Cedarina schachti
|
publication ID |
https://doi.org/ 10.5281/zenodo.189977 |
|
DOI |
https://doi.org/10.5281/zenodo.6225856 |
|
persistent identifier |
https://treatment.plazi.org/id/C76C4C39-586B-FFD8-C7DF-E218A5CDFC1B |
|
treatment provided by |
Plazi |
|
scientific name |
Cedarina schachti |
| status |
|
Implications of Cedarina schachti
Classification of Remopleurididae . The Superfamily Remopleuridoidea Hawle and Corda, 1847, includes taxa of late Cambrian and Ordovician age. It has traditionally been divided into a Cambrian-Ordovician Family Richardsonellidae Raymond, 1924 (= Kainellidae Ulrich and Resser, 1930 ; see Ludvigsen and Westrop in Ludvigsen et al., 1989), and an exclusively Ordovician Remopleurididae . Shergold (1980) erected the small subfamily Atratebiinae for three genera, which he regarded as family incertae sedis but of dikelocephaloidean affinities. This was followed by Peng (1992), though Dean (2006) assigned Taishania Sun, 1935, which had been included in Atratebiinae by Shergold (1980), to Pterocephaliidae Kobayashi, 1935 a. Jell (in Jell and Adrain, 2003) assigned the genera grouped as Atratebiinae by Shergold (1980) to Remopleurididae . Shergold (1980, p. 54) characterized the atratebiines as "dikelocephalacean trilobites whose morphology has remopleuridacean aspects."
Affinity of Atratebiinae aside, there has been general agreement on a Remopleuridoidea divided into two families. No modern phylogenetic analysis has been attempted for any component of the group (save for the inclusion of the superfamily itself as a terminal taxon in the early cladistic analysis of Fortey and Chatterton [1988]). The monophyly of the younger and more derived Remopleurididae seems highly likely as it is supported by a suite of characters such as large eyes, loss of the preglabellar field and fixigenal area, a distinctive librigena with a strong genal notch, a general streamlining of the body, and a distinctive pygidial morphology. These features are possibly associated with a nektobenthic life habit ( Fortey, 1985, pp. 227– 228). Richardsonellidae , however, is almost certainly rendered paraphyletic by traditional Remopleurididae and as such should not be recognized. It is possible that components of traditional Richardsonellidae may prove monophyletic, but this can only be assessed with careful, modern phylogenetic analysis. For these reasons, Jell and Adrain (2003) did not recognize separate families, referring all of the remopleuridoideans to a single Family Remopleurididae , and this course is followed herein.
The Cambrian "richardsonellids" have been further split by the proposal of several smaller family-group taxa which are (apparently) obvious components of the broader group and the recognition of which would likely create paraphyly. These are: Apatokephalinae Kobayashi, 1953 (which Shergold [1975] advocated classifying with the traditional remopleuridids, as opposed to the richardsonellids/kainellids); Loshanellidae Lu, 1975; and Apatokephalopsidae Zhou and Zhang, 1978. All are regarded here as synonyms of Remopleurididae .
Fortey and Chatterton (1988) also assigned the families Bohemillidae Barrande, 1872 , and Opipeuteridae Fortey, 1974 , to Remopleuridoidea. Bohemillids have been interpreted as adapted for a pelagic life habit and their morphology is similar to that of other such groups, including derived remopleuridids. Fortey and Owens (1987, pp. 128–129) acknowledged the difficulties in inferring higher relationship of such derived forms, but nevertheless suggested that they were remopleuridoideans, based on the presence of a mid-glabellar lateral bulge, librigenal morphology, very narrow cranidial anterior border, and spinose pygidium. The narrow border and librigenal morphology are not similar to plesiomorphic remopleuridids ("richardsonellids"), but rather to highly derived Ordovician forms. Remopleuridids have a thorax of either 12 or 11 segments, with a median axial spine on the eighth. This is true even of the most derived forms such as Hypodicranotus Whittington, 1952 , (see Ludvigsen and Chatterton, 1991). The thorax of Fenniops sabulon ( Fortey and Owens, 1987), the only bohemillid species for which thoracic information is available, apparently had only seven segments and lacked a spine ( Fortey and Owens, 1987, fig. 23), though no specimens are known with the pygidium articulated, so the segment count remains provisional. If this morphology is confirmed, it is very unlikely that bohemillids are remopleuridoideans. They may be derived cyclopygoidean asaphides.
Opipeuteridae Fortey, 1974 , was erected for Opipeuterella Fortey, 2005 (pro Opipeuter Fortey, 1974 , preoccupied). Fortey argued repeatedly (1974, 1975, 1979, 1980) that the taxon is a remopleuridoidean, but Laurie and Shergold (1996) demonstrated that it is ingroup Telephinidae Marek, 1952 View in CoL .
The earliest Laurentian taxa which have been considered remopleuridoideans are of Sunwaptan age, and almost all of the global Cambrian diversity of the family is of Sunwaptan equivalent age. Jell (in Jell and Adrain, 2003) reported that the type species of Tostonia Walcott, 1924 , T. iole ( Walcott, 1884) , was from the Hamburg Limestone which is of Marjuman age. Jell assigned the genus to Eurekiidae View in CoL , an otherwise exclusively Sunwaptan taxon. Previous workers (e.g., Wilson, 1954; Shergold, 1972) had considered the genus to be a richardsonelline remopleuridoidean. The specimens assigned to T. iole by Walcott (1925, pl. 18, figs. 10–14) are actually from the Sunwaptan Windfall Formation in the Eureka district of Nevada. Walcott's cranidia, one of which is the holotype, are likely conspecific, but they are almost certainly misassociated with his assigned pygidia. The latter represent a species of the remopleuridoidean Naustia Ludvigsen, 1982. Abundant silicified material of what is likely the same species has been collected from the Bullwhacker Member of the Windfall Formation at Barton Canyon, Cherry Creek Range, Nevada (J.M.A. and S.R.W., unpublished data).
Morphology of Cedarina schachti . The thoracic morphology of members of Remopleurididae is remarkably stable. Plesiomorphic taxa ("richardsonellines") have a thorax of 12 segments. The segments are of relatively simple morphology, with a prominent transverse pleural furrow. The pleural tips are free, and are turned posteriorly into tapering, blunt pleural spines. All species for which information is available have a single median axial spine located on the eighth segment. Examples include Pseudokainella keideli Harrington, 1938 ( Harrington and Leanza, 1957, figs 51, 52.6), Parakainella lata ( Kobayashi, 1935b) ( Harrington and Leanza, 1957, figs. 53, 54.3), Parakainella pustulosa Harrington and Leanza (1957, fig. 55), Apatokephalus exiguus Harrington and Leanza (1957, figs. 57, 58), Apatokephalops diggerensis ( Jell, 1985, pl. 25, figs. 10, 11), and Apatokephalus latilimbatus Peng (1990, pl. 11, figs. 4, 6). Highly derived Ordovician taxa with an inferred pelagic life habit ("remopleuridines") reduced the number of segments to 11, but retained a median spine on the eighth (e.g., Remopleurides eximius Whittington, 1959 , pl. 14, and other examples in the same work).
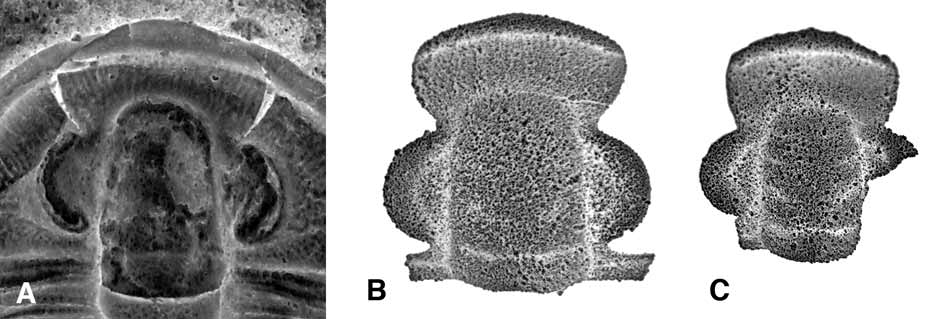
Cedarina has been regarded as a cedariid by all authors who have commented on it since it was proposed ( Lochman and Duncan, 1944; Lochman, 1950; Palmer, 1955; Deland and Shaw, 1956; Lochman and Hu, 1962; Rasetti, 1965; Hu, 1971, 1983; Hu and Li, 1971; Pratt, 1992; Stitt, 1998; Jell and Adrain, 2003). Species of Cedarina have been known only from cephalic and pygidial data. To this point, however, no information on the morphology of the thorax has been available. In contrast, the thoracic morphology of species of Cedaria Walcott, 1924 , is well understood. These species are nearly isopygous, with a thorax of seven segments, none of which bear an axial spine (e.g., Cedaria minor [ Walcott, 1916b, pl. 61, figs. 3, 3a, 3b], C. prolifica Walcott, 1924 [ Walcott, 1925, pl. 17, fig. 18; Robison, 1988, fig. 14.14]). The cedariid genus Carinamala Palmer, 1962 View in CoL , is presently known only from its type species, C. longispina Palmer, 1962 View in CoL . However, J.M.A. and S.R.W. have discovered articulated material of a new species of Carinamala View in CoL from the Lincoln Peak Formation in the Schell Creek Range, eastern Nevada (the same locality as for Cedaria clevensis n. sp.), and this taxon, too, is isopygous and has seven thoracic segments. Segments four through seven have axial nodes or short spines, but there is no long median spine. New finds in the Weeks Formation of western Utah provide the first knowledge of the thorax of a member of Cedarina . The thorax of C. schachti n. sp. is not closely similar to that of Cedaria or Carinamala View in CoL , but rather is extremely similar to the plesiomorphic condition of remopleuridids.
The thorax of C. schachti has ten segments ( Figs 6 View FIGURE 6 , 7 View FIGURE 7 ). The segments have a simple morphology ( Fig. 5 View FIGURE 5 ), featuring a well impressed pleural furrow with a transverse course and distal pleural regions that are tapered into blunt, posteriorly directed pleural spines. The eighth segment bears a long median axial spine. None of the other segments bear any axial nodes or spines. This morphology is all but identical to that seen in the articulated "richardsonellines" cited above. The only difference is that the remopleuridids have two additional thoracic segments posterior to the spine-bearing eighth.
Further, the cranidial morphology of C. schachti is highly reminiscent of that of the Sunwaptan remopleuridid Naustia Ludvigsen, 1982 ( Fig. 4 View FIGURE 4 ). We have discovered silicified material of an undescribed species of Naustia from the Windfall Formation in the Cherry Creek Range, eastern Nevada (see Adrain and Westrop, 2004). The silicified specimens record an ontogenetic sequence. Smaller cranidia ( Fig. 4 View FIGURE 4 B) are extremely similar to holaspid cranidia of C. schachti ( Fig. 4 View FIGURE 4 A) in all cranidial dimensions, the presence of radiating caecal trunks on the frontal areas, and the possession of large palpebral lobes with a prominent palpebral furrow. In C. schachti the anterior and posterior margins of the palpebral lobe are separated from the glabella by narrow strips of fixigena. The condition in remopleuridids is for the palpebral lobe to abut the glabella anteriorly and posteriorly. However, very small cranidia of Naustia have interocular fixigenae in these positions ( Fig. 4 View FIGURE 4 C), just as in C. schachti .
The only significant evolutionary steps required to transform from a Cedarina morphology to that of plesiomorphic remopleuridids is the release of an additional two thoracic segments and the loss of the rostral plate and development of a median connective suture. The pygidium of Naustia ( Ludvigsen, 1982, pl. 64, figs.
Q-S) is not tagmatized, but rather is a fused collection of seven unreleased segments. This contrasts strongly with the reduced number of segments (four or less) typical of remopleuridids. If Naustia is a basal member of the clade, it suggests derivation via increased segment generation, which is exactly the mechanism required to transform from the Cedarina morphology. The number of thoracic segments in the Naustia thorax is unknown, but the rear portion of the pygidium is closely similar to the pygidium of other Sunwaptan richardsonellines such as Elkanaspis Ludvigsen (1982, pl. 64, figs H-K). This raises the intriguing and testable possibility that Naustia may represent a transitional form with only ten thoracic segments, but with the remaining two segments generated but still incorporated into the holaspid pygidium. Transition from Naustia to more typical "richardsonellines" would then require only the release of the anterior two pygidial segments.
A further similarity between cedariids and remopleuridids is that they are two of the groups known to develop prominent pits in the anterior border furrow (see, e.g., Cedaria prolifica Walcott - Palmer, 1962, pl. 3, fig. 14). The significance of this pitting has not been fully appreciated and will be dealt with elsewhere, but unpublished evidence suggests that it may be a high level synapomorphy of a broader trilobite group.
The morphological case for a relationship between cedariids and remopleuridids seems very strong. There is, however, a significant gap in the fossil record, spanning the entire Steptoean Stage in Laurentia and equivalents on other paleocontinents, from which no obvious members of the clade are known. The only putative remopleuridid taxon that is older than the equivalent of the Laurentian Sunwaptan Stage is Oculeus Poletaeva and Romanenko, 1970 , which is known from the Mayan (Marjuman equivalent; Lejopyge laevigata Zone ) and Sakian (Steptoean equivalent) stages of the Siberian Platform and the Gorny Altay. The status of Oculeus as a remopleuridid, however, is dubious. The type species ( O. parvulus Romanenko in Poletaeva and Romanenko, 1970, pl. 11, figs. 1–5) is known from cranidia and a librigena. The species has extremely large eyes and huge palpebral lobes. The large eye area combined with a narrow frontal region are at least superficially similar to remopleuridids. However, the librigena is very different from that of early remopleuridids, with a very forwardly placed genal spine. It is unclear from the poorly preserved internal molds whether the glabella shows the lateral expansion characteristic ( Fortey and Chatterton, 1988, p. 201) of remopleuridids, but deeply incised glabellar furrows are present and in contact with the axial furrow. None of the other assigned species ( O. fidus Poletaeva in Poletaeva and Romanenko, 1970; O. parvulus Egorova in Egorova et al., 1982 [a homonym of the type species]; O. clivosus Gogin and Pegel in Pegel and Gogin, 1995) are known from sclerites other than cranidia. Clarification of the affinity of Oculeus must await more information, particularly on thoracic and pygidial morphology.
Implications for higher classification. If the hypothesis of relationship outlined above is corroborated, there are two major implications. First, obviously Cedariidae is rendered paraphyletic by Remopleurididae . A likely scenario is that genera of " Cedariidae " become successive sister taxa at the base of the remopleuridoidean tree, with a cedariform suture as a basal apomorphy. Cedaria and Carinamala may form a clade, depending on whether their shared seven-segment isopygous morphology is apomorphic. They may collectively be sister to Cedarina + Remopleurididae .
Second, Remopleurididae have been assigned by Fortey and Chatterton (1988) to a Suborder Asaphina , united with families such as Asaphidae Burmeister, 1843 , Nileidae Angelin, 1854 , and Ceratopygidae Linnarsson, 1869 . These authors considered ( Fortey and Chatterton, 1988, text-fig. 1) the key synapomorphy linking remopleuridids to Asaphina to be possession of an "asaphoid protaspis" - a spherical to ovoid larva with an enrolled doublure. Groups with such a larval type underwent metamorphosis after the protaspid period to more adult-like developmental stages.
Fortey and Chatterton's (1988) paper marked one of the first serious attempts to formulate testable and explicit higher level hypotheses of trilobite phylogeny and was a major advance in beginning to unravel the problem of trilobite "cryptogenesis" ( Stubblefield, 1959; Whittington, 1981) - the obscure origins and sister group relationships of most post-Cambrian clades. Nevertheless, recent discoveries suggest that remopleuridids are probably not Asaphina , and that similarities of the larvae are probably secondarily acquired.
The examples of asaphoid protaspides in remopleuridids given by Fortey and Chatterton (1988, p. 186, pl. 17, figs. 1–6) were drawn from Whittington's (1959) work on silicified Upper Ordovician species from Virginia. These are some of the latest and most derived members of the group, adults of which were highly specialized for a nektobenthic life habit. Tellingly, Fortey and Chatterton (1988, p. 182) noted that remopleuridids were the exception to a post-larval metamorphosis; unlike true Asaphina , in these most derived taxa the metamorphosis occurs during the meraspid period. As outlined above, these derived pelagic forms did not appear until the Ordovician. The plesiomorphic members of Remopleurididae - the "richardsonellines" - have none of the specialized pelagic adaptations of the taxa whose larvae were figured by Fortey and Chatterton (1988). Obviously, the larval morphology relevant to higher affinity of the family is that of the more basal members of the group.
Recent discoveries indicate that the basal condition for remopleuridid larvae is not asaphoid. Park and Choi (2008) demonstrated that larvae of the Korean Furongian "richardsonelline" Haniwa sosanensis Kobayashi, 1933 , are flattened, lack an enrolled doublure, and are conventionally adult-like. J.M.A. and S.R.W. have isolated silicified larvae of a Sunwaptan (Furongian) species of Elkanaspis from the Windfall Formation in eastern Nevada. These are exceedingly similar to those of Haniwa . This suggests, as is typical of other groups, that remopleuridids had a conserved larval morphology shared widely across the group. Further, it appears that the radical morphological changes for a pelagic life habit which took place in Ordovician "remopleuridines" also involved radical changes in the larvae, from an adult-like to an asaphoid form.
Hence, we consider that Remopleurididae is not a component of the Order Asaphida , although we support Fortey and Chatterton's (1988) approach and other general conclusions. Remopleuridids are more compellingly similar to cedariids and other taxa with pits in the anterior border furrow, but full exposition of this case is beyond the scope of the present work.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cedarina schachti
| Adrain, Jonathan M., Peters, Shanan E. & Westrop, Stephen R. 2009 |
Opipeuteridae
| Fortey 1974 |
Opipeuter
| Fortey 1974 |
Carinamala
| Palmer 1962 |
C. longispina
| Palmer 1962 |
Telephinidae
| Marek 1952 |
Tostonia
| Walcott 1924 |
Cedaria
| Walcott 1924 |
C. prolifica
| Walcott 1924 |
T. iole (
| Walcott 1884 |