Sagmatostreptus strongylopygus ( Attems, 1950 ) Attems, 1950
|
publication ID |
https://doi.org/ 10.5281/zenodo.201438 |
|
DOI |
https://doi.org/10.5281/zenodo.6182447 |
|
persistent identifier |
https://treatment.plazi.org/id/C91287FC-2A67-FFB9-ECE9-27DAFD36A6C6 |
|
treatment provided by |
Plazi |
|
scientific name |
Sagmatostreptus strongylopygus ( Attems, 1950 ) |
| status |
comb. nov. |
Sagmatostreptus strongylopygus ( Attems, 1950) View in CoL , new combination
Figures 1–16 View FIGURE 1 View FIGURES 2 – 6 View FIGURES 8 – 11 View FIGURES 12 – 16 .
Spirostreptus strongylopygus Attems, 1950 View in CoL , Ann. Naturh. Mus. Wien, 57: 198, figs. 1–4. Male holotype (NMW) labeled only “W-Usambara”
Spirostreptus strongylopygus: Krabbe, 1982 View in CoL , Abh. Naturw. Ver. Hamburg, NS 23: 213. — Hoffman, 1993, Biogeography and ecology of rain forests in eastern Africa, p. 106.
Name. The species name is a combination of two Greek words referring to the “rounded” (convex) form of the paraprocts.
Diagnosis. With the characters of the genus. The striking coloration in life is unique among known species of the entire order Spirostreptida .
Description. Male from Kihuhwi-Sigi Forest Reserve, body with 51 podous segments Specimen fragmented into six pieces, reconstructed length approximates 120 mm. Maximum metazonal diameter 10.0 mm, prozonal 9.4 mm, over much of body length. Coloration altered by preservation, currently nondescript gray with caudal margins of metazona maroon ( Fig. 1 View FIGURE 1 shows color of well-preserved specimen 25 years in alcohol).
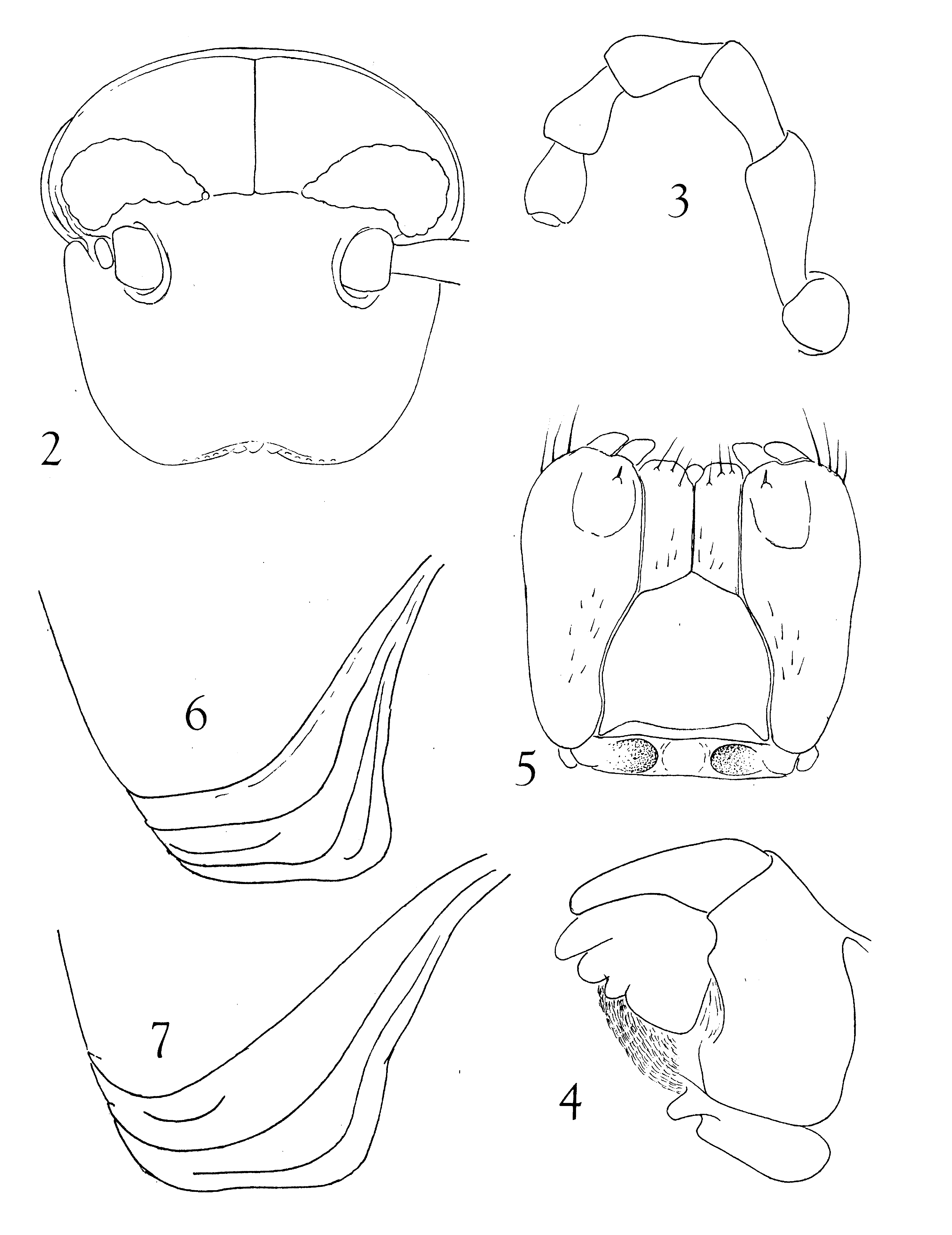
Head ( Fig. 2 View FIGURES 2 – 6 ) moderately convex, smooth, epicranial and interocellarial sutures evident; 10 labral pits, four clypeal punctures; ocellaria elongate subtriangular, ocelli disposed in 6 or 7 horizontal rows (at outer ends not easily distinguishable) as follows: 11-10-8-7 -5-3-2-1 = 46 on one side, 11- 11-10-7-5 -2 = 46 on the other; outer ventral ends of series obscure owing to curvature into postantennal fossa and reduction in size and pigmentation, ocelli nearest collum by far the largest and best defined. Interocellarial space 1.7 mm, interantennal space 2.7 mm.
Antennae ( Fig. 3 View FIGURES 2 – 6 ) ca. 7 mm, socket with fine margin along dorso-posterior edge., Mandible ( Fig. 4 View FIGURES 2 – 6 ): psectromere with three marginal lobes and 11 pectinate lamellae.
Gnathochiliarum ( Fig. 5 View FIGURES 2 – 6 ): sides of mentum convex near midlength, two small fields of short setae distally; stipes with dispersed setae medially and prominent distal stipital spur. Prebasilar sclerite transverse and complete, but thin and subtransparent, with two deep paramedian fossae between which notably convex.
Collum ( Fig. 6 View FIGURES 2 – 6 ) only slightly lobed anterolaterally, with three major striations, all extending dorsad to level of ocellaria.
Metazona of body rings slightly elevated above prozona, smooth and polished dorsally, Lower sides with 15– 20 longitudinal striae, surface below each stria slightly elevated and forming slight projection at caudal margin, most distinctly so on anteriormost rings. Ozopores small, from 5th ring, placed at midlength of metazona. Stricture shallow, without posterior definition, merging into metazonal surface; anterior margin present as sharp ridge defining edge of prozonum. Prozonal surface with fine irregular annular transverse striations, often in the form of stretched meshwork. Surface of prosternum smooth, stigmata small and indistinct.
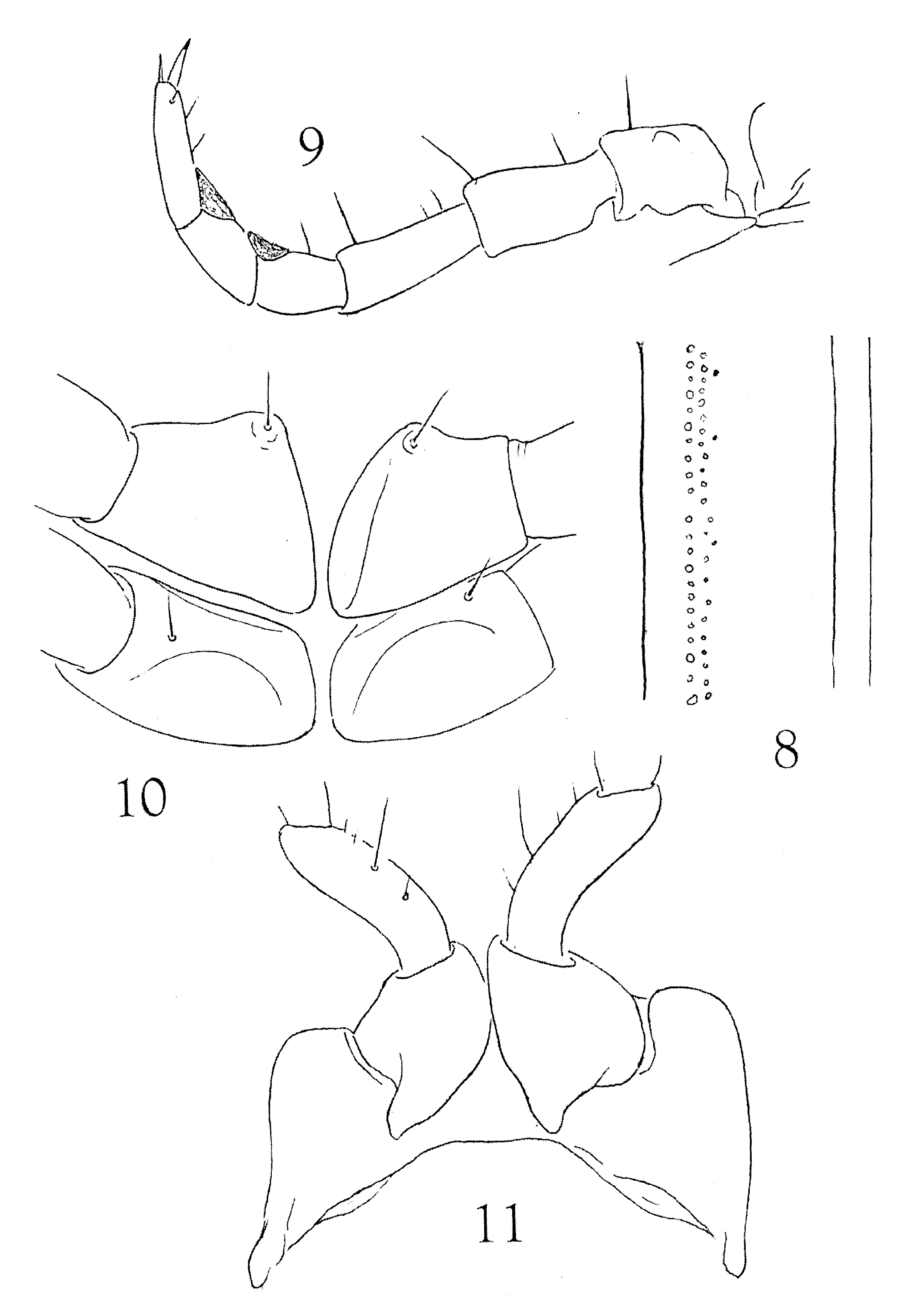
Endozona with poorly-defined small sigilla typically in two rows ( Fig. 8 View FIGURES 8 – 11 ). Terminal ring with transverse caudal edge, not produced into median epiproct. Paraprocts convex, smooth, with only indistinct indication of marginal thickening, no submarginal fossa present, therefore no true labium formed. Hypoproct narrowly transverse, of typical spirostreptid form.
Legs ( Fig. 9 View FIGURES 8 – 11 ) relatively long and slender (ca. 7.5 mm at midbody) distal three podomeres visible beyond sides of body in dorsal aspect, membranous pads present on postfemora and tibiae; prefemora long, with nearly straight dorsal profile; podomeres sparsely setose on ventral surface only. Procoxae slender, compressed, metacoxae larger, subtriangular in profile, contiguous along the flattened medial surface ( Fig. 10 View FIGURES 8 – 11 ).
1st pair of legs ( Fig. 11 View FIGURES 8 – 11 ) of typical spirostreptid form, anterior surface of coxae without evident setae.
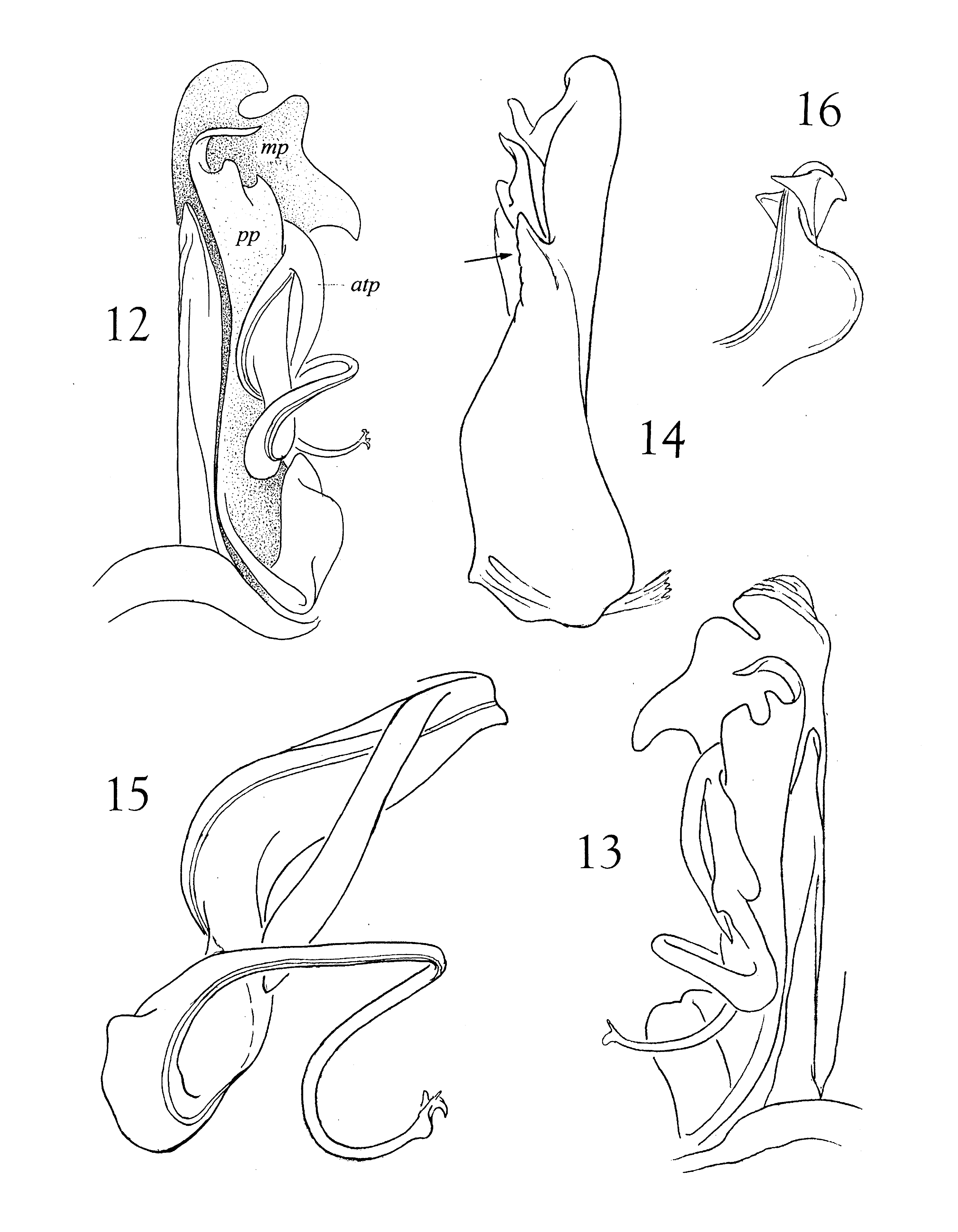
Gonopods ( Figs. 12–16 View FIGURES 12 – 16 ): mesosternum transverse, not medially produced; both proplica and metaplica with three apical projections; medial margin of metaplica produced into an acute projection. Telopodite placed on anterior side of gonopod, antetorsal process long and slender, originating near arculus. Torsate region extended, comprising over half of telopodite length ( Fig. 15 View FIGURES 12 – 16 ), distal third abruptly more slender and sinuously recurved, apex with a large rounded lobe and two smaller subtriangular projections ( Fig. 16 View FIGURES 12 – 16 ).
Female (Mount Lutindi): Substantially larger than male, maximum diameter 12.0 mm, body with 51 podous body rings. Peripheral characters similar to those of male; lateral end of collum (Fig, 7) similar to that of male but anterior corner more rounded.
Variation. Adults and subadults with 51–53 podous body rings. Diameter of adults 9.4–10.0 mm (males), 10.8–l 2.4 mm (females). Most specimens with a very characteristic color pattern consisting of 6–7 blackish dorsal “saddles” each covering ca 3 consecutive body rings and separated by ca 2 marked rings. “Saddles” triangular in lateral view, extending farthest ventrad posteriorly.
Distribution. Northeastern Tanzania (Usambara, Nguru, and Uluguru mountains) and Southeast Kenya (Map 1).
Material examined (sexes indicated as male/female). KENYA: Coast Province: Shimba Hills near Kwale, SW of Mombassa, 23 June 1970, R. Jacobson, H. Enghoff ( ZMUC 1/0).
TANZANIA: Morogoro Region and District: Kasanga Forest Reserve, South Uluguru Mountains, 7.10S, 37.45E, SW of Matombo, 27 July 2000, N. Doggart ( ZMUC 1/1). Nguru Mountains, NNW of Kwadihombo, without further data, K. M. Howell ( VMNH 1/0), Nguru Mountains, 600 m., September 1982, J. Kielland ( VMNH 1/0). Kanga Mountain. 1600 m., ca. 30 km NE of Kwadihombo, 17 November 1983, J.Kielland ( VMNH 0/2).
Tanga Region: East Usambara Mountains, Luheza and Korogwe Districts: Kambai Forest Reserve, 4.59S, 38.41E,?date, Frontier team ( VMNH 1/0). Mount Lutindi Forest reserve, 4.54S, 38.73E, 23 August 1986, K. M. Howell ( VMNH 1/1). Kihuhwi-Sigi Forest Reserve, 5.20S, 38.6E, 15–30 March 1981, S. M. Stuart ( VMNH 1/1). Handeni Hill Forest Reserve, 5.5S, 38.62E, Jan.–Mch. 1981, Frontier team ( VMNH 1/1). Kwamkoro-Kwamsambia Forest Reserve, 5.07 S, 38.38E 1999, Frontier team ( VMNH?/?). Mtai, Masimbi River, 11 August 1990, N. Cordeiro ( VMNH 1/1). Amani West Forest Reserve, 26 July 1774, I. B. & H. Enghoff ( ZMUC 1 juv), same locality, 27 December 1981, K. M. Howell ( VMNH 1/0). Amani, Sigi river, 500 m, 30 July 1974, 30 July 1974, I.B. & H. Enghoff ( ZMC 0/1). Amani, 1000 m., January 1976, O. Lumholdt & E. Wederkinch ( ZMC 1/0); same locality, 17 November 1993, L. Sørensen ( ZMUC 1/0), same locality, 27 October–11 November 1993, C. Griswold, N. Scharff, D. Ubick ( ZMUC 1 juv,; same locality, 11 April 1985, T. G. Nielsen ( ZMUC 0/2, 2 juv.); Amani, Mbomole Hill, 1000 m, 5–8 November 2001, C. Griswold, N. Scharff, D. Ubick ( ZMUC 2 juv), same locality, Nilo Forest Reserve, 920 m, 1 January 2001, Frontier Tanzania ( ZMUC 1/0). Kwamgumi Forest Reserve, 4.57S, 38.44E, 15 July 1995, L. Sørenson ( ZMC 0/1); same locality, 20 July 1995, L. Sorenson ( ZMC “several”; same locality and date, S. H. McKamey et al. ( ZMC 0/1).
Coast Region: Kisarawe District:: Pugu Forest Reserve, “late August”, 1982, Jan Kielland ( VMNH 1/0).
Notes on postembryonic development. Due to the unique color pattern, juveniles of this species can be identified. Many of the juveniles we have examined lack apodous rings in front of the telson and are therefore in the epimorphic phase of their postembryonic development (cf. Enghoff et al. 1993); they differ from adults in body size and lack of developed gonopods or vulvae. Nine juveniles with apodous rings (‘anamorphic specimens’) were found. These include one with 29 podous + 4 apodous rings and 4 vertical rows of ocelli and four with 33–34 podous + 5 apodous rings and 5 vertical rows of ocelli. The remaining anamorphic specimens had 45+3, 49+3, 50+1 and 51+2 body rings and apparently 11 or more vertical rows of ocelli which is highly unusual. In those other species of Spirostreptida which have been studied, one row of ocelli seems to be added at each moult, as is normal in juliformian millipeds ( Enghoff et al 1993). But in S. strongylopygus , the growth of the ocellarium appears to “run wild” between the stadium with 33–34+5 rings (5 eyerows) and the stadium with 45+3 rings (11 or more rows). Such a pattern has not been seen in any other spirostreptid and may be correlated with the large eyes and noncryptic habits of S. strongylopygus .
Biological notes. The only available information on the habits of S. strongylopygus is in an unpublished report by Enghoff & Enghoff (1976). The specimens observed by these authors were light brown in life, with conspicuous dark “saddles”, like the specimen shown in Fig.1 View FIGURE 1 . One male was found in a just felled tree together with Dendrostreptus macracanthus ( Attems, 1914) , one female was found in the top of a Costus plant, 1.5 m about ground, and one juvenile was found in decaying wood. Enghoff & Enghoff compared S. strongylopygus with the arboreal D. macracanthus (cf. Hoffman & Howell, 1983): both species are long-legged, have relatively large eyes and short, curved claws, and both secrete copiously from the ozadenes. All these traits are probably correlated with the relatively conspicuous habits of these two species, and the distinctive coloration of S. strongylopygus most likely has an aposematic function.
A female from Mount Lutindi collected 23 August contained numerous shelled eggs with an average diameter of 2.4 mm, probably ready for oviposition.
Affinities.In the present state of our knowledge of African spirostreptids, it seems futile to hazard any speculations on possible relationships of this taxon.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sagmatostreptus strongylopygus ( Attems, 1950 )
| Hoffman, Richard L. & Enghoff, Henrik 2011 |
Spirostreptus strongylopygus:
| Krabbe 1982 |
Spirostreptus strongylopygus
| Attems 1950 |