Ercolania annelyleorum, Wägele, Heike, Stemmer, Kristina, Burghardt, Ingo & Händeler, Katharina, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.199203 |
|
DOI |
https://doi.org/10.5281/zenodo.6204671 |
|
persistent identifier |
https://treatment.plazi.org/id/C72BF37D-E828-8748-05AD-ED72FDCEA8D3 |
|
treatment provided by |
Plazi |
|
scientific name |
Ercolania annelyleorum |
| status |
sp. nov. |
Ercolania annelyleorum View in CoL sp. nov.
Type material. All specimens were collected in front of Casuarina Beach, Lizard Island (North Queensland, Australia) in shallow water up to 1 m depth at high tide. Type material is deposited at the Australian Museum Sydney. Holotype (AM C.464067): 25th June 2007 (length of preserved specimen: 1.5 mm); three paratypes (AM C.464068, including SEM preparation of radula): 10th July 2006; 13th July 2006; 20th October 2008 (length of all paratypes between 1 and 2 mm). For further material collected and investigated see Table 2 View TABLE 2 .
Distribution. Up to now this species has only been recorded from Casuarina Beach , in front of the Lizard Island Research Station, in clusters of the alga Boodlea sp. ( Figs. 1 View FIGURE 1 C–E, material studied here) and from the Mariana Islands (see discussion).
Etymology. This species is dedicated to Dr. Anne Hoggett and Dr. Lyle Vail, the directors of the Lizard Island Research Station (LIRS, Great Barrier Reef, Australia) for continuously supporting our projects.
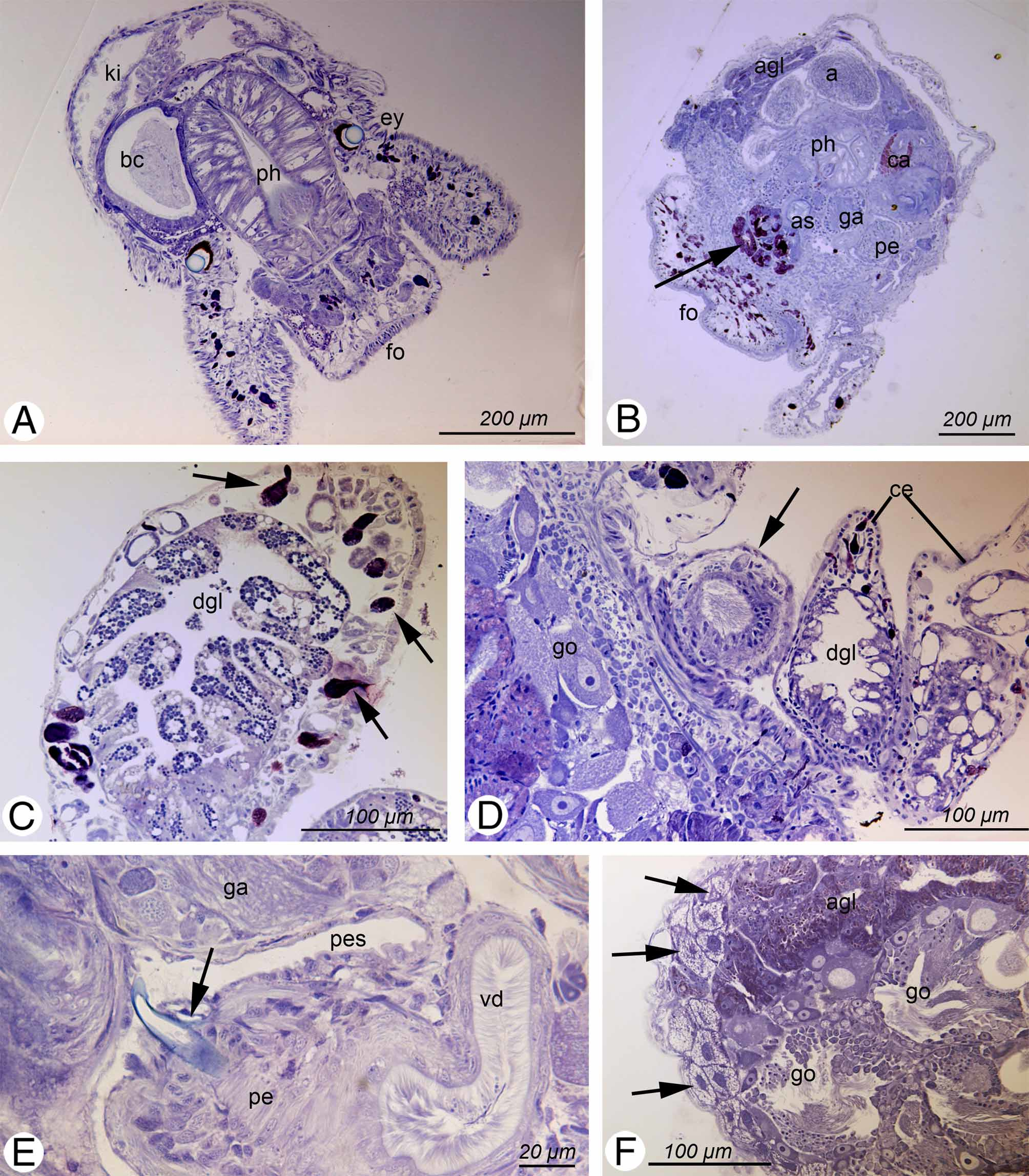
Description. External morphology and color of living specimens ( Fig.1 View FIGURE 1 ). Size up to 4 mm; body elongate; foot tapering posteriorly; anterior foot slightly extended to lateral sides with small notch, but without any propodial tentacles; rhinophores long, solid and digitiform; eyes located behind rhinophores; cerata clubshaped to elongated, in two to three rows, with smaller ones on outer side; cerata standing close, those of similar size in opposite position; central part of dorsal surface free of any cerata, without a prominent pericardial hump ( Figs. 1 View FIGURE 1 A–C).
Overall color of body translucent to whitish; green branches of digestive gland (color is due to undigested chloroplasts) shining through translucent body wall, especially in cerata and margin of the body. Two green lines beginning in posterior part of back and running parallel towards head; ending in anterior part of rhinophores, but not reaching into tips. These digestive gland ducts hardly branch. White dots spread over body, but more frequent and larger on distal parts of cerata and rhinophores. In posterior third of body red irregular formed patch visible dorsally between these two green lines, an additional red patch of different shape also visible in anterior third of dorsal surface of body. Branches of digestive gland brownish in animals starved for one day, their color therefore more translucent and light brown ( Fig. 1 View FIGURE 1 B). After longer starvation periods due to physiological experiments most cultivated specimens lost their greenish appearance, looked rather pale and were colored whitish to slightly pinkish ( Fig. 1 View FIGURE 1 C).
Description of anatomy and histology ( Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 ). Three specimens investigated (see Table 2 View TABLE 2 ). Specimens were bent slightly to the ventral side in the longitudinal axis. This is considered (and corrected) in the description of the anatomy.
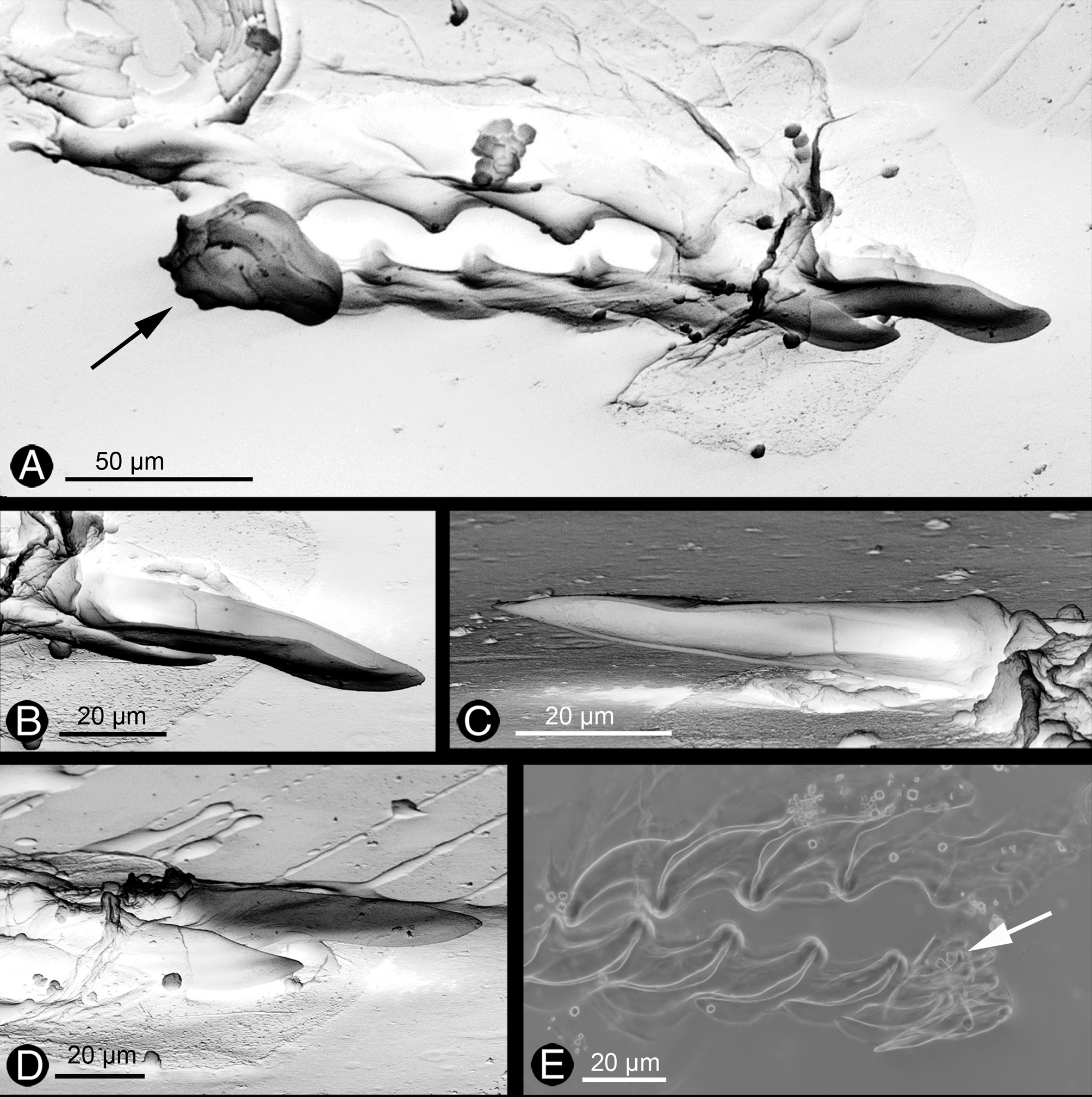
Digestive tract. Mouth opening between ventral part of head and anterior foot lip. Epithelium of oral tube with ciliated cells; subepithelial glandular follicles forming a layer around oral tube and entering the latter; cells with bluish to violet stained mucus. This glandular layer extends far back below pharynx and ascus ( Fig. 2 View FIGURE 2 B, arrow). Anterior outer pharynx (labial disc) covered by thin unarmed cuticle (labial cuticle), continuing interiorly. Pharynx on dorsal part forming a longitudinal buccal pump with an internal thin cuticular layer. Ascus separate from pharynx, surrounded by thick muscle layer and with teeth present in lumen ( Fig. 2 View FIGURE 2 B). Radula formula 0.1.0; at least four to five larger teeth in ascending limb and six to seven teeth in descending limb ( Figs. 3 View FIGURE 3 A – E). Leading tooth of sabot-shaped type, with a smooth cutting edge ( Figs. 3 View FIGURE 3 A, D). Several smaller teeth (six counted in one of the investigated radulae) and two pre-radular teeth in ascus present ( Fig. 3 View FIGURE 3 E). Salivary glands could not be found. Oesophagus originating from posterior pharynx, above ascus; here surrounded by nerve ring. Oesophageal epithelium composed of cylindrical ciliated cells, interspersed with few glandular cells, staining violet. Stomach lined by columnar, ciliated epithelium and hardly distinguishable from oesophagus. Digestive gland ramifying and running into cerata ( Figs. 2 View FIGURE 2 C, D), as well as into rhinophores, forming one central tubular rugose structure; no ramifications in foot observed. Epithelium of digestive gland filled with globular chloroplasts ( Fig. 2 View FIGURE 2 C). Intestine inconspicuous; anus lying dorsal in front of pericardium.
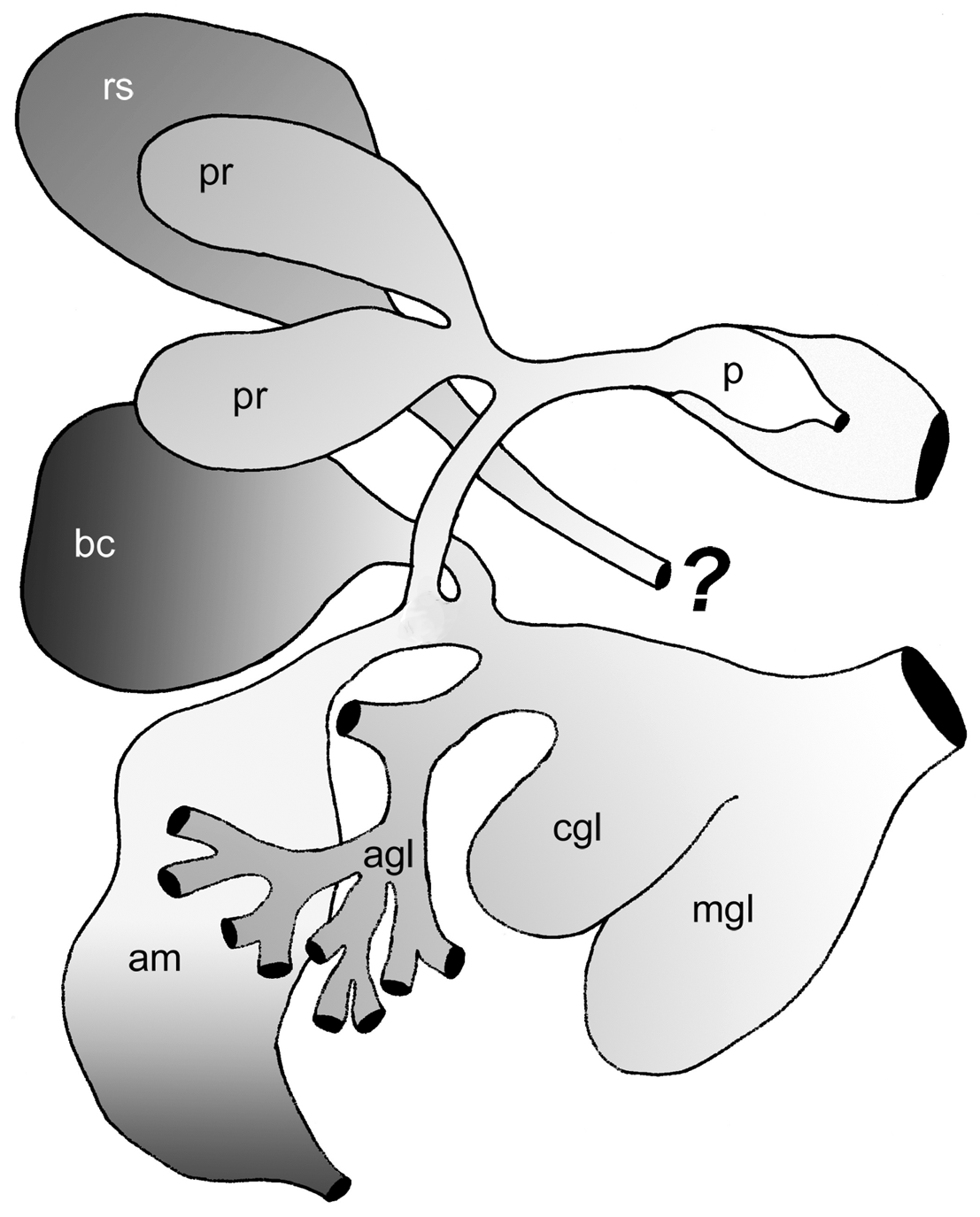
Genital system. A schematic outline of the distal genital system is given in Figure 4 View FIGURE 4 . Gonad partly lying lateral and dorsal, occupying most of body cavity in posterior third of animal. Gonad follicles with oogonia lying in periphery and spermatogonia in median part ( Fig. 2 View FIGURE 2 F). Ampulla large and coiled, with flat epithelium; lumen filled with sperm exhibiting elongate heads ( Fig. 2 View FIGURE 2 B). Bursa copulatrix extremely large, occupying most of space in head area to right of pharynx ( Fig. 2 View FIGURE 2 A); bursa entering proximal oviduct directly before beginning of nidamental glands. Epithelium composed of large cuboidal ciliated and partly apocrine secreting cells. Lumen filled with disintegrating sperm. A saclike elongate receptaculum seminis filled with parallel lying sperm ( Fig. 2 View FIGURE 2 D) present. Direct opening to outside not present, but connection to internal parts of genital system could not be observed. Epithelium of receptaculum consisting of flat to cuboidal cells. Nidamental glands comprising of three distinct areas: albumen, capsule and mucus gland. Albumen gland forming small tubes and running laterally into posterior part of body ( Fig. 2 View FIGURE 2 F), but never into the cerata. Cells cuboidal, apical part filled with violet staining granules. Capsule gland tubular and mainly situated in central part of body. Capsule gland cells filled with smaller vacuoles staining bright red ( Fig. 2 View FIGURE 2 B). Cells of medium size, i.e., smaller than mucus gland cells and larger than albumen gland cells. Mucus gland forming a thick tubular structure situated latero-dorsal and posterior of pharynx. Cells very large with red stained contents. Opening of nidamental glands in ciliated vestibule without any glandular structures.
Vas deferens with a separate prostate gland composed of two large limbs, each with a central duct surrounded by glandular cells, forming a subepithelial layer. Cell contents staining light bluish. Epithelium of distal muscular part of vas deferens (before entering penis) composed of ciliated cells ( Fig. 2 View FIGURE 2 E). Vas deferens inside penis lined by epithelium with long cilia. Muscular penis lying within sheath; distal part of penis with one hollow stylet, which is lightly curved ( Fig. 2 View FIGURE 2 E).
Excretory system. Kidney lying dorsal; forming two branches on lateral sides of pericardium which unite behind heart region into a dorsal sac-like structure ( Fig. 2 View FIGURE 2 A). Left branch reaching further anteriorly than the right one. Epithelium slightly folded, with larger cells containing several non-staining vacuoles. Nephroproct close to anus.
Nervous system and sensory organs. Nervous system located at transition of pharynx into oesophagus, forming a ganglionic ring with cerebral and pleural ganglia lying closely annexed and only partly fused. Pedal ganglia completely separated from cerebropleural complex. Additional two to three small ganglia present below the oesophagus. Eyes with pigment cup and globular, homogeneously light blue-stained lense; orientation to laterodorsal side ( Fig. 2 View FIGURE 2 A). Statocyst large, with one otolith.
Epithelia and glandular structures. Epidermis of cerata composed mainly of flat to cuboidal and ciliated cells. Large subepithelial glandular cells present, staining homogenously violet ( Fig. 2 View FIGURE 2 C). Epidermis of body composed of flat cells, without any subepithelial glandular structures ( Fig. 2 View FIGURE 2 A, B). A special glandular structure present mainly in posterior part of body; starting between albumen gland and epidermis in anterior half, then present on both lateral sides until posterior part of body. Glandular cells characterized by large nucleus and large non staining vacuoles ( Fig. 2 View FIGURE 2 F arrows). Cells not connected by any ducts and without openings to outside.
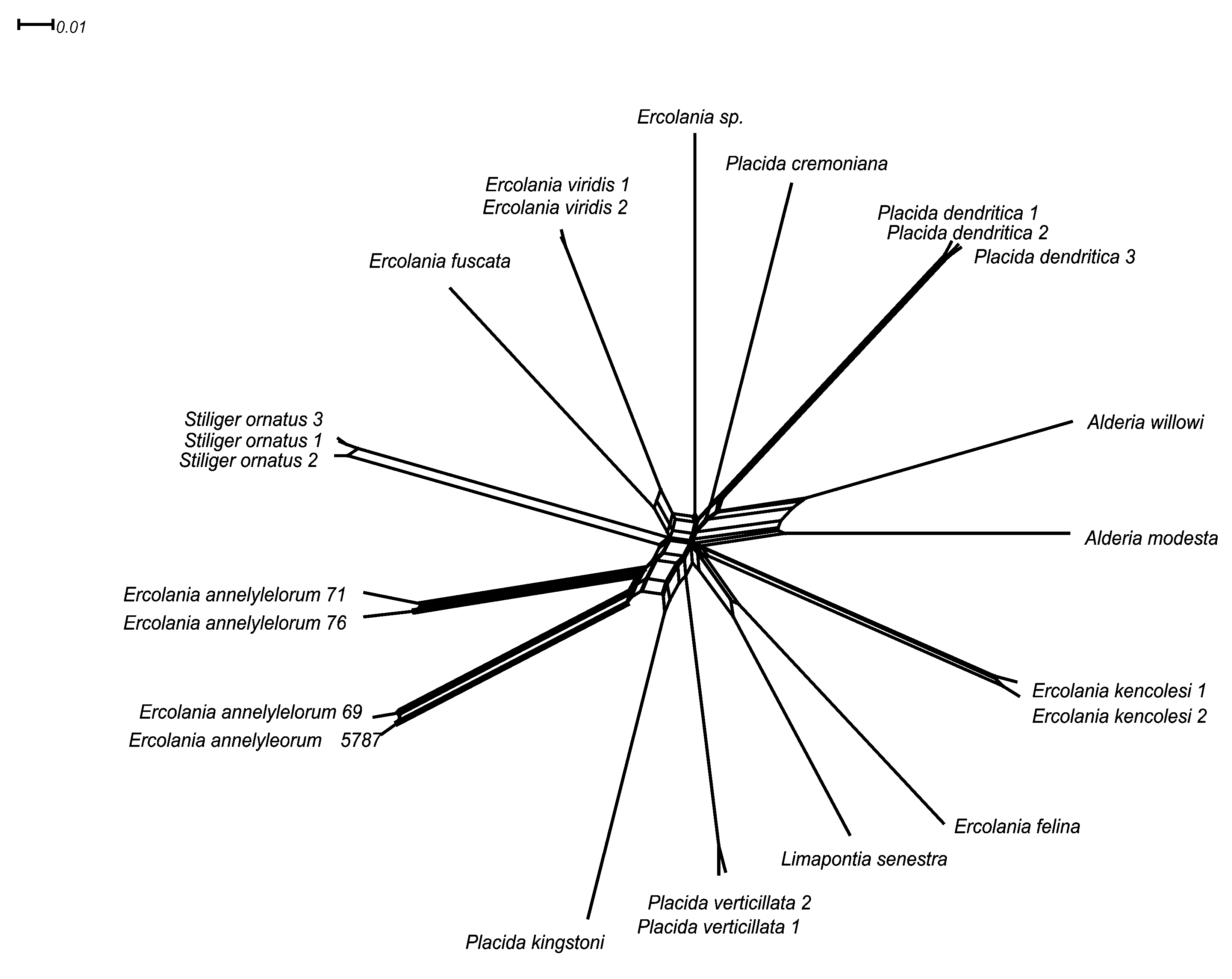
Molecular investigations ( Figs. 5–6 View FIGURE 5 View FIGURE 6 ). Partial CO1 sequences of four specimens were investigated. Three of them were collected from the same small algal clutch and at the same day (see Tab. 2 View TABLE 2 ). A fourth one was collected two years earlier at the same locality. The four sequences form two pairs, with a high sequence similarity within the pair (1.31 to 3.56%) and a high sequence divergence between the two pairs (17.07 to 17.64%) ( Tab. 3 View TABLE 3 ). This is also visualized in the NeighborNet analysis, where the two pairs are separated by similar distances as to other members of the Limapontiidae ( Fig. 5 View FIGURE 5 ).
The number of available sequences of other Ercolania species is still low, hence only five additional species out of the about 20 described ones (see Jensen 2007) could be included. Table 3 View TABLE 3 shows divergence of partial CO1 gene sequences of further three included Ercolania species, as well as limapontiid species against Ercolania annelyleorum sp. nov. The smallest sequence divergence of E. annelyleorum sp. nov. (69 and 5787) to any other limapontiid sequences included (uncorrected p-distances) is against the sequence of Ercolania fuscata (17.64 to 17.82%), and to Placida verticillata (71 and 76; 15.95%; see Table 3 View TABLE 3 ). Intraspecific variability of E. viridis (two sequences: 0.79%) and E. kencolesi (two specimens: 1.27%) is considerably lower and similar to the divergence observed within the two groups of E. annelyleorum sp. nov.. Intraspecific variability for three Stiliger ornatus sequences lies from 0.56% to 1.31 and 1.50%.
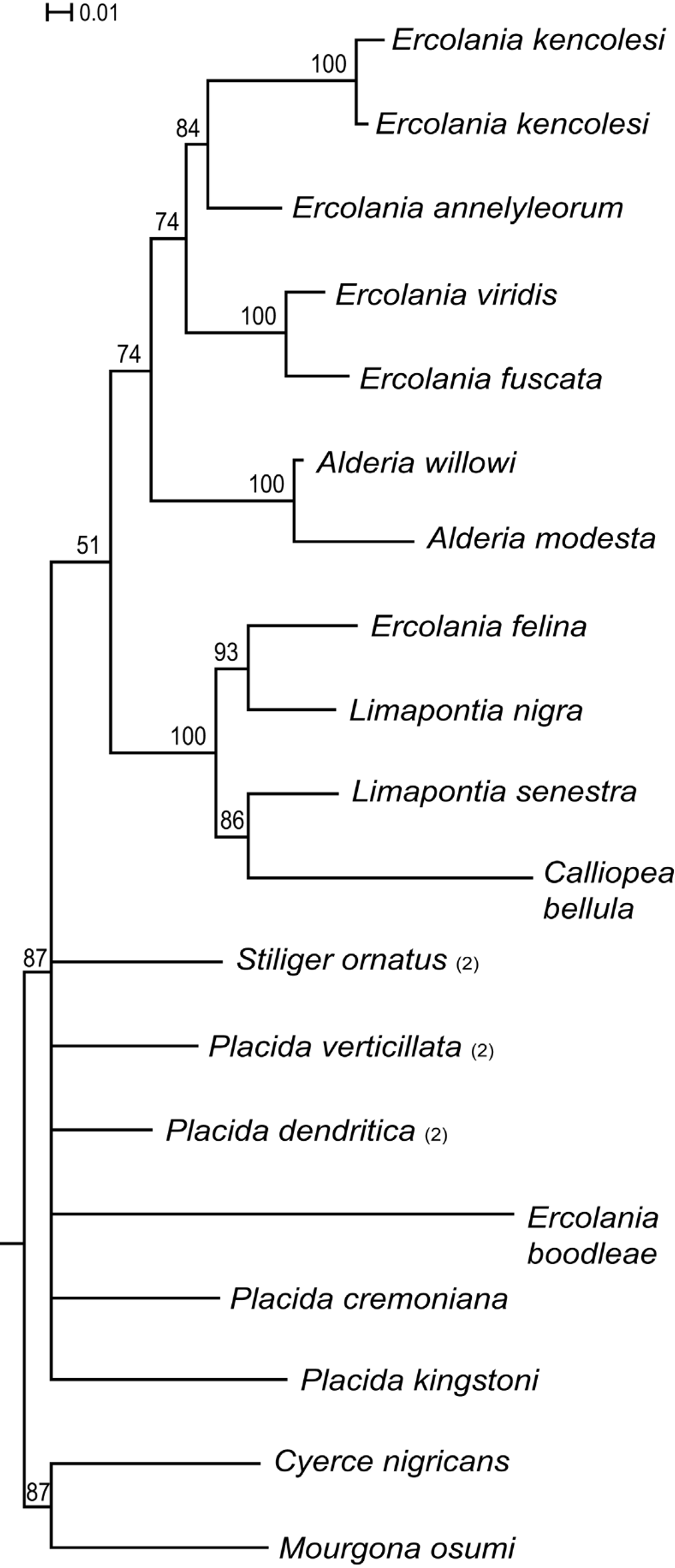
Figure 6 View FIGURE 6 shows the relationship of the new Ercolania species (only one specimen is included) within Limapontiidae . The genus Ercolania is not monophyletic, since E. boodleae and E. felina do not group with the other four Ercolania species. Sister taxon of E. annelyleorum sp. nov. is the endophytophageous species E. kencolesi . The specimen named Ercolania sp. in the analysis by Händeler et al. (2009) represents a further individual of the species E. kencolesi (here designated as E. kencolesi 2, since the overlapping parts of 28S rDNA are identical, sequence divergence between the 16S rDNA is lower than 0.5% (two of 413 positions) and overlapping parts of CO1 differ under 1.4% (three nucleotides in 1st position and six in 3rd position of 657) (see also Fig. 5 View FIGURE 5 ).
Notes on biology and photosynthetic activity. Ercolania annelyleorum sp. nov. was observed crawling and feeding on the algal species Boodlea sp. (Chlorophyta, Cladophorales ), which forms cushion-like patches. Branches of this alga are tough and the patches are rather stiff, forming compact shelters for the slugs ( Figs. 1 View FIGURE 1 D, E). Juvenile animals appeared in a big number (up to 33 individuals per cluster) in the algal patches, suggesting that larvae stay close to Boodlea sp., where they hatch from egg clutches inside the algal patches. Feeding and crawling on this alga has been observed. It was not possible to rear the larvae.
PAM measurements were taken of ten specimens. Data are shown in Table 4. Mean values of maximum quantum yield measurements for single specimens do not exceed 0.14, the average of all specimens does not exceed 0.10. Standard deviation of mean yield values of specimens is low. These low maximum quantum yield values result from background noise and are no indication of photosynthetic activity. Up to three days starved and pale animals of Ercolania annelyleorum sp. nov. were allowed to feed again on Boodlea sp. for four days. Their green coloration returned, but values of their photosynthetic activity never exceeded 0.2, indicating direct break down and digestion of chloroplasts.
TABLE 4. PAM measurements (PSII maximum quantum yield, Φ IIe-max) of ten specimens of Ercolania annelyleorum sp. nov.. For specimens no. 7 and 8 a joined mean value is given. Mean value and standard deviation are given for days of starvation.
Discussion. Taxonomy of Ercolania annelyleorum sp. nov.. Systematics of Limapontiidae has been under debate for long time (see Jensen 1993). According to the phylogenetic analyses of Sacoglossa ( Händeler et al. 2009) , the validity of different genera of the Limapontiidae ( Limapontia , Alderia , Placida and Ercolania ) seem to be solved, but validity of the limapontiid genus Stiliger still needs to be clarified. According to the present phylogenetic analysis of the Limapontiidae including more sequences from GenBank, validity of the genus Ercolania may also have to be reconsidered. Although distinct characters for the genus Ercolania were mentioned by Baba and Hamatani (1970), Marcus d. B.-R. (1982) rejected the fact of genus-specific characters for Stiliger or Ercolania and pointed out the high degree of variation in other genera. Until these taxonomic problems are solved, we follow Schmekel and Portmann (1982), Jensen (1985, 1993) and Grzymbowski et al. (2007) and assign the new species to the genus Ercolania due to the presence of digitiform rhinophores, the presence of smooth sabot shaped teeth, the presence of a curved penial stylet and the absence of the albumen gland in the cerata. The very similar Placida differs from Stiliger by the auriculate rhinophores, which are rolled or folded in Stiliger ( Jensen 1993) . Placida has an oesophageal crop, which could not be found in the specimens investigated here, and Stiliger lacks a penial stylet ( Jensen 1993). Future studies additionally based on molecular markers will reveal validity of these three different genera.
About 20 valid species of Ercolania are recognized. Few of them have been described from the Mediterranean Sea and the Atlantic ( Jensen 2007). Additional to the locality, these species ( E. fuscata ( Gould, 1870) , E. lozanoi Ortea, 1981 and E. nigra ( Lemche, 1935) are distinguished by their different coloration. One species, Ercolania coerulea Trinchese, 1892 , has been recorded from the Atlantic as well as the Pacific Ocean. Due to the inflated numerous cerata and the darker coloration, partly due to the much higher branching of the green digestive gland, that species differs considerably from the new species described here. Additionally, Sanders-Esser (1984) described the receptaculum seminis with a distinct opening to the outside, which is not present in our new species. Several species described from the Pacific Ocean (see Jensen 2007), like E. erbsa (Marcus & Marcus, 1970) , E. felina ( Hutton, 1882) , E. gopalai ( Rao, 1937) and E. margaritae Burn, 1974 , are distinguished by their differing coloration. Ercolania irregularis ( Eliot, 1904) is described by the author with posterior cerata which are twice as long as the front ones.
In the following, those species are discussed, which show a similar coloration to our newly described species. Ercolania boodleae ( Baba, 1938) is recorded to feed on the tubular alga Boodlea , similar to the new species described here. Ercolania boodleae is darkly green colored, nearly blackish, with distinct white stripes along the head. The eyes are situated within these stripes ( Baba & Hamatani 1970). Due to this coloration, it cannot be confounded with E. annelyleorum sp. nov.. Ercolania emarginata Jensen, 1985 is very similar in coloration to E. boodleae and can also not be confounded with the new Ercolania species. Besides, the radula teeth of E. emarginata show a distinct prominence and there is only one prostate lobe ( Jensen 1985). A very similar color and body shape to the new described sea slug can be observed in E. subviridis ( Baba, 1959) . The body of this species is translucent yellowish with fine green branches of digestive gland shining through the body wall. Within the cerata, several green branches of the digestive gland are visible as longitudinal lines, giving each ceras a characteristic green-lined appearance. Baba (1959) described a red marking at the anterior end of pericardial prominence which is also found on the new species. However, specimens of our species always exhibit an additional red patch in the posterior part of the body. Furthermore, only one central channel of digestive gland in each ceras with short pustule-like branches is present, and never several longitudinal stripes. The new species shows two distinct green branches on both sides of the head, which are missing in E. subviridis . Ercolania viridis ( Costa, 1866) usually shows much more inflated cerata and the digestive gland branches up to tertiary degree within the cerata, but does not reach into the rhinophores (unpublished results based on histological investigations of a Mediterranean specimen). Ercolania endophytophaga and E. kencolesi are also differentiated by their inflated cerata, the former also show peculiar teeth with knoblike apices ( Jensen 1999). Both can be separated also from the new species by their life style: they penetrate into algae and spend at least some time of their life cycle inside the algal tube. Ercolania translucens Jensen, 1993 has inflated cerata, a distinct pericardial prominence and the digestive gland does not reach into the rhinophores ( Jensen 1993). Although Jensen (1993) mentioned a reddish sphere in the neck region, this species lacks the second patch, but exhibits reddish-brown pigment stripes along the sides of the body and is therefore quite distinct from E. annelyleorum sp. nov. Ercolania nigrovittata ( Rao & Rao, 1963) is also described with only one pale-pinkish patch and not a second one in the rear. In contrast to our species, the digestive gland reaches far into the rhinophores, starting from a distinct patch over the head. The overall color is described as “faintly speckled grey over a faint orange background" ( Rao & Rao 1963: 233). Ercolania varians ( Eliot, 1904) from Zanzibar was described as “brilliant green”, although the color may vary. This coloration is due to “numerous lines of deeper color”, which can be interpreted as many digestive gland branches reaching throughout most parts of the body (“except at the sides of the body and in the centre of the back”) ( Eliot 1904: 290). Although he also mentioned crimson patches in some of his animals, it is not clear, whether there is only one patch or more on the dorsal body. Because of its general green color and a more elongate tooth, it is not considered to be the same species as the one described here. Ercolania gopalai ( Rao, 1937) can be excluded due to the lack of a bursa copulatrix in the genital system and the presence of a ventral oesophageal pouch ( Jensen 1985).
On the sea slug forum of Bill Rudman (www.seaslugforum.net), pictures are available of undescribed Ercolania species which show a similar color pattern to our specimens: Trowbridge (2005) depicted one specimen and several further ones are described in their appearance. According to the descriptions, the numbers of the red patches vary from zero to two, a variety that was not observed in our specimens. The digestive gland ducts in the cerata seem to branch more often than in our specimens and they are more inflated. The radula is very similar. Although these specimens are recorded from Okinawa, Japan, it can not be excluded that this is the species we describe here.
Tani (2006 a, b) followed with pictures of a similar specimen on the sea slug forum. The specimen was recorded from Saipan, Northern Mariana Islands on Boodlea coacta . The animal shows a distinct red patch in the median dorsal part of the body ( Tani 2006 b). Fujie (2007) depicted further specimens from the same locality and he also mentioned only one brown mark. Therefore an assignment of their specimens to our new species is difficult, although the overall appearance is certainly similar to our specimens. The same applies to a specimen depicted in Gosliner et al. (2008) under the designation Stiliger sp. 6, which might represent a member of the new species, but coloration has to be verified first.
Only few species are investigated by histological means. It is of interest that the new species described here and E. kencolesi exhibit a special gland ( Fig. 2 View FIGURE 2 F, this study; Fig. 2 View FIGURE 2 D in Grzymbowski et al. 2007) with undetermined function. The lack of any ducts leading to the outside indicates an endocrine function. Thorough investigation of Alderia modesta ( Lovén, 1844) and A. willowi Krug, Ellingson, Burton, and Valdés, 2007 (Krug &Wägele, unpublished results) did not reveal similar glandular structures. Further histological studies on other Ercolania species, and also related genera, will show, whether this gland is genus-specific or only present in few species.
Molecular characters. CO1 is one of the major barcoding genes to identify or characterize metazoan species (see http://www.barcodeoflife.org) and has been successfully applied for detection of cryptic speciation (e.g. Burns et al. 2008; Hajibabaei et al. 2006; Vaglia et al. 2008). Krug et al. (2007) recently discovered cryptic speciation in sacoglossan members of the genus Alderia by analysing CO1 data. The new species A. willowi is distinguishable from the other species involved ( A. modesta ) in ontogeny, morphology and geography (see Krug et al. 2007). The detection of two distinct clades in E. annelyleorum sp. nov. with a similar sequence divergence as is observed between limapontiid species (see Tab. 3 View TABLE 3 , Fig. 5 View FIGURE 5 ) in general allows several interpretations: 1. There are pseudogenes present in the species. This would result in different CO1 sequences in the same individual. Usually pseudogenes evolve quicker and since they are not translated, there should be mutations not only in the silent third position, but also in the first and second positions. The translation into aminoacid sequences showed only completely conserved aminoacids, mutations occurred exclusively as silent mutations. 2. There is a cryptic speciation ongoing in this Ercolania species, which shows no differentiation on morphology level yet. The two genetic distinct populations would then co-exist sympatrically in one and the same algal clutch. 3. There are two different mitochondrial lineages coexisting in the species, or even within one and the same individual. We do not know anything on population structures of this new species. Incomplete lineage sorting, as was recently shown for a butterfly family Lycaenidae ( Wiemers & Fiedler 2007) , and occasional introgressive hybridisation as a still ongoing genetic exchange, e.g., described in fish populations in ancient lakes (see e.g., Herder et al. 2006), are factors that certainly needs further investigation.
In the phylogenetic reconstruction based on a concatenated alignment of all available Limapontiidae sequences, Ercolania annelyleorum has a closer relationship to a species which penetrates into algal cells ( E. kencolesi ), but the position is uncertain as nodal support is not high (bootstrap value: 84) and many Ercolania species were not included. The genus Ercolania is polyphyletic. We do not consider the phylogeny of the Limapontiidae resolved, nevertheless, the molecular analyses revealed the distinctiveness of this new species and separation from all other included species.
Photosynthetic activity. Photosynthetic activity of Ercolania annelyleorum sp. nov. was analysed by PAM measurements under starving conditions. Maximum quantum yield values (Φ IIe-max) of the alga Boodlea sp. range between 0.6 and 0.7 indicating a “healthy” photosynthetic activity. The maximum quantum yield values (Φ IIe-max) of E. annelyleorum sp. nov. are very low from the very beginning of the experiments. Feeding experiments with Boodlea sp. and subsequent starving experiments clearly show that digestion of chloroplasts occurs within few hours. This is also recognizable by the loss of the green color within the first days of starvation. Similar to the results on E. kencolesi (see Grzymbowski et al. 2007), it can be concluded that chloroplasts are not retained in the new species described here. Clark et al. (1990) reported that freshly collected animals of E. coerulea retain plastids in digestive gland diverticula for at least two hours of starvation, but no photosynthate was detectable by isotope tracer techniques in that other species, supporting the hypothesis of direct digestion.
TABLE 2. Synopsis of investigated specimens and collecting data of Ercolania annelyleorum sp. nov. Material is listed according to collecting dates. FSW: preservation in formaldehyde / seawater; PAM: measurements of photosynthetic activity; LM: light microscopy; SEM: scanning electron microscopy; ZFMK Zoologisches Forschungsmuseum Alexander Koenig in Bonn, Germany; ZMS Zoologische Staatssammlung Munich.
| Date of collection | Date and kind of preservation | PAM | Type of investigation |
|---|---|---|---|
| 0 7.07.2006 | 0 8.07.0 6 FSW | Yes | Histology (ZSM 20100675) |
| 0 7.07.2006 | 0 8.07.0 6 EtOH | Yes | Gene analyses: 28S, 16S, CO1 (internal number 5787) (publ. in Händeler et al. 2009 as Ercolania sp.) |
| 10.07.2006 | 10.07.0 6 EtOH | Yes | Paratype AM C.464068 |
| 13.07.2006 | 0 1.08.0 6 FSW | Yes | Paratype AM C.464068 |
| 25.06.2007 | 28.06.0 7 FSW | Radula preparation (LM) (lost) | |
| 25.06.2007 | 0 1.07.0 7 FSW | Histology | |
| 25.06.2007 | 0 1.07.0 7 FSW | Histology | |
| 25.06.2007 | 0 1.07.0 7 EtOH | 2 specimens not investigated | |
| 25.06.2007 | 0 2.07.0 7 EtOH | Holotype AM C.464067 | |
| 20.10.2008 | 21.10.0 8 EtOH | Gene analysis: CO1 (internal number 71) Radula preparation (LM) (Fig. 3 E) Voucher material: ZFMK–DNA SacSti 0 0 0 1 | |
| 20.10.2008 | 21.10.0 8 EtOH | Yes | Paratype AM C.464068 (Radula only) Gene analysis: CO1 (internal number 76) Radula preparation (LM and SEM (Fig. 3 A–D) |
| 20.10.2008 | 22.10.0 8 EtOH | - | Gene analysis: CO1 (internal number 69) |
TABLE 3. Divergence of CO 1 sequences between individuals of Ercolania annelyleorum sp. nov. (Ea) and related species (only species presenting lowest and highest values are listed). Ercolania fuscata showed lowest values of all Ercolania species and Placida verticillata lowest values in total against E. annelyleorum sp. nov.. Placida dendritica showed highest values against E. annelyleorum sp. nov..
| Ea 71 | Ea 69 | Ercolania fuscata | Placida verticillata | P. dentritica 2 | |
|---|---|---|---|---|---|
| Ea 71 | 0.00 | 17.63 | 18.76 | 15.95 | 21.95 |
| Ea 76 | 3.56 | 17.07 | 18.57 | 15.95 | 22.51 |
| Ea 5787 | 17.64 | 1.31 | 17.82 | 19.32 | 23.45 |
| Ea 69 | 17.63 | 0.00 | 17.64 | 19.70 | 23.26 |
| Ercolania felina | 21.39 | 22.51 | 19.70 | 20.45 | 21.95 |
| Ercolania viridis | 17.82 | 18.95 | 15.76 | 16.14 | 19.51 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.