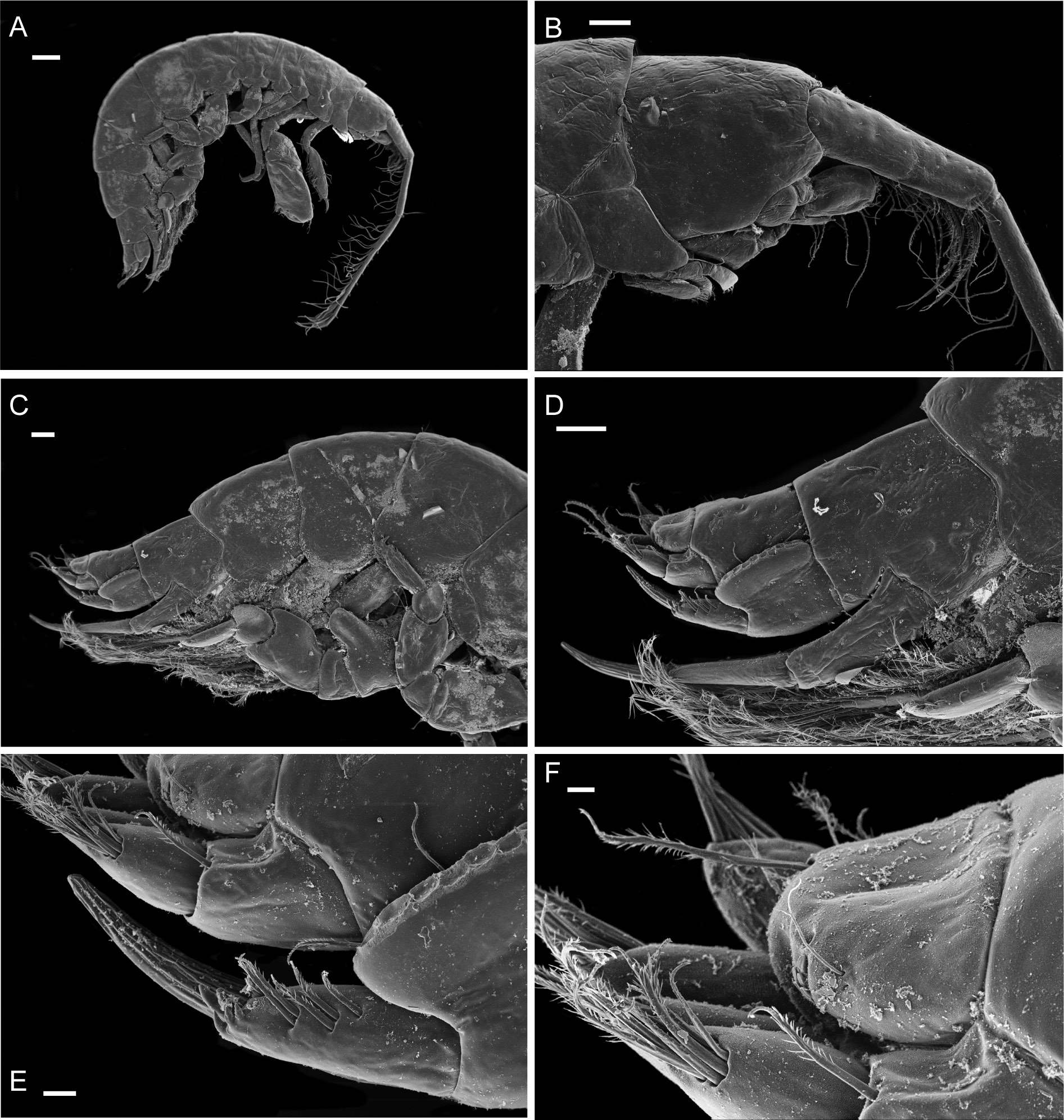
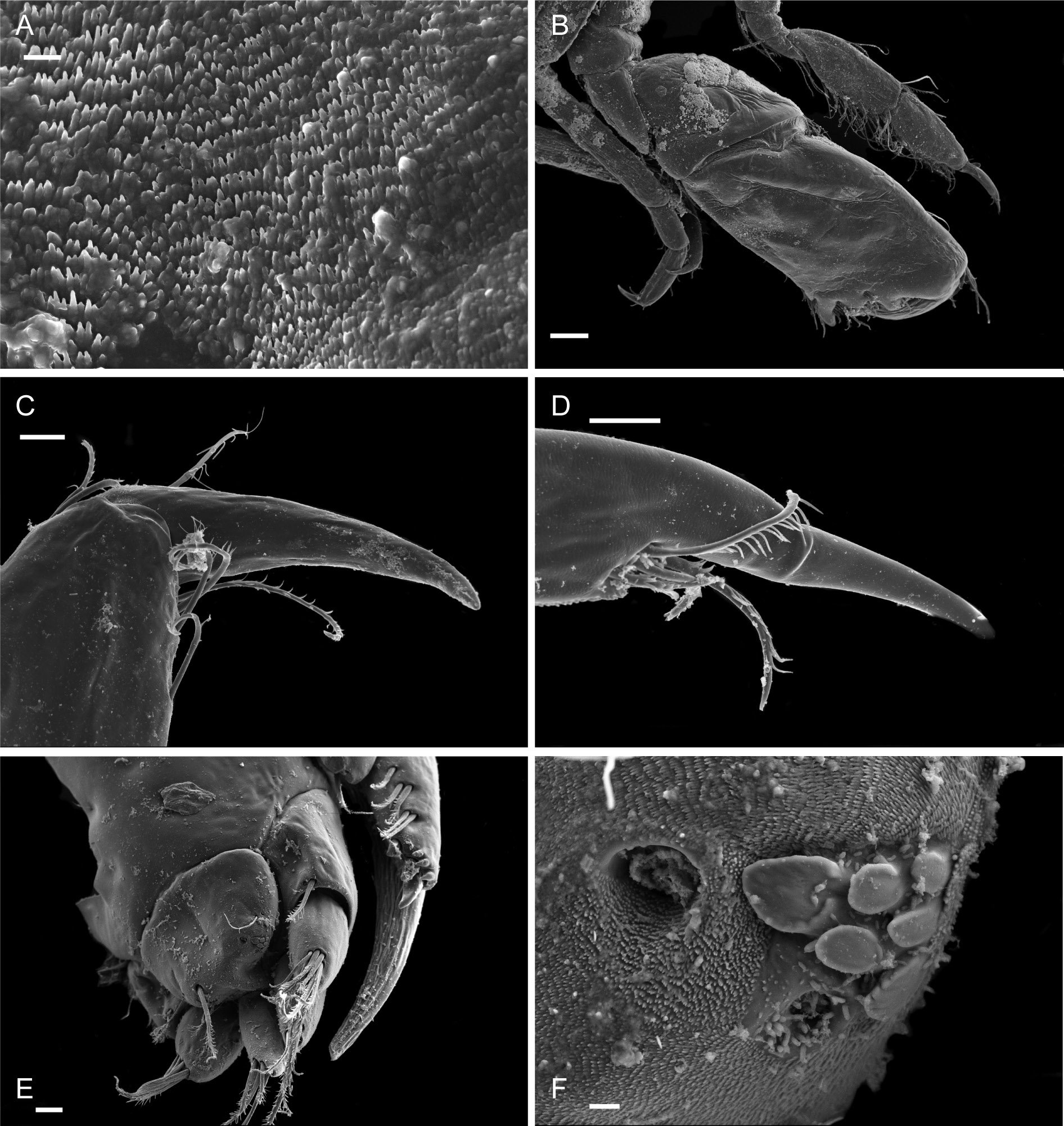
Figs 3-8 View Fig View Fig View Fig View Fig View Fig View Fig
Diagnosis
As for genus.
Etymology
Latin: ‘tutus’ = safe.
Material examined
Holotype
NEW ZEALAND: NIWA 88918 View Materials , ovigerous ♀, 1.72 mm long, station G675, 19 Jan. 1970, 45.4500° S, 171.4000° E, 792 m. GoogleMaps
Paratypes
NEW ZEALAND: Same collection data as for holotype. NIWA 52919, juvenile ♂, 0.64 mm long; NIWA 89534, 4 juveniles, about 0.4 mm long.
Additional material
NEW ZEALAND: Several dry specimens. NIWA 89536, st. G705, 23 Jan. 1970, 46.0667° S, 172.4750° E, 1500 m; NIWA 89533, st. TAN1208/58, 24 Jun. 2012, 42.8107° S, 179.8265° E, 1005 m; NIWA 89535, st. TAN0705/155, 16 Apr. 2007, 42.9989° S, 176.3483° W, 648 m; NIWA 89538, st. C620, 2 May 1961, 43.6667° S, 174.7833° W, 752 m; NIWA 89537, st. E827, 24 Oct. 1967, 46.5917° S, 166.7417° E, 530 m.
Description
Female (holotype, 1.72 mm long)
HEAD. Lateral cephalic lobes weakly produced forward, subtriangular, anteroventral excavation weak. Eye present. Antenna 1 & 2 subequal in length, about half body length. Antenna 1 slender, posterior margin with long slender setae; peduncular articles in the ratios 3:4:3; flagellum less than half length of peduncle, with 7 articles, accessory flagellum with one long and one tiny article. Antenna 2 peduncular articles 4 and 5 subequal; flagellum shorter than peduncle, with 6 articles. Labrum lacking epistome. Mandible molar triturative; palp three-articulate; article 1 short; article 3 a little longer than article 2, elongate sub-ovoid with dense distal setae and a few more sparsely on the posterior distal margin. Labium with subacute mandibular projections. Maxilla 1 inner plate with marginal setae; palp biarticulate with 5 distal robust setae. Maxilla 2 inner plate with oblique setal row. Maxilliped palp much longer than outer plate; dactylus rounded, short, lacking nail.
PEREON. Gnathopod 1 coxa weakly produced forward, sub-acute; basis slender, anterior margin weakly concave; carpus slender, longer than propodus; propodus slender, palm oblique, evenly continuous with posterior margin; dactylus slender, two thirds length of propodus. Gnathopod 2 larger than gnathopod 1; coxa subquadrangular, equal in depth with coxa 1; basis stout, a little over twice as long as broad, anterior margin concave; carpus short, stout, as broad as long; massive propodus nearly twice length of carpus, palm with three processes, a triangular distal spine and two rounded mid-palmar humps; dactylus a little shorter than palm. Pereopods 3-4 slender; coxae subtriangular, about half depth of coxae1-2; dactylus short, about one third length of propodus. Pereopod 5-7 coxae very shallow; carpus small, lunate; merus large and broad; dactylus with accessory spine on anterior margin. Pereopod 7 basis of posterodistal margin with strong projection.
PLEON. Epimera 1-3 rounded. Urosome segments 1 and 2 coalesced. Uropod 1 biramous, inner ramus longer than peduncle, weakly curved with deep longitudinal ridges and completely lacking setae; outer ramus a little shorter than peduncle with distal robust setae and one sub-distal seta. Uropod 2 posterior margin with a strongly developed flange with a pitted edge; inner ramus longer than peduncle, weakly curved with deep longitudinal ridges and completely lacking setae; outer ramus a little shorter than peduncle, with distal robust setae. Uropod 3 peduncle short, rami subequal in length with peduncle, with terminal setae. Telson dorsoventrally thickened with one long dorsal seta on each side; subdistal margin with a small group of flat-topped projections visible under SEM.
Male
(Sexually dimorphic characters.) Not different from female, except for much smaller body size.
The bryozoan host
Onchoporoides Ortmann, 1890 is a nominally monotypic genus of Calwelliidae (Cheilostomata) that is known only from the southwestern Pacific. The sole included species, O. moseleyi , was first collected by the R.V. Challenger from a depth of 951 m on the Kermadec Ridge north of Macauley Island. Additional specimens were subsequently taken by the then New Zealand Oceanographic Institute (now NIWA) from 526 m in the Solander Trough, at the western approaches to Foveaux Strait, and from 2677 m in the Hikurangi Trough east of Cook Strait ( Gordon 1989). This paper adds additional records, within this depth range, mostly from stations east of South Island. Gordon & d’Hondt (1997) also reported the species from the Loyalty Basin.
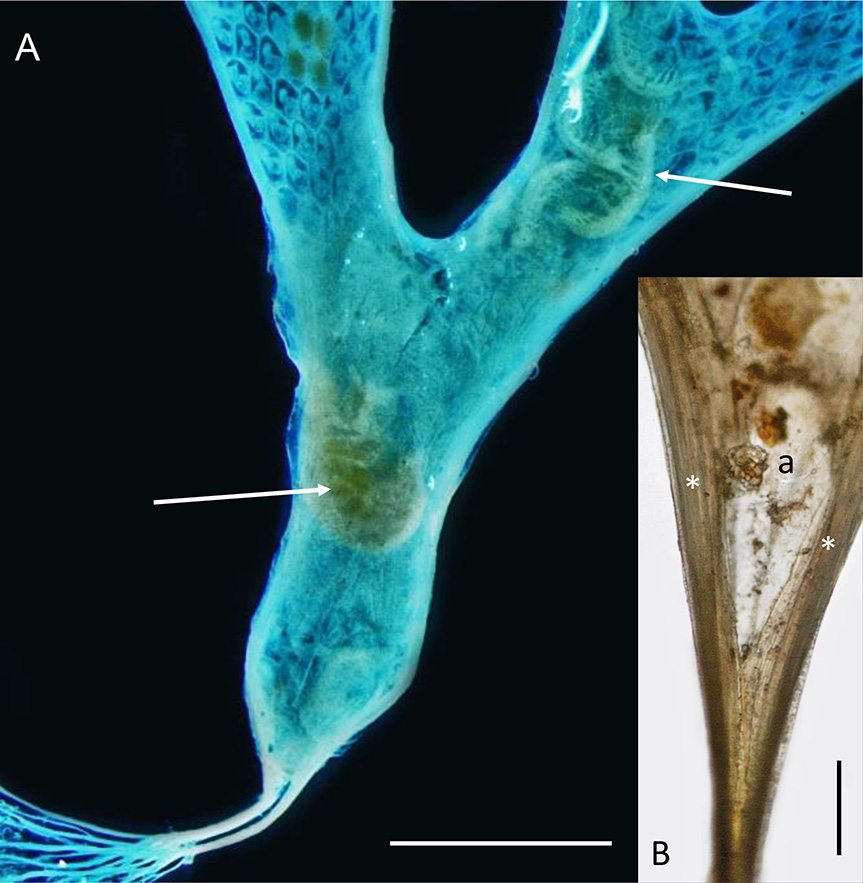
Onchoporoides moseleyi forms planar erect colonies up to 52 mm high. These can branch dichotomously to give an overall flabellate form and the whole colony is anchored in soft substrata by root-like rhizoids (non-feeding zooidal polymorphs). Like the zooids in the colony, each rhizoid has a calcified body wall. A tubular kenozooid (also a non-feeding bryozoan polymorph) runs down the lateral margin of the colony on each side, converging proximally to form a short stem that divides proximally into numerous individual rhizoids ( Fig. 2A View Fig ) with small lateral processes. The founding individual of a colony (ancestrula) is unusually long (2 mm) for bryozoans. It tapers proximally into two rhizoid-like processes that anchor it. The colony grows by distal budding of new zooids from the ancestrula and its daughter zooids.
The zooids of O. moseleyi open only on one face, with resident amphipods dwelling on the abfrontal side ( Fig. 2A View Fig ) where they apparently do not interfere with the life processes of the bryozoan. Enclosing the amphipods is a semitransparent membrane that is attached to each outer margin, conforming to the shape of the colony. The space enclosed by the membrane is interpreted to be a colony-wide extrazooidal basal coelom and its membrane an ectocyst (cuticularized epithelium). Gordon (1989) noted the apparent universality of the membrane in the colonies he examined, with the possible exception of very small colonies in which he failed to detect a membrane, perhaps because of its transparency. Busk (1884) described the species on the basis of a single specimen 19 mm high and did not mention an abfrontal membrane or any association with amphipods.
Examination of growing margins of O. moseleyi by light microscopy and SEM indicates that the basal coelom and ectocyst are laterally contiguous with the continuous marginal kenozooid (‘extrazooidal marginal coelom’ of Cook & Chimonides 1981) on each side of the branch. The basal coelom probably originates from the vicinity of the ancestrula ( Fig. 2B View Fig ) when it buds the first daughter zooids distally, each with a lateral kenozooid and the presumed inception of the basal coelom and ectocyst between them. Later in colony ontogeny, the first settler amphipod (possibly a hermaphrodite or a female with an attached male companion) is inferred to cut an entrance slit in the ectocyst, presumably using its gnathopods, in order to insert itself beneath, but not so destructively as to wholly interfere with the integrity of the basal coelom (though there must be some leakage or loss of coelomic fluid). One dried colony shows a longitudinal slit in the ectocyst where it would otherwise attach to the lateral kenozooid, with the tips of amphipod appendages protruding, but it is not clear if the tear is a consequence of drying or made by the amphipod. Self-repair is well documented in bryozoans (e.g., Ryland 1970) and a slit made by an amphipod to enter the basal coelom would quickly repair. We believe it much more likely that resident amphipods would remain within the basal coelom, surviving on nutrient transfer from the bryozoan, rather than constantly exiting and returning or sustaining an opening in the basal membrane for feeding. Continual loss of coelomic fluid would compromise the integrity of the extrazooidal basal coelom, whereas it is evident from the continued differentiation of the distal branch tips of the colony by intussusception that this must not be the case.
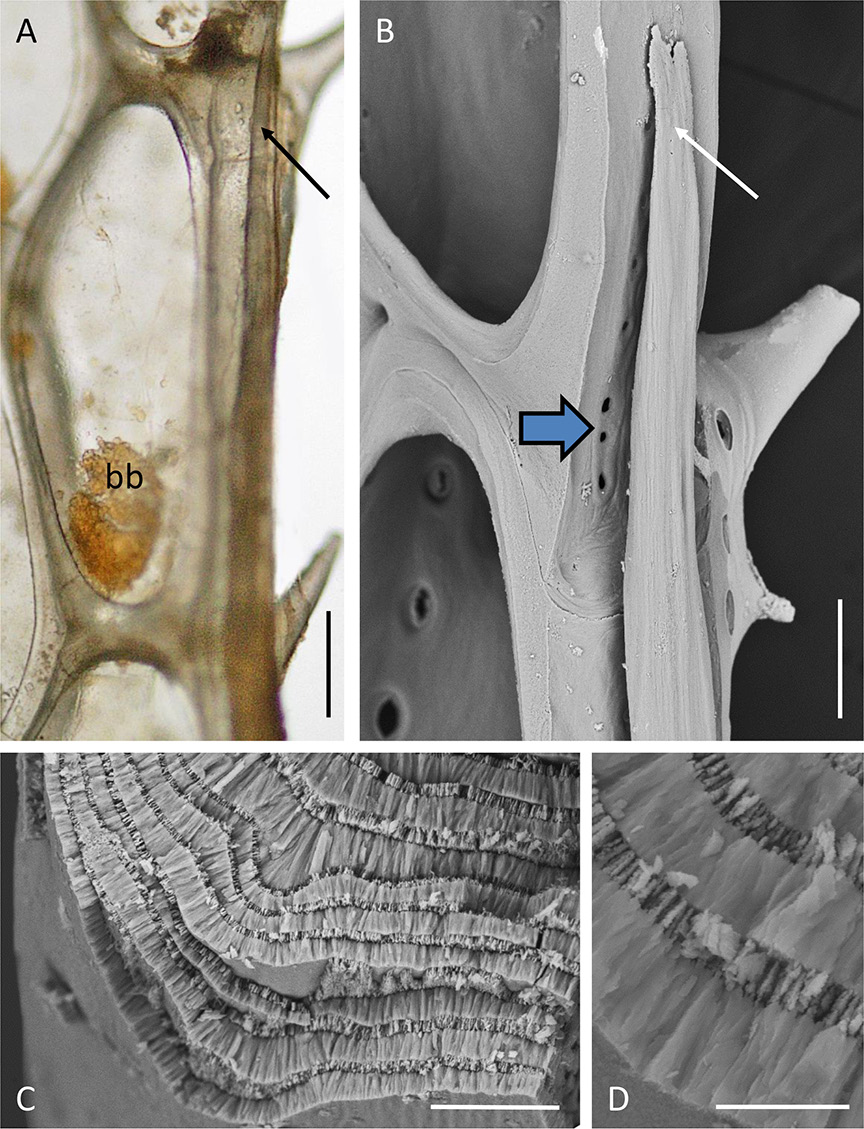
There are no communication pores in autozooidal basal walls (which are, however, uncalcified, allowing diffusion across the thinly cuticularised epithelium). There are such pores in the lateral walls, including those abutting the marginal kenozooidal tube as it differentiates on each side of a branch. At their distal tips, the marginal kenozooids are hollow and filled with coelomic fluid. Proximal to each tip the tapered distal end of a developing intracoelomic calcified rod is visible ( Fig. 9A-B View Fig ). This rod occupies the remainder of the kenozooid, fully occupying the coelomic cavity. A transverse fracture of the rod shows it to have a wall-perpendicular prismatic fabric (cf. Weedon & Taylor 2000) made up of thick and thin layers ( Fig. 9C-D View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |