Macaca assamensis (McClelland, 1839)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6867065 |
|
DOI |
https://doi.org/10.5281/zenodo.6863167 |
|
persistent identifier |
https://treatment.plazi.org/id/CE199B17-FFCF-FFCB-FF2E-6408FC2BF821 |
|
treatment provided by |
Jonas |
|
scientific name |
Macaca assamensis |
| status |
|
15. View Plate 36: Cercopithecidae
Assamese Macaque
French: Macaque d’'Assam / German: Assam-Makak / Spanish: Macaco de Assam
Other common names: Assam Macaque; Eastern Assamese Macaque (assamensis), Western Assamese Macaque (pelops)
Taxonomy. Macacus assamensis McClelland, 1839 ,
India, Assam.
M. assamensis is a member of the sinica species group of macaques that also includes M. sinica , M. radiata , and M. thibetana ; their distributions are allopatric or parapatric and extend from Sri Lanka to east-central China. The glans penis of this group is highly distinctive: the apex is sagittate, it is narrowed towards the tip, and its length is less than 30 mm. The subspecies recognized here are clearly different in the relative tail length of the adult males. A population differing in general body form and coloration has been identified in Nepal and may represent a new subspecies. Two subspecies recognized.
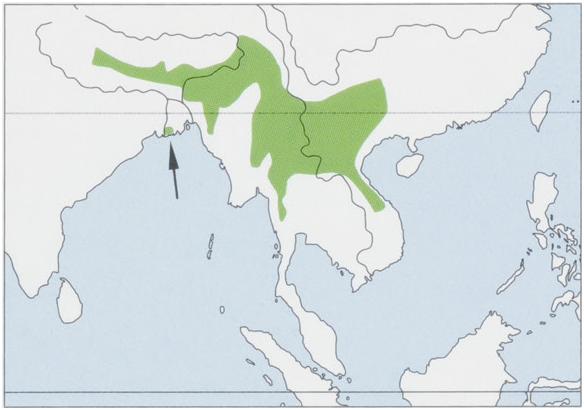
Subspecies and Distribution.
M. a. pelops Hodgson, 1840 — Himalayas up to 3100 m above sea level, from C Nepal (W limit Tipling, 83° 36’ E) E through NE India (N West Bengal, Sikkim, W Assam states), and Bhutan (E limit M.a. River, 90° 58” E), with a widely disjunct record, of what may be a geographic relict, in coastal SW Bangladesh (Sundarbans). View Figure
Descriptive notes. Head-body 53.2-73 cm (males) and 43-7-58:7 cm (females), tail 19-36 cm (males) and 17-29.3 cm (females); weight 7.9-16.5 kg (males) and 4.9-8.7 kg (females). The Assamese Macaque is much larger than the southern species of the sinica species group and more sexually dimorphic. Sexual dimorphism of headbody length of male “Eastern Assamese Macaques” (M. a. assamensis ) may exceed that of the “Western Assamese Macaque” (M. a. pelops). Pelage on dorsal and lateral surfaces of the trunk is brown, varying individually from golden brown to dark chocolatebrown, usually with scapular regions somewhat brighter, paler, and longer-haired than lower back regions. Bright burnt-orange coloration (erythrism) may occur on the anterior dorsal trunk during the cool season in western Thailand. Short, coarse, drab dorsal pelage is reported late in the dry season. Color of outer surfaces of limbs and tail is similar to the adjacent surface of the trunk. Ventral surfaces of trunk and limbs are thinly haired, pale buffy to whitish, with exposed ventral skin pigmented pale bluish. Crown is about the color of the back, with hair variably smooth, tufted, or forming a rudimentary cap. Buffy to whitish cheek tufts and chin whiskers usually prominent in adults, with blackish marginal hairs around the face. Face is thinly haired, with pale pinkish to whitish skin above the orbits and brownish to purplish skin on the muzzle. Early infant pelage is distinctively drab grayish brown.
Habitat. Subtropical mid-elevation montane broadleaf evergreen rainforest. In Bhutan, the preferred habitat of the Assamese Macaque is mostly tropical wet evergreen and semi-evergreen forest in foothills and subtropical and temperate broadleaf forests in higher hills and mountains. In western Thailand, it has also been observed in mixed deciduous/bamboo and dry evergreen forest, patchily distributed from lowland to mountainous areas, and in hill forest. Limestone outcroppings, rocky cliffs, or protruding crags with sparse vegetation, sometimes near watercourses, are commonly important features of various habitats and may be preferred sleeping sites of the Assamese Macaque. In hill forest in rolling plateau (1000 m above sea level) in western Thailand, protruding rocky crags, or fig trees ( Ficus , Moraceae ) adjacent to them, may be preferred sleeping sites. Two large accumulations of macaque feces were recorded near cliff faces suggesting habitual use as sleeping sites. Frequently, the Assamese Macaque is, or was, the most common macaque in hills and mountains throughout its distribution. Human encroachment is causing a change in the landscapes where it is found. In West Bengal, some groups are semi-wild or provisioned, inhabiting areas adjacent to or in human settlements. At a dam site in Kanchanaburi Province, western Thailand, near its known southernmost distribution (14° 41° N), Assamese Macaques resort to begging and nuisance behavior to obtain food from tourists.
Food and Feeding. Assamese Macaques are predominantly vegetarian, although animal food, including insects and small vertebrates such as lizards, may be consumed. Fruit appears to be the principal natural food. Bamboo shoots are also eaten. Diets of mostly leaves are reported in Bangladesh and Nepal. Crop raiding, especially for corn, occurs in many regions throughout the distribution of Assamese Macaques. Figs are among fruits consumed in western Thailand. When feeding in trees, they gather food either by breaking off terminal branches that bear several fruits or by plucking individual fruits. They feed on fruits of the same species as the Lar Gibbon (Hylobates lar). Human disturbance of their native habitat is resulting in the use of new foods. In Darjeeling District, West Bengal, the Assamese Macaque eats fruits, leaves, seeds, petioles, bases of leaves, flowers, rhizomes, epiphytic roots, and cotyledons from 63 different plant species, of which 52 are wild and the rest agricultural. Fruits such as banana, orange, apple, mango, guava, papaya, litchi, cucumber, and tomato and items such as bread, biscuits, snacks, rice grain, grams, groundnuts, and molasses may be obtained directly or indirectly from humans. One study group located away from human habitation near riverbank and thick vegetation consumed 90-1% natural food daily. A second study group inhabiting open-valley land interspersed with human settlements and tea plantations consumed less (743%). Artificial foodstuffs made up the remainder of the diet in both groups. A preference for natural vegetation may persist despite gradual dependence on artificial food items.
Breeding. Cyclical ovarian swelling of sexual skin in females of the sinica species group is relatively modest. In the Assamese Macaque, swellings are reported to occur only in adolescence, although a subcaudal swelling has been noted during pregnancy in captivity. Gray, blue, or purple coloration of the sexual skin has been noted in all four species in the sinica species group, and a bluish perineal streak has been reported in a pregnant captive Assamese Macaque. A small number of records available from eastern India and Nepal to northern Vietnam suggests that births probably peak in summer (rainy season), and thus mating probably peaks in winter (dry season). A distinct four-month mating season (October—January/February) occurs in wild populations in north-eastern Thailand. Based on fecal hormone analysis,it appears that female Assamese Macaques synchronize mating, not ovarian cycles; receptivity 1s socially mediated (significantly associated with the number of other females mating on a given day) and induced before onset of ovulation and maintained after conception. Conceptions are spread over the mating season, with few occurring at the beginning. Females are promiscuous, initiating 70% of copulations and rarely refusing to copulate. Males are single-mount ejaculators. Extended sexuality may enhance benefits that a female receives from many males, although she may mate repeatedly with a “primary partner.” Harassment by other females is rare. A single young is born every 1-2 years after a gestation of ¢.5-5 months. A captive female conceived until c.18 years of age, but her last viable infant was born when she was c.14 years old. Longevity in captivity is ¢.28 years.
Activity patterns. Assamese Macaques are rarely observed on the ground in the forest but will raid cultivated crops and explore areas of human habitation (both rural and urban). When frightened or disturbed, they may flee through the canopy and then descend to the ground and withdraw quietly through underbrush, or flee first through trees and then on the ground. In Darjeeling District, the time spent in daily feeding was nearly 1-5 hours in two study groups, with 51% of this time occurring during a morning feeding session. Maximum feeding occurred in trees at heights of 5 m and 13 m. Although it varies seasonally, overall feeding time averaged 13-3% of the daily routine. An activity budget recorded in Nepal in March-April during two successive years averaged 45-5% feeding, 27% walking, 16-5% resting, and 11% grooming. A provisioned free-ranging group of Assamese Macaques in Golpara, India, spent 36% of its time feeding, 30% resting, 20% grooming, and 15% moving, and in a second study during the same year spent 43% foraging, 22% walking, 18% resting, and 17% grooming. An estimated time budget for a provisioned group at a temple in northern Thailand in the month of September was ¢.31% resting, 27% traveling, 17% feeding, 15% playing, 8% grooming, 1% engaged in aggressive behaviors, and 0-3% engaged in sexual activities. Differences in nutritive values of foods may be responsible for differences in percentages of time spent feeding and foraging.
Movements, Home range and Social organization. Home ranges of all species in the sinica species group may be relatively stable. In some areas (Sikkim, Darjeeling, India, and Guangxi, Yunnan, China), Assamese Macaques migrate seasonally between higher elevations in summer and lower elevations in winter. Groups forage for seasonally available fruit in phalanx or file formation in a mosaic of mixed deciduous/bamboo and dry evergreen forest in western Thailand. Typical of the genus, Assamese Macaques live in multimale-multifemale groups. Male transfer is assumed. Group size averages.20 individuals (range 7-50 individuals). Single individuals (probably males) have been seen in Bhutan and Nepal. The mean sex ratio of adult males to adult females was 1:2-3 in a small sample of three groups in West Bengal and northern Thailand and 1:1-6 and 1:1-9 in seven groups in Nepal during successive years. Another group studied in northern Thailand consisted of 53 individuals, including 13 males and twelve adult females, and 55 individuals, including 15 males and 14 adult females, in consecutive years. Large adult males may be important in detecting and providing protection against potential predators, including humans.
Status and Conservation. CITES Appendix II. Classified as Near Threatened on The IUCN Red List, including both subspecies. The extent of human population pressure and economic development differentially affect the status of the Assamese Macaque throughoutits distribution. Reduction and degradation of habitat and hunting and poaching constitute the most serious threats. Human encroachment on its native habitat has increased crop raiding and other pest behaviors. The Assamese Macaque is the most abundant primate in Bhutan. Existing levels of harvesting of forest resources are unlikely to be of serious concern. There is no organized poaching or trade of any primate in Bhutan. Rapid development in some areas and hydroelectric projects and the presence of villages in protected areas may constitute problems in the future. In contrast, the Assamese Macaque in India and Bangladesh is considered endangered and critically endangered, respectively. During the past 20 years, populations there have declined significantly because of habitat fragmentation and degradation, along with hunting for food and traditional medicine. Selective logging is a major cause of habitat loss, and the loss offruiting trees and human encroachment contribute to the decline in habitat quality. In 2002, the number of adult males and females was estimated at less than 300. In China, the Assamese Macaque was quite numerous and common in south-eastern Yunnan in the 1950s and 1960s but subsequently became rare. A significant decline began in Guangxi in the 1980s. Before declaration of the 1988 Wildlife Protection Law, in which the Assamese Macaque was listed as a Protected Animal of the First Class, its bones and fur were commonly brought by state purchasing stations for traditional medicine. Local hunters continue to poach Assamese Macaques as if they were the Rhesus Macaque ( M. mulatta ), which can be trapped and hunted legally as a crop raider. Body parts of the Assamese Macaque are not uncommon at county markets. Traditional shifting cultivation by cultural minorities has long been a major disturbance to wildlife habitat but on a minor scale compared with technologybased agriculture, including rubber plantations. In China, the Assamese Macaque is now confined mainly to the upper elevations of steep mountains and karst hills with relatively degraded vegetation. It is classified variously as endangered or extremely threatened in China and may have numbered no more than 5000-7000 individuals around the year 2000. In Laos, the Assamese Macaque is usually the most common macaque in hills and mountains and is considered to be potentially at risk. It may be affected by opportunistic hunting to supply bonesfor traditional medicine in Vietnam. Although such threats are incipient, agriculture, especially commodity crops, mining, and hydropower, are causing significant loss of forest. The Assamese Macaque was classified as vulnerable in the Red Data Book of Vietnam in the 1990s. Poaching for traditional medicine, stimulated by increasing affluence in Vietnam and East Asia,is putting extreme pressure on all primate populations in Vietnam, and no new conservation measures have been introduced. Direct threats to the habitat include logging, gold mining, and shifting cultivation by minority peoples. The Assamese Macaque may be losing preferred habitat because limestone outcroppings are being mined for the production of cement for the construction boom in the country. Factors associated with substantial economic development, such as timber extraction, large-scale farms and plantations, irrigation and hydroelectric projects, highway construction, mining, resettlement programs for hill tribes and others, and recreation and tourism have significantly reduced forest cover in Thailand. The shifting cultivation of hill tribes and ethnic Thais has also resulted in large areas of forest being cleared. Illegal hunting for sale (market hunting) and poaching for food near villages can directly impact primate populations including Assamese Macaques. The Western Conservation Corridor in western Thailand may offer some protection to both habitat and wildlife. Forest exploitation, hunting, and political unrest of cultural minorities have impacted Assamese Macaques in Myanmar.
Bibliography. Abegg & Thierry (2002a), Aggimarangsee (1992), Ahsan (1994), Bernstein & Cooper (1999), Chalise (2003), Choudhury (2008a), Dathe (1983), Duckworth et al. (1999), Eudey (1979, 1980, 1981, 1991), Fooden (1971, 1982, 1986, 1988), Flrtbauer et al. (2011), Gippoliti (2001), Groves (2001), Hamada, Kawamoto, Kurita et al. (2009), Hamada, Kawamoto, Oi et al. (2009), Jintanugool et al. (1985), Kingsada et al. (2010), Minh et al. (2010), Mitra (2002, 2002/2003), Molur et al. (2003), Sarkar & Bhattacharjee (1996), Timmins & Duckworth (2013), Vietnam, Ministry of Science, Technology and Environment (1992), Wehrenberg et al. (1980), Zhang Rongzu & Quan Guogiang (1996), Zhang Yongzu et al. (2002).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.