Haliclona (Rhizoniera) fugidia, Muricy, Guilherme, Esteves, Eduardo L., Monteiro, Leandro C., Rodrigues, Beatriz Roma & Albano, Rodolpho M., 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3925.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:F02FAC72-AD74-496D-9811-B8B4313A783E |
|
DOI |
https://doi.org/10.5281/zenodo.5618318 |
|
persistent identifier |
https://treatment.plazi.org/id/CD0A878E-3D2D-8E65-FF29-7979FD88F82D |
|
treatment provided by |
Plazi |
|
scientific name |
Haliclona (Rhizoniera) fugidia |
| status |
sp. nov. |
Haliclona (Rhizoniera) fugidia View in CoL sp. nov.
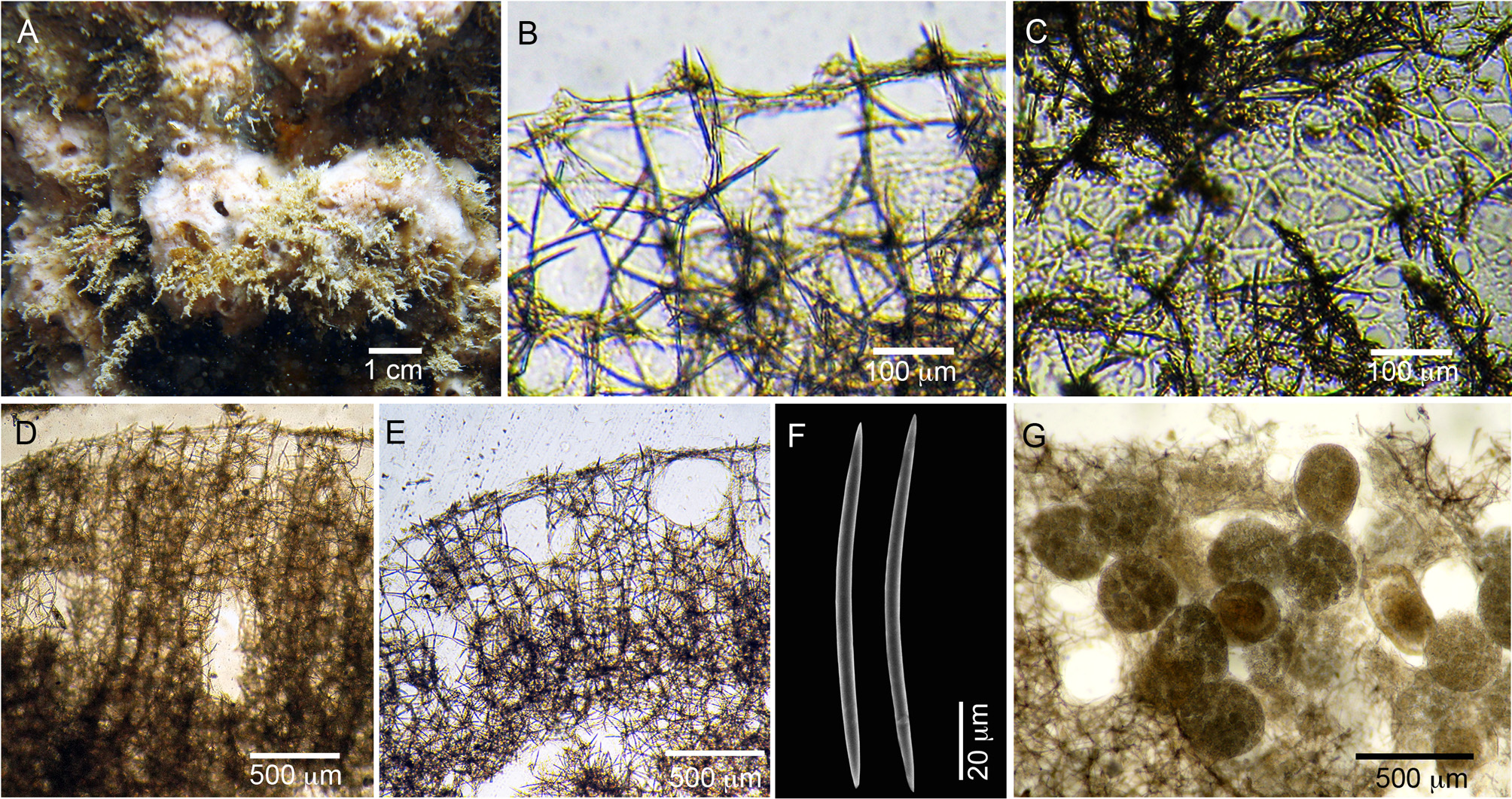
( Figure 2 View FIGURE 2 ; Table 1 View TABLE 1 )
Diagnosis. Haliclona (Rhizoniera) brownish-pink to salmon in color, thickly encrusting, without oscular tubes and without tangential ectosomal reticulation. The ectosomal skeleton is absent and the surface is thin, translucent and pierced by abundant pores, clearly visible in LM. The choanosomal skeleton is a uni- to paucispicular, ladder-like reticulation of oxeas with low spongin, more organized near the sponge surface and denser and more disorganized, sub-halichondrioid in the interior of the sponge. Oxeas fusiform, acerate, 86–124 µm in length. Microscleres absent.
Material examined. Holotype: MNRJ 16846, Vermelha Beach, Rio de Janeiro, 4 m depth, coll. Jessica Pinho & Guilherme Muricy, July 7, 2013. Paratype: MNRJ 16860-B, collection data as for the holotype.
Description ( Fig. 2 View FIGURE 2 A). Shape thickly encrusting to massive, irregular, 3‒8 cm wide by 1‒2 cm thick. Color brownish pink to light salmon in vivo, becoming cream to light brown after fixation. Oscules circular, randomly dispersed, with slightly elevated rims, 1‒3 mm in diameter. Surface irregular, uneven, with a few superficial channels. Consistency compressible, slightly elastic.
Skeleton: Ectosome without tangential ectosomal reticulation. The ectosomal skeleton is absent and the surface is lightly reinforced by spongin, with dispersed debris and only few oxeas at the extremities of the choanosomal primary tracts, which slightly surpass the surface ( Fig. 2 View FIGURE 2 B–C). Pores abundant, closely spaced, clearly visible on the surface, 20‒60 µm in diameter ( Fig. 2 View FIGURE 2 C). Subectosomal and choanosomal lacunae common. Choanosomal skeleton formed by a ladder-like reticulation of uni- to paucispicular tracts of oxeas, connected by irregular unispicular secondary lines, forming irregular or rectangular meshes 60‒150 µm wide ( Fig. 2 View FIGURE 2 B, D‒E). The amount of spongin is low and there is no fibre development in both primary and secondary lines. The reticulation is more organized and ladder-like near the surface and becomes denser and more disorganized towards the choanosome ( Fig. 2 View FIGURE 2 D–E).
Spicules: oxeas smooth, fusiform, slightly curved, with acerate tips: 78.2–105.2 (± 11.0)–126.1 µm in length by 1.3–3.6 (± 0.9)–5.5 µm in width ( Table 1 View TABLE 1 , Fig. 2 View FIGURE 2 F).
Ecology. Haliclona (Rhiz.) fugidia sp. nov. appears to be very rare in Rio de Janeiro, being found so far only at Vermelha Beach, on vertical surfaces near the sandy bottom between 4‒8 m depth. It is very difficult to find, camouflaged near the rocky/sand interface and covered with sediment and epibionts. It can be easily mistaken underwater for Pachychalina alcaloidifera Pinheiro et al., 2005 , which it resembles externally.
Reproduction. Spherical to ovoid embryos 200‒430 µm in diameter were found in the two specimens, both collected in July 2013 ( Fig. 2 View FIGURE 2 G).
Distribution. Provisionally endemic to Rio de Janeiro, SE Brazil, where it is known from a single location (Vermelha Beach).
Etymology. The name fugidia comes from Latin fugitīvu, meaning "fugitive", "elusive", due to the great difficulty in finding the species in the field.
Taxonomic remarks. Haliclona is one of the most diverse genera of Porifera, with over 420 currently accepted species (van Soest et al., 2014). It is divided in six subgenera ( Gellius , Halichoclona , Haliclona , Reniera , Rhizoniera , and Soestella ; de Weerdt, 2002), but 218 species are not allocated to any subgenus in the World Porifera Database so far (WPD; van Soest et al., 2014, accessed in December 1, 2014). It is challenging to classify our new species from Rio de Janeiro into these subgenera, because its skeleton does not fit precisely in any of them. It clearly differs from subgenera H. ( Reniera ) and H. ( Halichoclona ), which have (sub)isotropic choanosomal skeletons without well-defined primary lines (de Weerdt, 2000, 2002), and from the subgenus H. ( Soestella ), in which the choanosomal skeleton is sub-anisotropic, with irregular, ill-defined choanosomal primary lines and paucispicular secondary lines, and there is a tendency to form rounded meshes in the ectosome (de Weerdt, 2002).
The new species is closer to the subgenera H. ( Haliclona ), H. ( Gellius ) and H. ( Rhizoniera ) in the presence of an anisotropic reticulation with clearly defined ascending primary lines and unispicular connecting secondary lines.
However, in Haliclona the reticulation is very regular throughout the choanosome, regularly connected by unispicular secondary lines and with moderate to abundant spongin. The oxeas are usually short and stout, cigarshaped. The secondary lines of the new species seem to be more irregular and in the deeper choanosome the reticulation is very irregular, sub-halichondrioid, more similar to that of H. ( Gellius ). One of the main characters leading to H. ( Gellius ) in the key to the subgenera of Haliclona proposed by de Weerdt (2002) is that the choanosomal anisotropic reticulation becomes more disorganized and sub-halichondrioid from the surface towards the interior of the sponge, similarly to that of the new species. This character however is not mentioned any further in the Systema Porifera chapter, neither in the subgenus diagnosis and definition nor in the redescription of the type species (de Weerdt, 2002). Nevertheless, a transition from a more or less regular anisotropic reticulation near the ectosome to an irregular sub-halichondrioid reticulation deep inside the choanosome is present in at least some species of the subgenus H. ( Gellius ), including H. (Gell.) angulata ( Bowerbank, 1866) , H. (Gell.) rava (Stephens, 1902) , and the type species H. (Gell.) fibulata ( Schmidt, 1862) . In the subgenus H. ( Gellius ), however, the oxeas are usually very large (160–1000 µm, more often 300–500 µm long) and microscleres are often present in the form of sigmas and toxas, while H. (Rhiz.) fugidia sp. nov. has small oxeas (86–124 µm long) and no microscleres. In the subgenus H. ( Rhizoniera ) the primary lines are usually pauci- to multispicular with scarce spongin, whereas in the new species they are uni- to paucispicular with moderate to low amount of spongin. As in the new species, in the subgenus H. ( Rhizoniera ) the microscleres are absent, megascleres are slender, relatively small oxeas, the choanosomal meshes are irregular, and the ectosomal tangential skeleton is usually absent. Also, in some species the choanosomal skeleton becomes more disorganized towards the base of the sponge (e.g., Haliclona (Rhiz.) viscosa ( Topsent, 1888) ; cf. de Weerdt, 1986). Therefore, it was with hesitation that we decided to allocate the new species in the subgenus Haliclona (Rhizoniera) . We made small emmendments in the subgenus definition to accomodate the new species. It is clear, however, that the current subgeneric classification of the genus Haliclona must be improved. Recent phylogenetic analysis based on 28S and 18S rDNA sequences ( McCormack et al., 2002; Redmond et al., 2007, 2013) indicate that the family Chalinidae , the genus Haliclona and most of its subgenera are all probably non-monophyletic. Therefore, below we will compare the new species not only to those of the subgenus H. ( Rhizoniera ), but to all 34 species of Haliclona currently known from the Tropical Western Atlantic, most of which were tabulated by Bispo et al. (2014).
Van Soest et al. (2014) list 83 valid species in the subgenus Haliclona (Gellius) , of which only three occur in the TWA. Haliclona (Gell.) calcinea ( Burton, 1954) from 720–800 m depth in Grenada is massive rounded, dark brown in spirit; H. (Gell.) megasclera Lehnert & van Soest (1996) from 78 m depth in Jamaica is massive, grey, and agglutinates Halimeda leaves; and H. (Gell.) tenerrima Burton, 1954 from 3.5 m depth in Belize was described from a fragment, pale yellow in spirit. The oxea of the three species are much larger than in the new species (500 x 16 µm, 282–370 x 9–12 µm and 280 x 7 µm, respectively). Two of them also have microscleres: H. (Gell.) calcinea has sigmas and toxas and H. (Gell.) tenerrima has toxas. H. (Gell.) megasclera has raphid-like growth stages of the oxea (Lehnert & van Soest, 1996), which are absent in the new species.
Seven species of the subgenus H. ( Halichoclona ) are known from the TWA: H. (Halich.) albifragilis ( Hechtel, 1965) , from Florida, Barbados, Venezuela, Jamaica, Bonaire and Curaçao, is well characterized by the combination of an opaque white color, friable consistency and slender oxea (de Weerdt, 2000). H. (Halich.) dura Sandes et al., 2014 from Sergipe State, NE Brazil, is thick encrusting, dark brown, and has a hard, incompressible consistency. H. (Halich.) lernerae Campos et al., 2005 from 94 m depth at the North Brazilian continental shelf is massive with tube-like projections. H. (Halich.) magnifica de Weerdt et al., 1991 from Belize, Florida and Puerto Rico has a massive base from which arise thick walled tubes. H. (Halich.) perforata ( Pulitzer-Finali, 1986) from Puerto Rico has a tangential ectosomal reticulation of unispicular tracts. H. (Halich.) stoneae de Weerdt, 2000 form thick cushions with large oscules and has a subisotropic tangential ectosomal reticulation with paucispicular tracts. H. (Halich.) vansoesti de Weerdt et al., 1999 has a whitish ectosome and a violet to pink choanosome, and the oscules are placed on top of short tubular projections. All these species further differ from the new species by the subisotropic choanosomal reticulation, without well-defined primary lines, and by the detachable ectosome separated from the choanosome by large subdermal cavities, typical of the subgenus (de Weerdt, 2002).
Twenty-three species are currently accepted in the subgenus H. ( Haliclona ) in the World Porifera Database, of which only one occurs in the Tropical Western Atlantic (cf. de Weerdt, 2000; van Soest et al., 2014): H. (Halicl.) epiphytica Zea & de Weerdt, 1999 from the Colombian Caribbean forms cream-colored, thin incrustations (0.1–1.1 cm thick), exclusively epibiotic on seaweeds, has a tangential ectosomal reticulation, and has thick, short oxeas (63–97 µm long; Zea & de Weerdt, 1999; de Weerdt, 2000). Although not allocated to any subgenera by the WPD (van Soest et al., 2014), two other species from Southern Brazil have been included in the subgenus Haliclona due to their ladder-like reticulate skeleton with unispicular secondary lines: H. (Halicl.) catarinensis Mothes & Lerner, 1994 and H. (Halicl.) lilaceus Mothes & Lerner, 1994 (cf. Muricy et al., 2011; Bispo et al., 2014). H. (Halicl.) catarinensis differs from H. (Rhiz.) fugidia sp. nov. by the orange-gray color, the absence of superficial canals, subectosomal and choanosomal lacunae, the multispicular ascending spicule tracts (vs. uni- to paucispicular tracts in the new species), and the larger oxeas (111‒161/3‒9 vs. 86‒124/1‒6 µm). H. (Halicl.) lilaceus is distinguishable by its violet color, thin encrusting shape (1 mm thick), microconulose surface, and multispicular ascending tracts ( Mothes & Lerner, 1994). These two species from S Brazil need revision for a more detailed description of their skeleton and external morphology.
The subgenus H. ( Reniera ) is represented in TWA by eight species. H. (Ren.) chlorilla Bispo et al., 2014 from NE Brazil forms delicate, anastomosing branches, dark green to black in color. H. (Ren.) implexiformis ( Hechtel, 1965) , widely distributed in the Caribbean, is bright violet-pink. H. (Ren.) manglaris Alcolado, 1984 , known from several Caribbean localities and also from NE Brazil, is green or bright turquoise-green and form laterally spreading sheets with low volcano-shaped elevations. H. (Ren.) mucifibrosa de Weerdt et al., 1991 from Belize, Florida, Colombia and Jamaica is greyish purple to bluish gray, has an irregularly massive base and short thick-walled oscular chimneys. H. (Ren.) portroyalensis Jackson et al., 2006 from Jamaica is purple and digitate. H. (Ren.) ruetzleri de Weerdt, 2000 from Belize is light brown, with extremely delicate, slender anastomosing branches. H. (Ren.) strongylophora Lehnert & van Soest, 1996 from Jamaica is encrusting, dark brown, and its megascleres are strongyles. H. (Ren.) tubifera ( George & Wilson, 1919) , known from the Caribbean to North Carolina, has abundant volcano- or chimney-shaped oscular elevations. All these species are easily distinguishable from the new species by the (sub)isotropic choanosomal reticulation, without well-defined primary lines, typical of the subgenus H. ( Reniera ).
The subgenus H. ( Soestella ) has 21 species, of which 11 occur in TWA: H. (Soest.) brassica Sandes et al., 2014 from NE Brazil is lamellate and its spicules are strongyles and raphides. H. (Soest.) caerulea ( Hechtel, 1965) is widely distributed from the Caribbean to Bahia State, NE Brazil (Hajdu et al., 2011); it usually forms irregular cushions with thin digitations ranging from yellowish green to light blue in color and has sigmas. H. (Soest.) lehnerti de Weerdt, 2000 from Jamaica is dark red and has conspicuous aquiferous canals at the surface. H. (Soest.) luciensis de Weerdt, 2000 from Santa Lucia is dark brown and has raphides. H. (Soest.) melana Muricy & Ribeiro, 1999 from Santa Lucia and Brazil is black and has toxas. H. (Soest.) piscaderensis (van Soest, 1980) from Jamaica and Curaçao is greyish yellow to light purplish brown and has sigmas. H. (Soest.) peixinhoae Bispo et al., 2014 from Bahia State, NE Brazil, forms dense aggregations of erect tubes with slender torn-like projections. H. (Soest.) smithae de Weerdt, 2000 form yellow-green oscular mounds and has raphides. H. (Soest.) twincayensis de Weerdt et al., 1991, from Belize, Florida, Guadeloupe and Venezuela, forms slender branches, whitish grey to pink in vivo. H. (Soest.) vermeuleni de Weerdt, 2000 from E Caribbean to North Carolina is blue and forms small outgrowths (oscular chimneys, blind digitations, and lobes). H. (Soest.) walentinae Diaz et al., 2007 from Panama is thinly encrusting, dark brown to purple externally and tan internally. All these species further differ from the new species by the irregular primary lines with a tendency to form rounded meshes both in the choanosomal and ectosomal skeletons, typical of the subgenus H. ( Soestella ) (de Weerdt, 2002).
The subgenus H. ( Rhizoniera ) has 17 valid species, of which only two are currently recognized from TWA: H. (Rhiz.) curacaoensis (van Soest, 1980) from Curaçao, Belize, South Carolina, Florida and Grenadines and H. (Rhiz.) mammillaris Mothes & Lerner, 1994 from South Brazil. Although these are the species most similar to H. (Rhiz.) fugidia sp. nov., both clearly differ from it in external morphology: H. (Rhiz.) curacaoensis is bluish-purple and has an encrusting base with closely-packed oscular mounds; H. (Rhiz.) mammillaris is encrusting, white in spirit, and its oscules are located on top of mamiliform projections. Other five species of H. ( Rhizoniera ) occur in other regions in the Atlantic: H. (Rhiz.) canaliculata Hartman, 1958 from the NW Atlantic (Connecticut), is thinly encrusting, has a ridged surface, multispicular primary lines and multispicular "horizontal" spicule tracts (cf. Hartman, 1958). H. (Rhiz.) indistincta ( Bowerbank, 1866) from NE Atlantic is greenish-brown. H. (Rhiz.) pedunculata Boury-Esnault et al., 1994 from Alboran Sea is stipitate and has two categories of sigmas. H. (Rhiz.) rosea ( Bowerbank, 1866) from NE Atlantic has somewhat wavy primary lines and rather long oxeas (150–220 µm long; de Weerdt, 1986). Finally, H. (Rhiz.) viscosa ( Topsent, 1888) from NE Atlantic and Mediterranean is greyishpurple verging to yellow towards the base, has chimney- or volcano-shaped osculiferous elevations and is extremely slimy (cf. de Weerdt, 1986).
TABLE 1. Spicule measurements (oxeas length and width in µm) of the type specimens of Haliclona (Rhizoniera) fugidia sp. nov. from Rio de Janeiro State, SE Brazil. Measurements are expressed as minimum – average (± standard deviation) – maximum. N = 30.
| Specimen | Length | Width |
|---|---|---|
| MNRJ 16846 | 86.4–108.6 (± 8.7)–123.5 | 1.3–3.5 (± 1.1)–5.5 |
| MNRJ 16860-B | 78.2–101.8 (± 11.7)–126.1 | 2.5–3.6 (± 0.7)–5.1 |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.