Mycodiplosis puccinivora Jiao, Bu & Kolesik, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4661.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:73F86A48-BF25-46D2-874E-F8607A5BEA91 |
|
persistent identifier |
https://treatment.plazi.org/id/1F06E27A-2760-4617-8FF8-2DBAEBE97CED |
|
taxon LSID |
lsid:zoobank.org:act:1F06E27A-2760-4617-8FF8-2DBAEBE97CED |
|
treatment provided by |
Plazi |
|
scientific name |
Mycodiplosis puccinivora Jiao, Bu & Kolesik |
| status |
sp. nov. |
Mycodiplosis puccinivora Jiao, Bu & Kolesik sp. nov.
( Figs 1–27 View FIGURES 1–8 View FIGURES 9–13 View FIGURES 14–18 View FIGURES 19–27 )
urn:lsid:zoobank.org:act:1F06E27A-2760-4617-8FF8-2DBAEBE97CED
Material examined. Holotype male, China, Hainan Province, Chengmai County, Jinjiang Town, Chengmai State Forest Farm of Hainan Province (19.183°N, 111.017°E), larva collected 10-20.X.2015 while feeding on rust Maravalia pterocarpi ( Pucciniomycetes : Pucciniales : Chaconiaceae ) growing on leaf of Dalbergia tonkinensis ( Fabaceae) , adult reared 26.X. 2015 in laboratory by Jiao Wang and Sheng-Song Su , deposited in NKUM on a permanent micrscope slide. GoogleMaps
Paratypes. 3 males, 10 females, 5 pupae, 3 larvae, same data as holotype .
Other material. 5 females, same data as holotype but reared 10-20.XI.2015 .
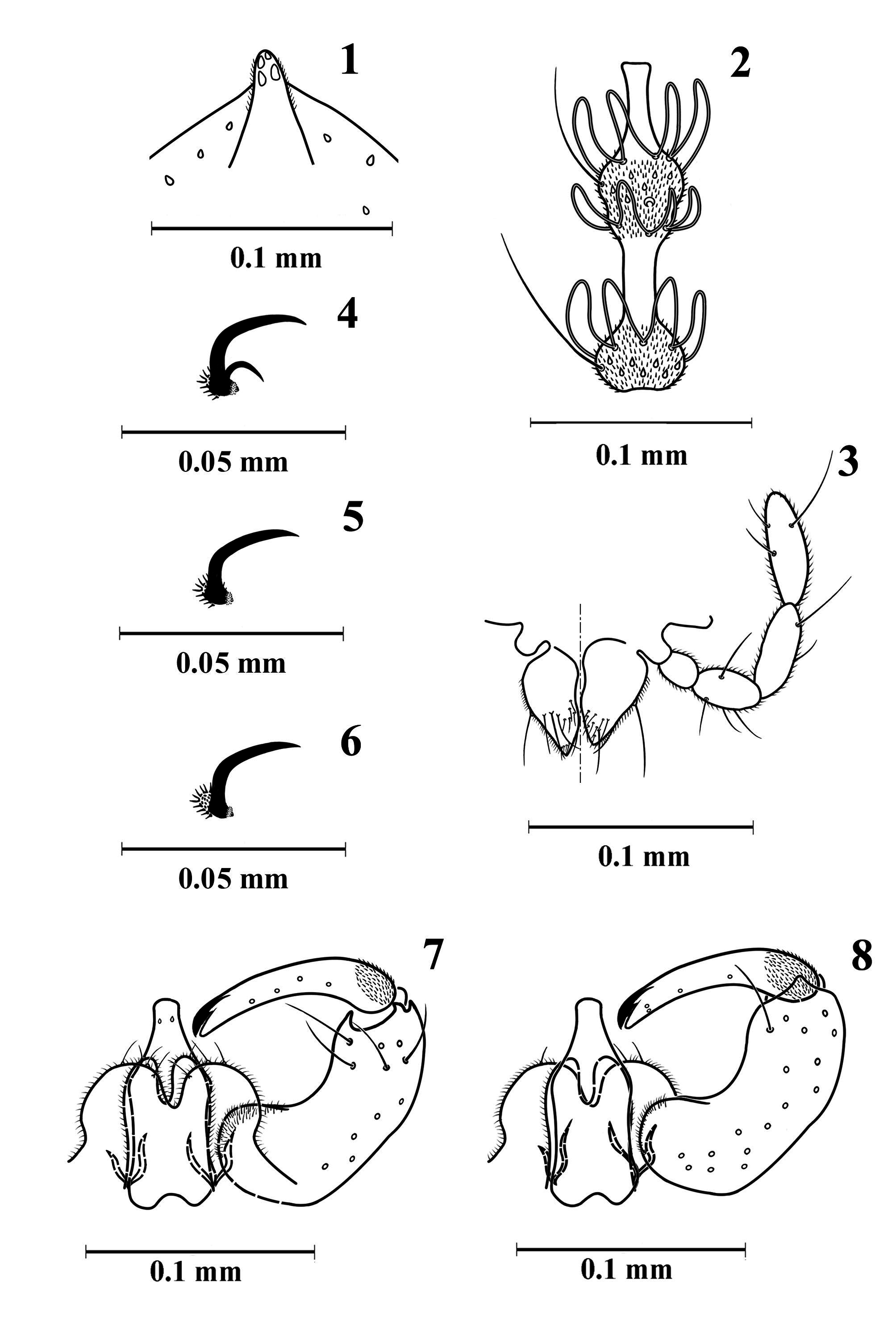
Diagnosis. The new species has toothed tarsal foreclaws and simple mid- and hindclaws ( Figs 4–6 View FIGURES 1–8 ); male flagellomeres ( Fig. 2 View FIGURES 1–8 ) have one whorl of circumfilar loops on the basal and two loops on the distal node, circumfilar loops on the basal node reach midlength of the internode, loops of the anterior whorl on the distal node reach midlength of the node, loops of the posterior whorl reach 3/4 of neck’s length; female flagellomeral necks have sparse microtrichia basally but are otherwise bare ( Fig. 9 View FIGURES 9–13 ); male terminalia ( Figs 7, 8 View FIGURES 1–8 ) have a bottle-shaped aedeagus, wide, rounded cerci that are separated by an incision reaching midlength, hypoproct wide, divided into two discrete, apically rounded lobes; setae on the larval terminal segment are 2/3 length of segment. The new species is morphologically closest to M. coniophaga (Winnertz) and M. buhri Holz , from which it differs in the following. In M. coniophaga the gonostyle is equally narrow and the male cerci are triangular while in M. puccinivora the gonostyle is wider basally and the male cerci are rounded. In M. buhri the male cerci and hypoproct are triangular and the larval head is more than 2x longer than wide while in M. puccinivora the male cerci and hypoproct are rounded and the larval head is only slightly longer than wide. The new species differs from M. hemileiae Barnes , the only Mycodiplosis previously recorded from east and south-east Asia ( Chen et al. 1990), which has the narrow and pointed lobes of the male hypoproct ( Barnes 1939).
Description. Male ( Figs 1–8 View FIGURES 1–8 , 20 View FIGURES 19–27 ). Colour: eyes black, remaining head and thorax dark brown, abdomen orangey brown. Wing length 1.1–1.3 mm, width 0.5 mm (n = 4).
Head. Eye bridge 6–7 facets long. Maxillary palpus 4-segmented, segments 1–3. progressively longer, segment 4 slightly longer than 3 ( Fig. 3 View FIGURES 1–8 ). Occipital protuberance short ( Fig. 1 View FIGURES 1–8 ). Antenna: pedicel subglobular, slightly smaller than scape; 12 flagellomeres, first and second fused; flagellomeres binodal, with one whorl of looped circumfila on basal node and two on distal node, distal node widened at posterior half; flagellomere ( Fig. 2 View FIGURES 1–8 ) with internode slightly longer than basal node, neck as long as distal node; circumfilar loops on basal node reaching midlength of internode, loops of anterior whorl on distal node reaching midlength of distal node, loops of posterior whorl on distal node reaching 3/4 length of neck.
Thorax. Wing ( Fig. 10 View FIGURES 9–13 ) sparsely covered with narrow scales; R 1 joining C slightly proximad wing midlength; R 5 joining C posteriad wing apex; Rs faded posteriorly, positioned closer to juncture of C with R 1 than arculus; Cu forked; M 3+4 and Cu 2 folds visible. Legs densely covered with narrow scales and sparse setae; tarsal claws ( Figs 4–6 View FIGURES 1–8 ) toothed on forelegs, simple on mid- and hindlegs; empodium distinctly shorter than claws; pulvilli minute.
Abdomen. First through sixth tergites rectangular with irregular posterior row of long setae, few lateral and central setae, covered with scattered scales, and anterior pair of trichoid sensilla; seventh tergite slightly narrower than sixth; eighth tergite much smaller than seventh; second through seventh sternites rectangular, with irregular posterior row of long setae, lateral and central setae slightly denser than those on corresponding tergites, and anterior pair of closely set trichoid sensilla; seventh sternite slightly narrower than sixth; eighth sternite much smaller than seventh. Terminalia ( Figs 7, 8 View FIGURES 1–8 ): gonocoxite elongate, with low, round mediobasal lobe densely covered with setulae; gonostyle long, slender, evenly bowed inwardly, slightly and gradually narrowed beyond base, basal fifth covered with dense microtrichia, remainder with sparse short setae, with large distal tooth; cerci wide, round, much shorter than aedeagus, with few apical setae; hypoproct as long as cerci, rounded lobes divided by narrow U-shaped incision, each lobe with apical seta; aedeagus stout, bottle-shaped, as long as gonocoxite, with two sensory pores distally.
Female ( Figs 9–13 View FIGURES 9–13 , 19 View FIGURES 19–27 ). Wing length 1.4–1.5 mm, width 0.6 mm (n = 10).
Head. Flagellomeres cylindrical, flagellomere ( Fig. 9 View FIGURES 9–13 ) with node slightly constricted at midlength, neck 1/3 length of node, with sparse microtrichia basally, otherwise bare; circumfila appressed, consisting of interconnected two horizontal and two longitudinal bands. Abdomen. Eight tergite and sternite membranous, not sclerotized, with no trichoid sensilla. Terminalia ( Figs 11–13 View FIGURES 9–13 ) short, retractable; cerci large, covered with dense setulae and scattered setae; distally with two of thick, blunt setae; hypoproct half length of cerci, wide and ovoid in dorso-ventral view, covered with setulae, apically with pair of setae ( Fig. 11 View FIGURES 9–13 ).
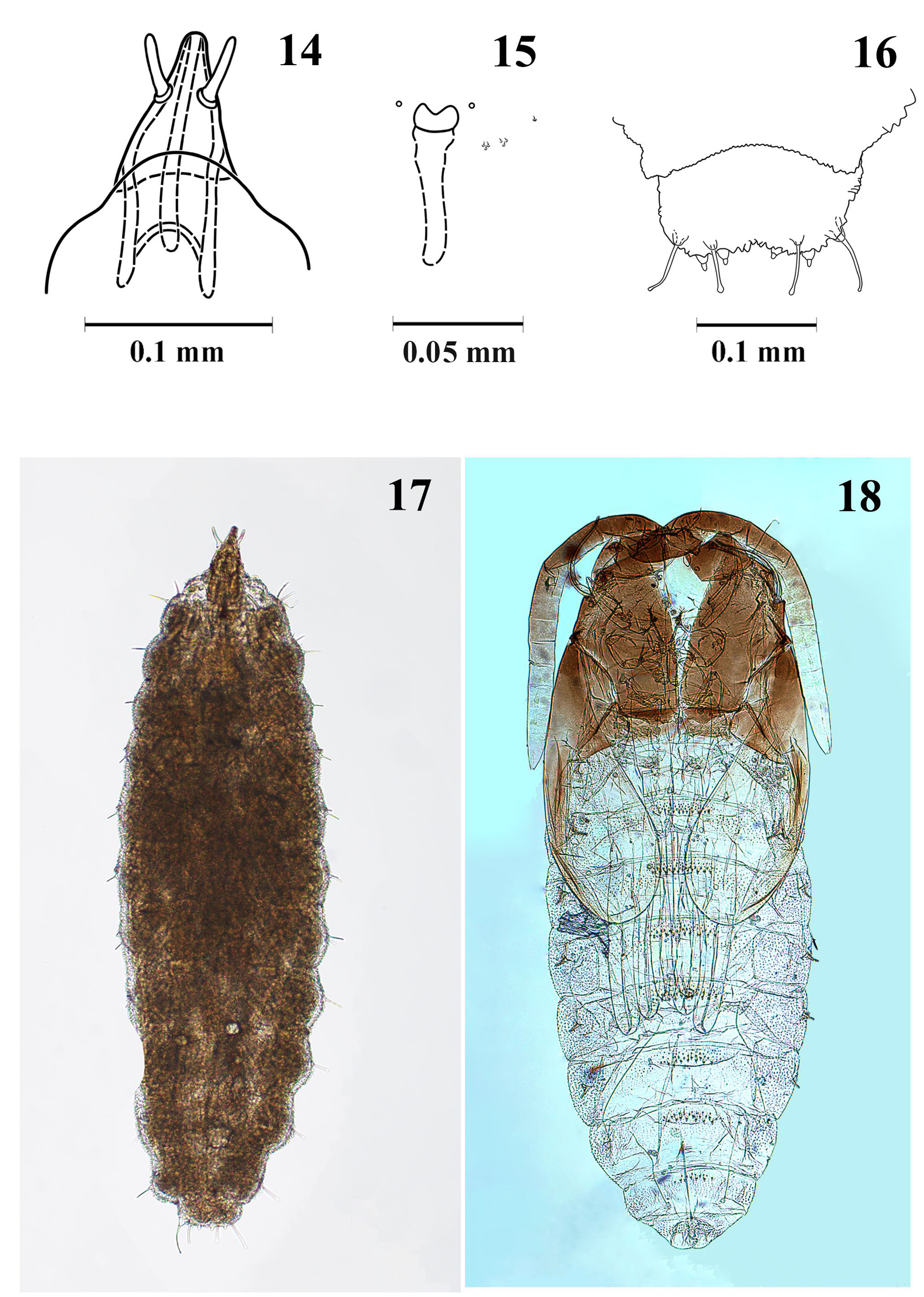
Pupa ( Figs 18 View FIGURES 14–18 , 25–27 View FIGURES 19–27 ). Head, thorax, anterior part of antennae and wing sheaths dark brown, rest pale. When adult already present, wings orangey brown, abdomen orangey brown at early developmental stage ( Fig. 25 View FIGURES 19–27 ) and pinkish white later ( Fig. 26 View FIGURES 19–27 ). Body length 1.2–1.4 mm (n = 5). Antennal base with tiny angular horn; cephalic setae short and thin. Prothoracic spiracle long, about 12 times as long as wide at base, curved, gradually tapering, trachea ending at apex. Abdomen ventrally with extensive fields of small verrucae; dorsally with small anterior fields of large, simple spines; spiracles on abdominal segments 2–6 tube-like ( Fig. 18 View FIGURES 14–18 ).
Larva ( Figs 14–16 View FIGURES 14–18 , 22–24 View FIGURES 19–27 ). Colour orangey red. Length 1.2–1.3 mm (n = 3). Antennae tapered, 4 times as long as wide at base ( Fig. 14 View FIGURES 14–18 ). Sternal spatula ( Fig. 15 View FIGURES 14–18 ) with two rounded to slightly angular anterior teeth, separated by wide, shallow V-shaped incision; lateral papillae consisting of two triplets and one single, all setose. Terminal segment with four pairs of papillae: two pairs short, corniform; two pairs with long, clavate setae, 2/3 length terminal segment ( Fig. 16 View FIGURES 14–18 ).
Egg ( Fig. 21 View FIGURES 19–27 ). Spindle-form, translucent, 0.2–0.3 mm in length.
DNA sequences. The COI sequences of the type population of the new species showed 99.13–100% intrapopulation similarity and an overall 98.99–100% similarity to the sequences of the polyphagous Mycodiplosis sp. 1 collected in Japan and Thailand by Yukawa et al. (2018) ( Tabs 1 View TABLE 1 , 2 View TABLE 2 ). These high levels of similarity suggest that all the mentioned populations compared here (# 1–5 in Tabs 1 View TABLE 1 , 2 View TABLE 2 ) belong to the new species described here. The similarity of the new species to other congeners sequenced by Yukawa et al. (2018) (# 6–8 in Tabs 1 View TABLE 1 , 2 View TABLE 2 ) was substantially lower, ranging from 91.62% to 95.34%. Hence, the intraspecific divergence in COI sequence within the new species was 0–1.01% while the interspecific divergence from M. coniophaga was 6.53–7.68%, from Mycodiplosis sp. feeding on Puccinia sp. on Vitis vinifera in Japan 4.66–4.84%, and from Mycodiplosis sp. feeding on Puccinia sp. on Plumeria rubra in Vietnam 7.87–8.38%.
Additionally, in BOLD, twelve COI sequences of adult Cecidomyiidae , trap-collected 6-vi and 7-vii-2014 at Chittagong, Bangladesh (22.4685°N, 91.7808°E), showed 98.96–100 % similarity to collections #1–5 ( Tabs 1 View TABLE 1 , 2 View TABLE 2 ) of the new species. In BOLD these sequences ( GMBCD 058-15, 090-15, 091-15, 1335-15, 1391-15, 1679-15, 2052- 15, 2381-15, 2527-15, 700-15, 785-15, 1923-15) are accompanied by a photograph of a male ( GMBCD 058-15) and a female ( GMBCD 700-15) taken prior to the DNA extraction. Based on the high similarity of the sequences and the observable morphology of photographed specimens we conclude that these Bangladeshi specimens, assigned to a Barcode Index Number (also known as “ BOLD species”) ACW1917), belong to M. puccinivora . Thus, the intraspecific divergence of the 36 currently published COI sequence of the new species is 0–1.04%. Furthermore, publicly yet unreleased in BOLD, COI sequence belonging to 19 Cecidomyiidae specimens from Chittagong, Bangladesh, one from Negeri Sembilan, Malaysia and one from Queensland, Australia are assigned to ACW1917. The BOLD-generated phylogenetic tree, based on Kimura 2 Parameter distance model, showed the Malaysian and the Australian sequences well within the group of the twelve publicly released Bangladeshi sequences. Once the Malaysian and the Australian sequences are officially released in BOLD, they will constitute new country records for M. puccinivora . Among BOLD sequences, the closest to M. puccinivora was RRMFI 1969-15. COI- 5P (= GenBank KT605292 View Materials ) with an interspecific divergence relative to the new species of 3.94–4.17%. This sequence belongs to sample BIOUG 23315-B10 assigned to a Barcode Index Number AAH3662, a cosmopolitan Cecidomyiidae species with unknown life history. The new species differs from AAH 3662 in the shape of the gonostyle, which is slightly and gradually narrowed beyond base in M. puccinivora ( Figs 7, 8 View FIGURES 1–8 ) as opposed to strongly and abruptly narrowed in AAH3662 (see image of a male sample PCPP 10-0813 in BOLD).
Note. Of the seven sequences of the 28S ribosomal RNA gene fragment provided by Henk et al. (2011) for rust-feeding Mycodiplosis larvae, three have an erroneous entry in the GenBank ( HQ 256896–898) and thus are unavailable, while the four sequences that are correctly entered ( HQ 256899 View Materials –902) show a similarity to each other of 98.62–99.87%. This high level of similarity is comparable to that of the COI gene in M. puccinivora , suggesting that the fungal rusts sampled ( Arthuriomyces peckianus and Uromyces ari-triphylli , both pathogenic on an unidentified plant in USA, and Puccinia malvacearum pathogenic on Alcea rosea (Malvaceae) in Spain) were fed on by a single, polyphagous, cosmopolitan Mycodiplosis species.
Etymology. The specific name puccinivora refers to the fact that the larvae feed on fungal rusts of the class Pucciniomycetes.
Distribution. Mycodiplosis puccinivora is currently known from China, Japan, Thailand, Bangladesh and possibly also from Malaysia and Australia. China: Jinjiang Town, Chengmai County, Hainan Province, 10-20.X.2015, from Maravalia pterocarpi on Dalbergia tonkinensis . Japan: Yamaguchi City, Yamaguchi Prefecture, Honshu, 10.X.2005, from Puccinia coronata on Lolium multiflorum ; Miyakonojo City, Miyazaki Prefecture, Kyushu, 11.X.2005, from Puccinia sp. on Zea mays ; Iriomote Island, Okinawa Prefecture, 11. VI.2008, from Puccinia arachidis on Arachis hypogaea . Thailand: Tombom Sameung, Chiang Mai Province, 16.XI.2005, from Puccinia allii on Allium fistulosum . Bangladesh: Chittagong, trap-collected 6-vi and 7-vii-2014. DNA sequences of specimens from Malaysia and Australia are awaiting public release in BOLD and thus we consider the occurence in these two countries as yet unconfirmed.
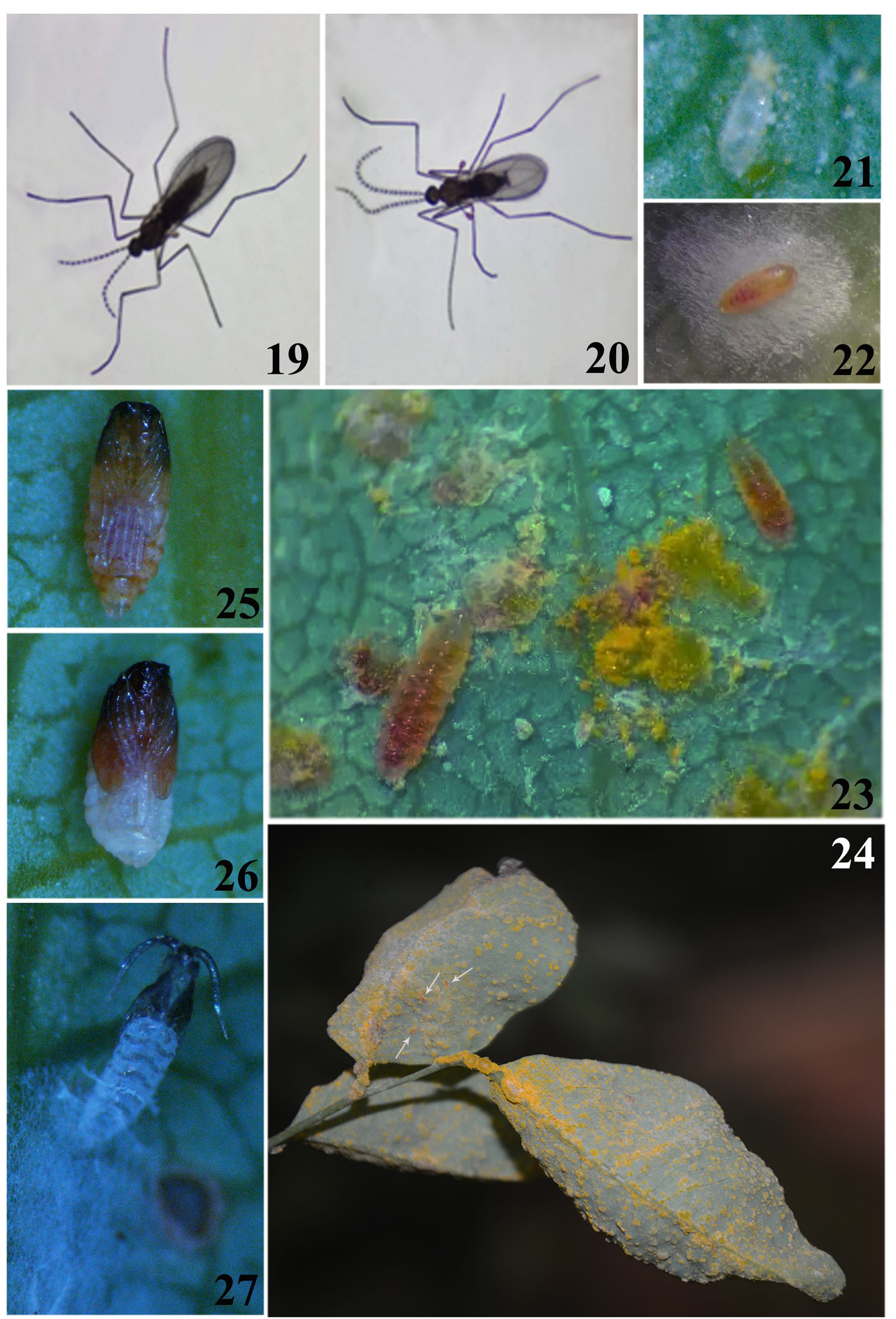
Life history ( Figs 19–27 View FIGURES 19–27 ). On Hainan Island in 2014 and 2015 there were four to five, usually overlapping, generations of M. puccinivora per year with the larva within a cocoon being the overwintering stage. Following copulation, females scattered eggs near fresh, yellow uredinia of the rust ( Fig. 21 View FIGURES 19–27 ). In the laboratory, most adults died within three days after emergence. Following eclosion, the larvae were observed to feed on the uredinia ( Figs 23, 24 View FIGURES 19–27 ). As a consequence of the larval feeding, the yellow uredia turned grey and the uredospores appeared empty, shrunk or ruptured under the microscope. Up to 50 larvae were found on a single rust-infested leaf. The mature larvae of the non-overwintering generations spun loose cocoons on the plant to pupate in ( Fig. 22 View FIGURES 19–27 ), while the larvae of the overwintering generation dropped to the ground to create a cocoon and to pupate in the soil later. If there were no more uredinia available on the leaf, the larvae of the intermediate generations entered the dormant period in advance and spun the cocoon on the plant. Each of the non-overwintering generations spanned about 45 days while the overwintering generation distinctly longer. The new species is currently known to feed on the following rust fungi: Maravalia pterocarpi on sua, Puccinia coronata on annual ryegrass, Puccinia sp. on maize, Puccinia arachidis on peanut and Puccinia allii on spring onion.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |